Duplex Analysis of Cannulated Vessels in Peripheral Veno-Arterial Extracorporeal Membrane Oxygenation
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
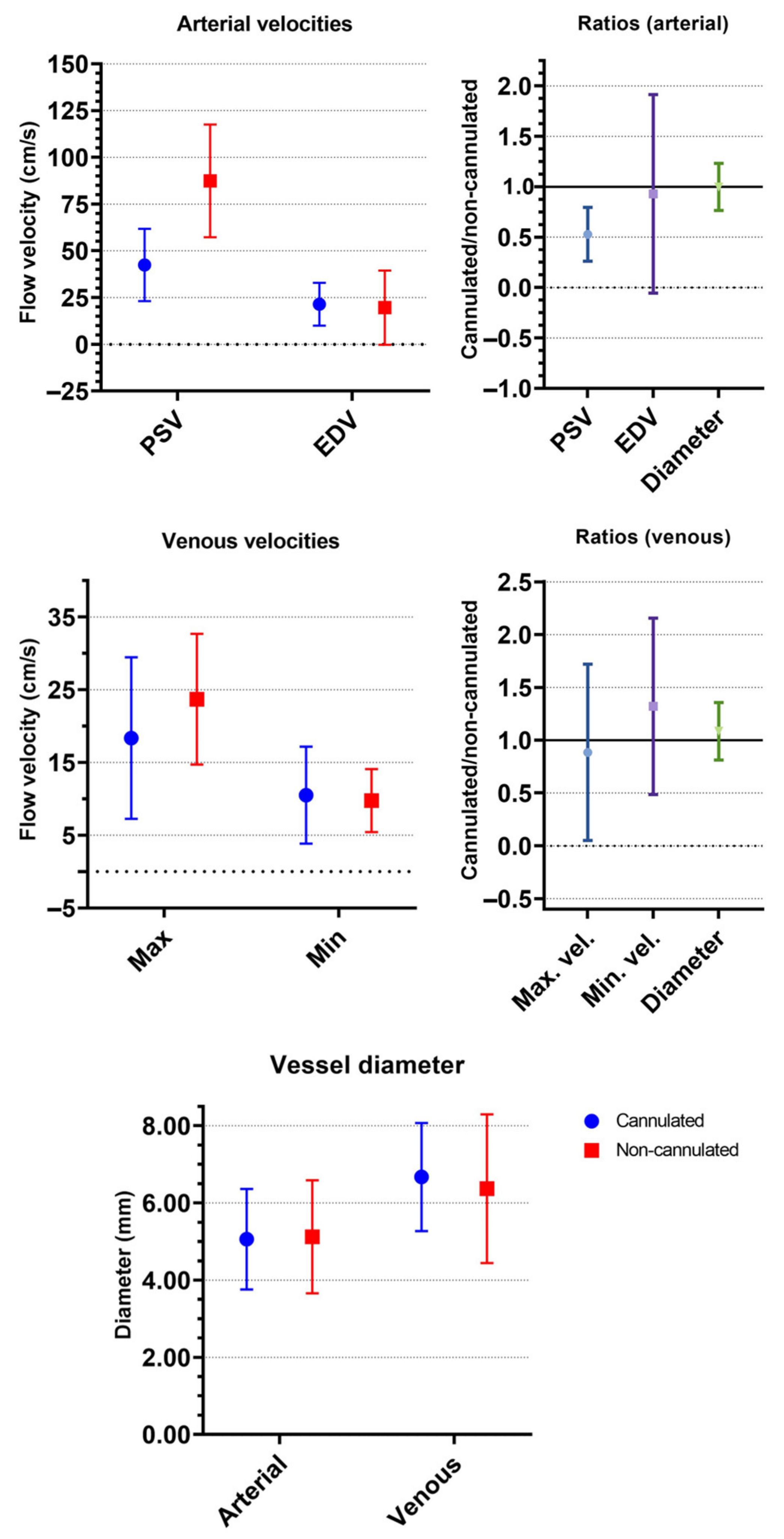
3.2. Duplex Analysis of Superficial Femoral Arteries
3.3. Duplex Analysis of Femoral Veins
3.4. Sub-Analysis of Limb Ischemia
3.5. Sub-Analysis of IABP
3.6. Outcomes
4. Discussion
4.1. Main Findings
- Normal PSV with, in most patients, multiphasic flow patterns in the SFAs on the non-cannulated side.
- Respirophasic (with or without cardiac cycles) flow patterns in the FVs on the non-cannulated side.
- Decreased PSV and monophasic flow patterns in the SFAs on the cannulated side.
- Non-phasic flow patterns in the majority of the FVs on the cannulated side.
- Suggestions that continuous flow, with a small difference between the maximum and minimum flow velocity in the FVs on the cannulated side and a large vein diameter ratio, indicate venous stasis, which might be a risk factor for limb ischemia.
4.2. Arterial and Venous Duplex Parameters
4.3. Duplex Parameters in Limb Ischemia and IABP Support
4.4. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitman, G.J. Extracorporeal membrane oxygenation for the treatment of postcardiotomy shock. J. Thorac. Cardiovasc. Surg. 2017, 153, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sauer, C.M.; Yuh, D.D.; Bonde, P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015, 61, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Broman, L.M.; Taccone, F.S.; Lorusso, R.; Malfertheiner, M.V.; Pappalardo, F.; Di Nardo, M.; Belliato, M.; Bembea, M.M.; Barbaro, R.P.; Diaz, R.; et al. The ELSO Maastricht Treaty for ECLS Nomenclature: Abbreviations for cannulation configuration in extracorporeal life support—A position paper of the Extracorporeal Life Support Organization. Crit. Care 2019, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Juo, Y.Y.; Skancke, M.; Sanaiha, Y.; Mantha, A.; Jimenez, J.C.; Benharash, P. Efficacy of Distal Perfusion Cannulae in Preventing Limb Ischemia During Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. Artif. Organs 2017, 41, E263–E273. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Yang, I.X.; Ling, R.R.; Syn, N.; Poon, W.H.; Murughan, K.; Tan, C.S.; Choong, A.; MacLaren, G.; Ramanathan, K. Vascular Complications of Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Regression Analysis. Crit. Care Med. 2020, 48, e1269–e1277. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hou, D.; Wang, J.; Cui, Y.; Wang, X.; Xing, Z.; Jiang, C.; Hao, X.; Du, Z.; Yang, X.; et al. Vascular complications in adult postcardiotomy cardiogenic shock patients receiving venoarterial extracorporeal membrane oxygenation. Ann. Intensive Care 2018, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Bonicolini, E.; Martucci, G.; Simons, J.; Raffa, G.M.; Spina, C.; Lo Coco, V.; Arcadipane, A.; Pilato, M.; Lorusso, R. Limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation: A narrative review of incidence, prevention, monitoring, and treatment. Crit. Care 2019, 23, 266. [Google Scholar] [CrossRef] [PubMed]
- Extracorporeal Life Support Organization. ECLS Registry Report April 2021. Available online: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx (accessed on 21 February 2022).
- Lorusso, R.; Shekar, K.; MacLaren, G.; Schmidt, M.; Pellegrino, V.; Meyns, B.; Haft, J.; Vercaemst, L.; Pappalardo, F.; Bermudez, C.; et al. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO J. 2021, 67, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.F.; Cannata, A.; Vitali, E.; Lanfranconi, M. Prevention of limb ischemia and edema during peripheral venoarterial extracorporeal membrane oxygenation in adults. J. Card Surg. 2009, 24, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Sharma, A.M.; Scissons, R.; Dawson, D.; Eberhardt, R.T.; Gerhard-Herman, M.; Hughes, J.P.; Knight, S.; Marie Kupinski, A.; Mahe, G.; et al. Interpretation of peripheral arterial and venous Doppler waveforms: A consensus statement from the Society for Vascular Medicine and Society for Vascular Ultrasound. Vasc. Med. 2020, 25, 484–506. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, A.; Scolletta, S.; Lorusso, R.; Taccone, F.S. Hybrid extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S707–S715. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.C.; Tonna, J.E.; Nanjayya, V.; Nixon, P.; Abrams, D.C.; Raman, L.; Bernard, S.; Finney, S.J.; Grunau, B.; Youngquist, S.T.; et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement from the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, H.; Brandl, R. Importance of ultrasound for diagnosing periphereal arterial disease. Ultraschall Med. 2009, 30, 334–374. [Google Scholar] [CrossRef] [PubMed]
- Kotval, P.S. Doppler waveform parvus and tardus. A sign of proximal flow obstruction. J. Ultrasound Med. 1989, 8, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lyons, S.; Hedgire, S. Ultrasound imaging of the arterial system. Cardiovasc. Diagn. Ther. 2019, 9, S2–S13. [Google Scholar] [CrossRef] [PubMed]
- de Smet, A.A.; Ermers, E.J.; Kitslaar, P.J. Duplex velocity characteristics of aortoiliac stenoses. J. Vasc. Surg. 1996, 23, 628–636. [Google Scholar] [CrossRef][Green Version]
- Denault, A.Y.; Aldred, M.P.; Hammoud, A.; Zeng, Y.H.; Beaubien-Souligny, W.; Couture, E.J.; Jarry, S.; Gebhard, C.E.; Langevin, S.; Lamarche, Y.; et al. Doppler Interrogation of the Femoral Vein in the Critically Ill Patient: The Fastest Potential Acoustic Window to Diagnose Right Ventricular Dysfunction? Crit. Care Explor. 2020, 2, e0209. [Google Scholar] [CrossRef] [PubMed]
- Alimoğlu, E.; Erden, A.; Gürsel, K.; Olçer, T. Correlation of right atrial pressure and blood flow velocities in the common femoral vein obtained by duplex Doppler sonography. J. Clin. Ultrasound 2001, 29, 87–91. [Google Scholar] [CrossRef]
- Abu-Yousef, M.M.; Mufid, M.; Woods, K.T.; Brown, B.P.; Barloon, T.J. Normal lower limb venous Doppler flow phasicity: Is it cardiac or respiratory? AJR Am. J. Roentgenol. 1997, 169, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Tansey, E.A.; Montgomery, L.E.A.; Quinn, J.G.; Roe, S.M.; Johnson, C.D. Understanding basic vein physiology and venous blood pressure through simple physical assessments. Adv. Physiol. Educ. 2019, 43, 423–429. [Google Scholar] [CrossRef] [PubMed]
| Overall Population | |
|---|---|
| N = 19 | |
| Gender | |
| Male | 11 (57.9%) |
| Female | 8 (42.1%) |
| Age (years) | 56 ± 14 |
| Body Mass Index (kg/m²) | 27.1 ± 5.4 |
| Body Surface Area (m²) | 1.95 ± 0.16 |
| Type of organ failure | |
| Cardiac | 15 (78.9%) |
| Cardiac and kidney | 3 (15.8%) |
| Cardiac and pulmonary | 1 (5.3%) |
| History | |
| Hypertension | 7 (36.8%) |
| Myocardial infarction at presentation | 6 (31.6%) |
| Endocarditis | 5 (26.3%) |
| Recent atrial fibrillation | 5 (26.3%) |
| Hypercholesterolemia | 5 (26.3%) |
| Asthma | 3 (15.8%) |
| Diabetes | 3 (15.8%) |
| Myocardial infarction (<90 days before presentation) | 2 (10.5%) |
| Cardiothoracic surgery (<1 year before presentation) | 2 (10.5%) |
| Recent acute kidney injury (<90 days before presentation) | 2 (10.5%) |
| Peripheral artery disease | 1 (5.3%) |
| Deep venous thrombosis | 1 (5.3%) |
| Chronic obstructive pulmonary disease | 1 (5.3%) |
| Cardiothoracic surgery (<90 days before presentation) | 1 (5.3%) |
| Overall Population N = 19 | |
|---|---|
| Indication | |
| Postcardiotomy, ventricular failure | 12 (63.2%) |
| Cardiogenic shock, right ventricular failure | 2 (10.5%) |
| ECPR, right ventricular failure | 2 (10.5%) |
| Post-myocardial infarction, ventricular septum rupture | 1 (5.3%) |
| Post-myocardial infarction | 1 (5.3%) |
| Respiratory failure, pulmonary hypertension | 1 (5.3%) |
| Mode | |
| V-A | 16 (84.2%) |
| V-AV | 2 (10.5%) |
| VV-A | 1 (5.3%) |
| Mean ECMO settings (at initiation) | |
| Blood flow (L/min) | 3.6 ± 0.6 |
| Air flow (L/min) | 2.5 ± 1.1 |
| Fraction inspired oxygen (%) | 57 ± 22 |
| pH | 7.30 ± 0.17 |
| pCO2 (mmHg) | 4.6 ± 1.3 |
| pO2 (mmHg) | 18 ± 7.2 |
| HCO3− (mmol/L) | 16.6 ± 5.5 |
| Norepinephrine (mcg/kg/min) | |
| None | 1 (5.0%) |
| 0–0.2 | 4 (21.1%) |
| 0.25–0.5 | 8 (42.1%) |
| >0.5 | 6 (31.6%) |
| Hemodynamic support agents (mean, count) | 1.6 ± 0.8 |
| IABP (during duplex) | |
| No | 13 (68.4%) |
| Yes | 6 (31.6%) |
| Overall Population, N = 19 | ||
|---|---|---|
| Arterial Cannula | Venous Cannula | |
| Cannulation Mode | ||
| Unilateral | 16 (84%) | |
| Bilateral | 3 (16%) | |
| Cannula size | ||
| 15 Fr | 2 (11%) | |
| 19 Fr | 12 (63%) | |
| 21 Fr | 5 (26%) | 2 (11%) |
| 23 Fr | 2 (11%) | |
| 25 Fr | 15 (79%) | |
| DPC (at time of duplex) | ||
| No | 1 (5%) | |
| Yes | 17 (89%) | |
| After | 1 (5%) | |
| Limb NIRS at initiation of ECMO | ||
| Side with arterial cannula | 64 ± 18% | |
| Side without non-arterial cannula | 59 ± 21% | |
| Superficial Femoral Artery | Overall Population | No Limb Ischemia | Limb Ischemia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cannulated | Non-Cannulated | Ratio | Cannulated | Non-Cannulated | Ratio | Cannulated | Non-Cannulated | Ratio | |
| N = 19 | N = 19 | N = 15 | N = 15 | N = 4 | N = 4 | ||||
| PSV, mean (cm/s) | 42.4 ± 19.4 | 87.4 ± 30.1 | 0.53 ± 0.27 | 39.7 ± 15.7 | 87.3 ± 31.8 | 0.49 ± 0.19 | 52.7 ± 30.4 | 87.8 ± 27 | 0.69 ± 0.48 |
| EDV, mean (cm/s) | 21.4 ± 11.4 | 19.6 ± 19.8 | 0.93 ± 0.98 | 18.6 ± 9 | 16.2 ± 18.4 | 0.73 ± 0.8 | 32.1 ± 14.5 | 32.5 ± 22.3 | 1.68 ± 1.38 |
| Diameter, mean (mm) | 5.1 ± 1.3 | 5.1 ± 1.5 | 1.00 ± 0.23 | 5.1 ± 1.4 | 5.2 ± 1.5 | 0.98 ± 0.23 | 5.1 ± 0.9 | 4.6 | 1.24 |
| Flow pattern | |||||||||
| Multiphasic | 0 (0%) | 8 (42.1%) | 0 (0%) | 6 (40%) | 0 (0%) | 2 (50%) | |||
| IABP | 0 (0%) | 4 (21.1%) | 0 (0%) | 4 (26.7%) | 0 (0%) | 0 (0%) | |||
| Monophasic | |||||||||
| High resistive | 0 (0%) | 2 (10.5%) | 0 (0%) | 1 (6.7%) | 0 (0%) | 1 (25%) | |||
| Intermediate resistive | 2 (10.5%) | 4 (21.1%) | 2 (13.3%) | 3 (20%) | 0 (0%) | 1 (25%) | |||
| Low resistive | 4 (21.1%) | 1 (5.3%) | 3 (20%) | 1 (6.7%) | 1 (25%) | 0 (0%) | |||
| Minimal phasic | 10 (52.6%) | 0 (0%) | 7 (46.7%) | 0 (0%) | 3 (75%) | 0 (0%) | |||
| Plus IABP signal | 3 (15.8%) | 0 (0%) | 3 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Blood flow during duplex, mean (L/min) | 3.09 ± 0.94 | 3.15 ± 0.96 | 2.85 ± 0.9 | ||||||
| DPC | |||||||||
| Flow (mL/min) | 261.9 ± 55.21 | 253.63 ± 59.34 | 295 ± 7.07 | ||||||
| Placement | |||||||||
| Direct | 17 (89.5%) | 15 (100%) | 2 (50%) | ||||||
| Delayed | 1 (5.3%) | 0 (0%) | 1 (25%) | ||||||
| None | 1 (5.3%) | 0 (0%) | 1 (25%) | ||||||
| Femoral Vein | Overall Population | No limb Ischemia | Limb Ischemia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cannulated | Non-Cannulated | Ratio | Cannulated | Non-Cannulated | Ratio | Cannulated | Non-Cannulated | Ratio | |
| N = 19 | N = 18 | N = 15 | N = 14 | N = 4 | N = 4 | ||||
| Maximum velocity, mean (cm/s) | 18.4 ± 11.1 | 23.7 ± 9 | 0.89 ± 0.83 | 17.9 ± 11.6 | 22.6 ± 7.2 | 0.92 ± 0.93 | 20.3 ± 10.3 | 27.5 ± 14.4 | 0.77 ± 0.42 |
| Minimum velocity, mean (cm/s) | 10.5 ± 6.7 | 9.8 ± 4.3 | 1.32 ± 0.84 | 9.6 ± 6.2 | 9.1 ± 4.2 | 1.3 ± 0.73 | 14 ± 8.2 | 12.2 ± 4.4 | 1.39 ± 1.29 |
| Diameter, mean (mm) | 6.7 ± 1.4 | 6.4 ± 1.9 | 1.08 ± 0.27 | 6.7 ± 1.5 | 6.6 ± 2 | 1.03 ± 0.25 | 6.8 ± 1 (n = 2) | 4.8 ± 0.3 (n = 2) | 1.41 ± 0.12 |
| Flow pattern | |||||||||
| Respirophasic and cardiac cycle | 1 (5.3%) | 5 (27.8%) | 1 (6.7%) | 4 (28.6%) | 0 (0%) | 1 (25%) | |||
| Respirophasic | 3 (15.8%) | 6 (33.3%) | 3 (20%) | 4 (28.6%) | 0 (0%) | 2 (50%) | |||
| Decreased | 3 (15.8%) | 1 (5.6%) | 3 (20%) | 1 (7.1%) | 0 (0%) | 0 (0%) | |||
| Pulsatile (cardiac cycle) | 3 (15.8%) | 4 (22.2%) | 2 (13.3%) | 3 (21.4%) | 1 (25%) | 1 (25%) | |||
| Continuous | 9 (47.4%) | 2 (11.1%) | 6 (40%) | 2 (14.3%) | 3 (75%) | 0 (0%) | |||
| Superficial Femoral Artery | Without IABP | With IABP | ||||
|---|---|---|---|---|---|---|
| Cannulated | Non-Cannulated | Ratio | Cannulated | Non-Cannulated | Ratio | |
| N = 13 | N = 13 | N = 6 | N = 6 | |||
| PSV, mean (cm/s) | 48.7 ± 18.8 | 89.2 ± 22.9 | 0.59 ± 0.29 | 28.8 ± 13.1 | 83.6 ± 44.5 | 0.39 ± 0.16 |
| EDV, mean (cm/s) | 25.3 ± 11.1 | 25 ± 14.7 | 1.31 ± 0.82 | 13 ± 7.1 | 8 ± 25.7 | 0.1 ± 0.82 |
| Diameter, mean (mm) | 5.0 ± 1.2 | 5.3 ± 1.5 | 0.94 ± 0.18 | 5.1 ± 1.6 | 4.8 ± 1.5 | 1.09 ± 0.29 |
| Flow pattern | ||||||
| Multiphasic | 0 (0%) | 7 (53.8%) | 0 (0%) | 1 (16.7%) | ||
| IABP | 0 (0%) | 0 (0%) | 0 (0%) | 4 (66.7%) | ||
| Monophasic | ||||||
| High resistive | 0 (0%) | 2 (15.4%) | 0 (0%) | 0 (0%) | ||
| Intermediate resistive | 2 (15.4%) | 4 (30.8%) | 0 (0%) | 0 (0%) | ||
| Low resistive | 4 (30.8%) | 0 (0%) | 0 (0%) | 1 (16.7%) | ||
| Minimal phasic | 7 (53.8%) | 0 (0%) | 3 (50%) | 0 (0%) | ||
| Plus IABP signal | 0 (0%) | 0 (0%) | 3 (50%) | 0 (0%) | ||
| Blood flow during duplex, mean (L/min) | 3.10 ± 0.94 | 3.07 ± 1.01 | ||||
| DPC | ||||||
| Flow (mL/min) | 287.5 ± 19.94 | 223.5 ± 72.15 | ||||
| Placement | ||||||
| Direct | 11 (84.6%) | 6 (100%) | ||||
| Delayed | 1 (7.7%) | 0 (0%) | ||||
| None | 1 (7.7%) | 0 (0%) | ||||
| Femoral Vein | Without IABP | With IABP | ||||
|---|---|---|---|---|---|---|
| Cannulated | Non-Cannulated | Ratio | Cannulated | Non-Cannulated | Ratio | |
| N = 13 | N = 12 | N = 6 | N = 6 | |||
| Maximum velocity, mean (cm/s) | 20.3 ± 13.1 | 23.3 ± 10.5 | 1.03 ± 1 | 14.2 ± 1.8 | 24.5 ± 5.5 | 0.6 ± 0.15 |
| Minimum velocity, mean (cm/s) | 10.8 ± 7.7 | 9.6 ± 4.5 | 1.46 ± 0.99 | 10 ± 4 | 10 ± 4.4 | 1.03 ± 0.25 |
| Diameter, mean (mm) | 6.6 ± 0.1.6 | 6.2 ± 2.2 | 1.11 ± 0.31 | 6.8 ± 1.1 | 6.6 ± 1.5 | 1.04 ± 0.21 |
| Flow pattern | ||||||
| Respirophasic and pulsatile | 1 (7.7%) | 2 (16.7%) | 0 (0%) | 3 (50%) | ||
| Respirophasic | 2 (15.4%) | 5 (41.7%) | 1 (16.7%) | 1 (16.7%) | ||
| Decreased | 0 (0%) | 1 (8.3%) | 3 (50%) | 0 (0%) | ||
| Pulsatile | 3 (23.1%) | 3 (25%) | 0 (0%) | 1 (16.7%) | ||
| Continuous | 7 (53.8%) | 1 (8.3%) | 2 (33.3%) | 1 (16.7%) | ||
| Overall Population N = 19 | |
|---|---|
| Bleeding at cannulation site | 3 (15.8%) |
| Compartment syndrome/fasciotomy | |
| During ECMO | 1 (5.3%) |
| After decannulation | 1 (5.3%) |
| Thrombectomy | |
| During ECMO | 3 (15.8%) |
| After decannulation | 1 (5.3%) |
| Arterial vessel repair | |
| During ECMO | 1 (5.3%) |
| After decannulation | 1 (5.3%) |
| ICU mortality | 9 (47.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simons, J.; Agricola, S.; Smets, J.; Metz, R.; Mariani, S.; Vleugels, M.-J.; Smeets, R.R.; van Mook, W.N.K.A.; Mees, B.; Lorusso, R. Duplex Analysis of Cannulated Vessels in Peripheral Veno-Arterial Extracorporeal Membrane Oxygenation. Medicina 2022, 58, 671. https://doi.org/10.3390/medicina58050671
Simons J, Agricola S, Smets J, Metz R, Mariani S, Vleugels M-J, Smeets RR, van Mook WNKA, Mees B, Lorusso R. Duplex Analysis of Cannulated Vessels in Peripheral Veno-Arterial Extracorporeal Membrane Oxygenation. Medicina. 2022; 58(5):671. https://doi.org/10.3390/medicina58050671
Chicago/Turabian StyleSimons, Jorik, Sandra Agricola, Jeroen Smets, Renske Metz, Silvia Mariani, Marie-José Vleugels, Reinier R. Smeets, Walther N. K. A. van Mook, Barend Mees, and Roberto Lorusso. 2022. "Duplex Analysis of Cannulated Vessels in Peripheral Veno-Arterial Extracorporeal Membrane Oxygenation" Medicina 58, no. 5: 671. https://doi.org/10.3390/medicina58050671
APA StyleSimons, J., Agricola, S., Smets, J., Metz, R., Mariani, S., Vleugels, M.-J., Smeets, R. R., van Mook, W. N. K. A., Mees, B., & Lorusso, R. (2022). Duplex Analysis of Cannulated Vessels in Peripheral Veno-Arterial Extracorporeal Membrane Oxygenation. Medicina, 58(5), 671. https://doi.org/10.3390/medicina58050671






