Epidemiologic Characteristics of Children with Diabetic Ketoacidosis Treated in a Pediatric Intensive Care Unit in a 10-Year-Period: Single Centre Experience in Croatia
Abstract
:1. Introduction
2. Materials and Methods
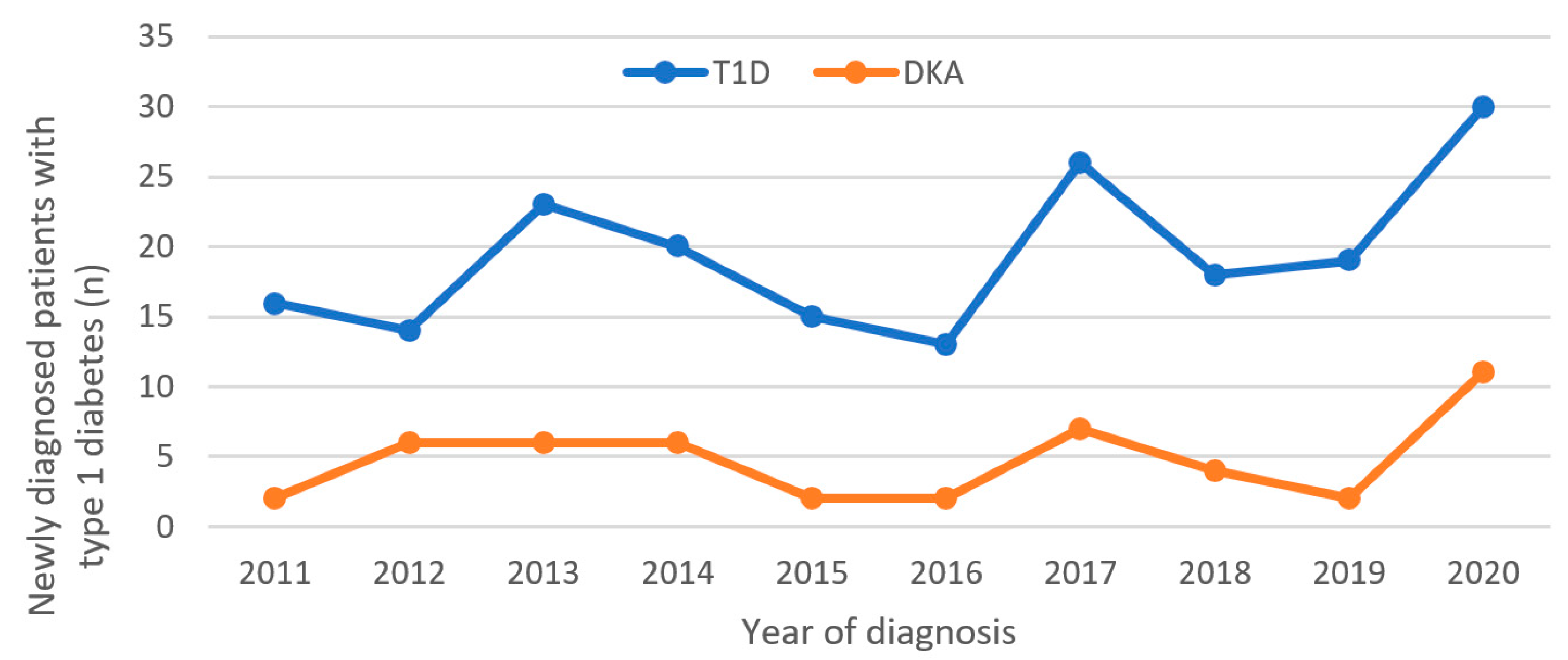
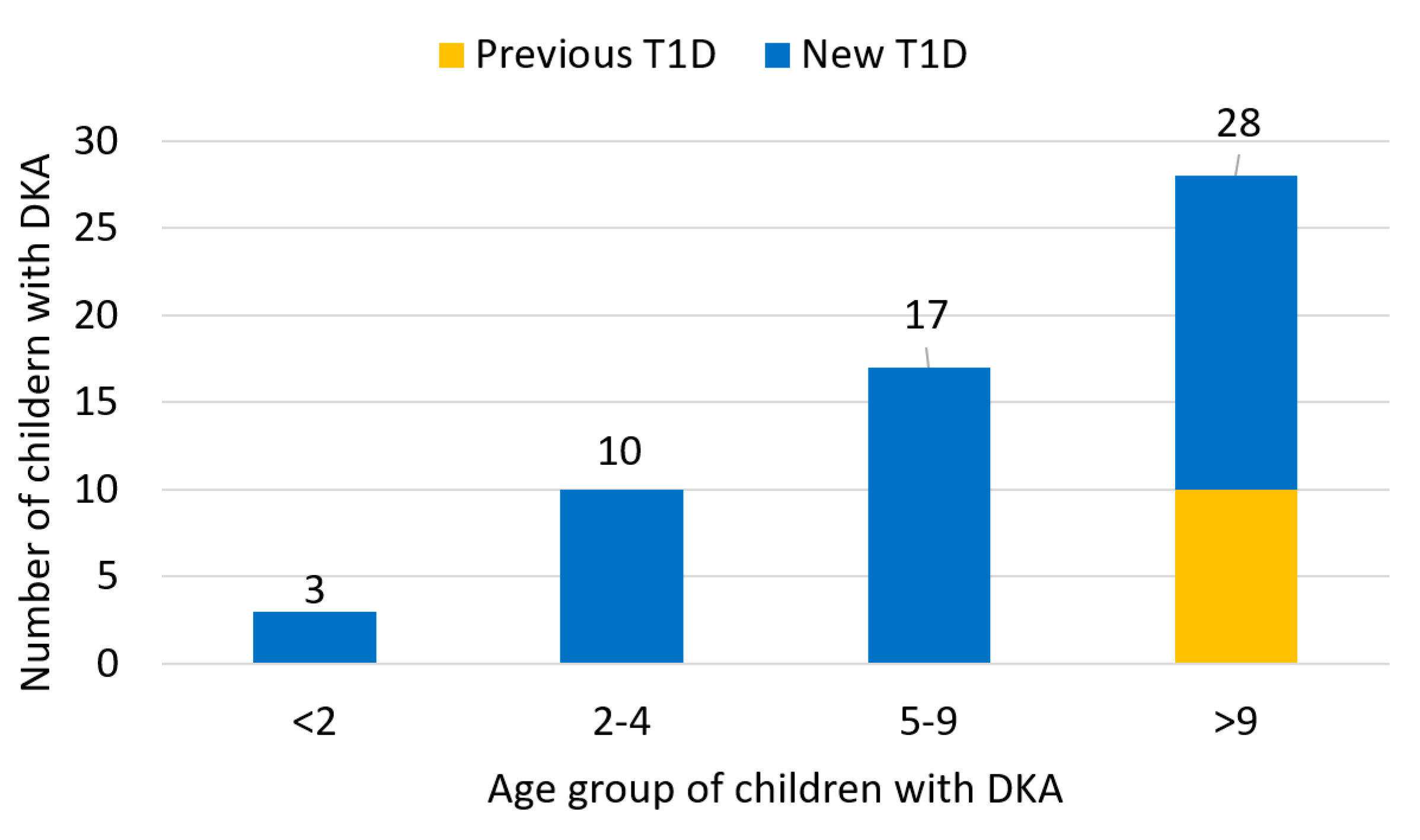
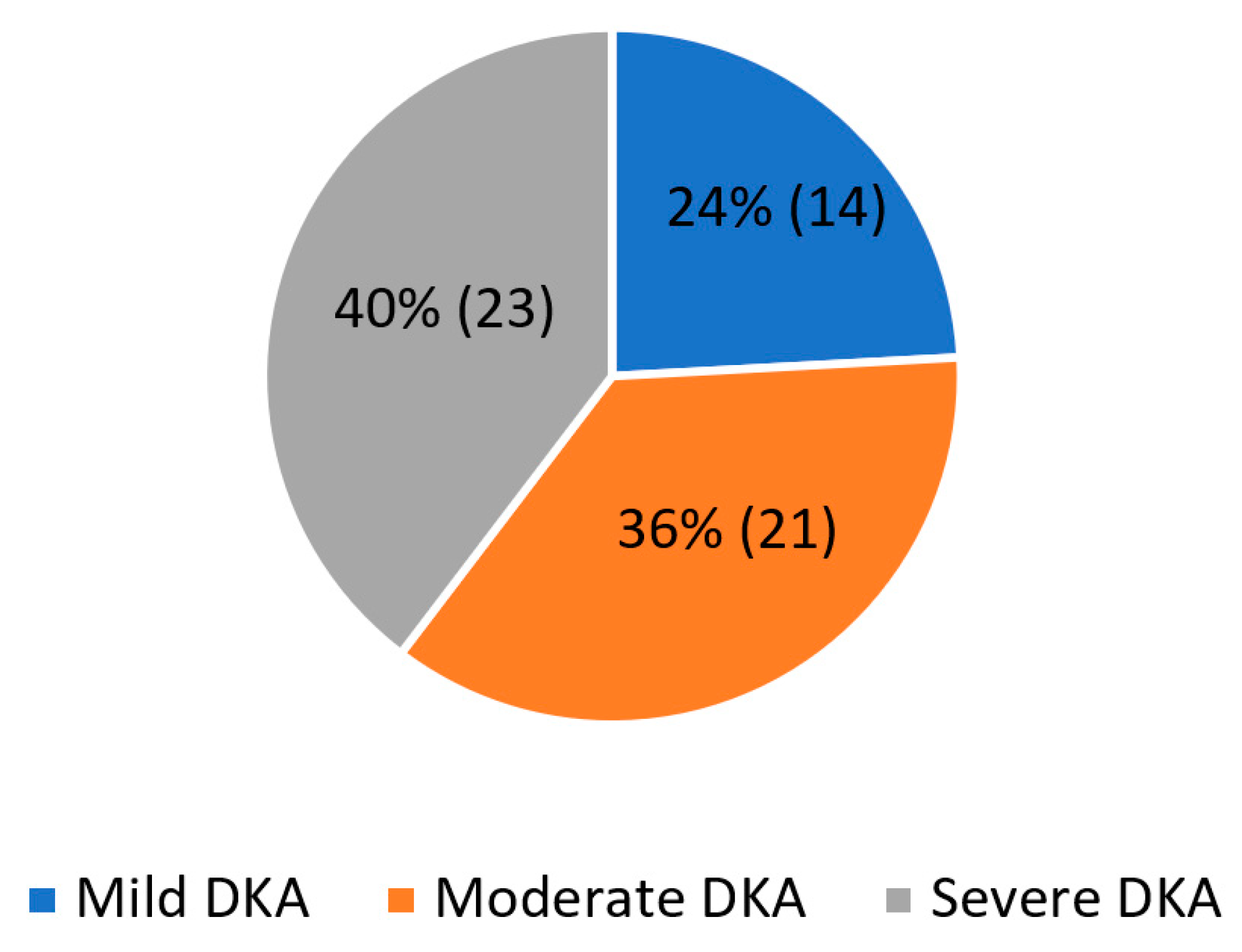
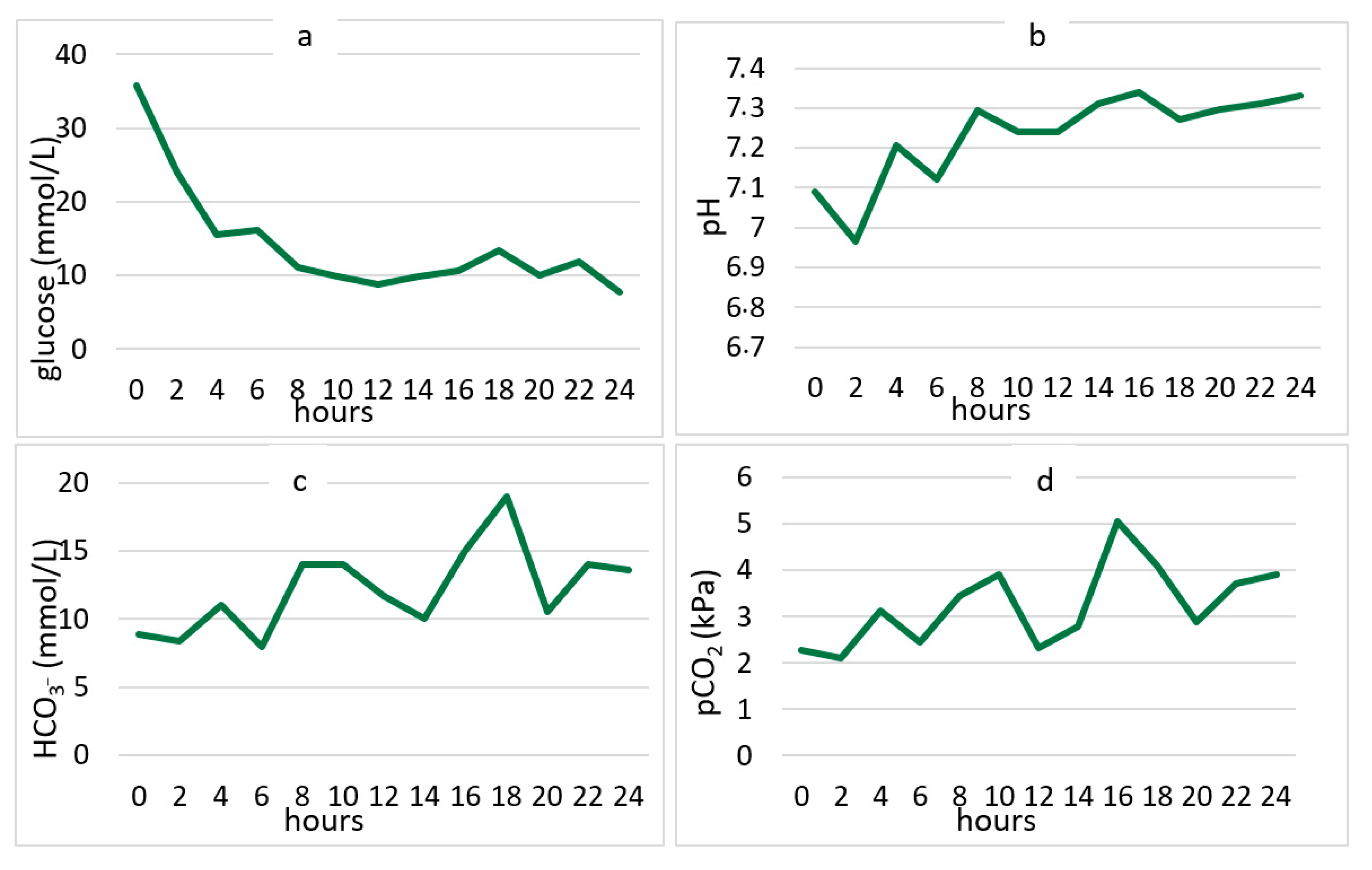
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfsdorf, J.I.; Glaser, N.; Agus, M.; Fritsch, M.; Hanas, R.; Rewers, A.; Sperling, M.A.; Codner, E. ISPAD clinical practice consensus guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr. Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Klingensmith, G.J.; Connor, C.G.; Ruedy, K.J.; Beck, R.W.; Kollman, C.; Haro, H.; Wood, J.R.; Lee, J.M.; Willi, S.M.; Cengiz, E.; et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr. Diabetes 2016, 17, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zucchini, S.; Scaramuzza, A.E.; Bonfanti, R.; Buono, P.; Cardella, F.; Cauvin, V.; Cherubini, V.; Chiari, G.; d’Annunzio, G.; Frongia, A.P.; et al. A multicenter retrospective survey regarding diabetic ketoacidosis management in Italian children with type 1 diabetes. J. Diabetes Res. 2016, 2016, 5719470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabelea, D.; Rewers, A.; Stafford, J.M.; Standiford, D.A.; Lawrence, J.M.; Saydah, S.; Imperatore, G.; D’Agostino RBJr Mayer-Davis, E.J.; Pihoker, C.; SEARCH for Diabetes in Youth Study Group. Trends in the prevalence of ketoacidosis at diabetes diagnosis: The SEARCH for diabetes in youth study. Pediatrics 2014, 133, e938–e945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Yuan, J.; Chiavaroli, V.; Dong, G.; Huang, K.; Wu, W.; Ullah, R.; Jin, B.; Lin, H.; Derraik, J.G.B.; et al. 10-Year Incidence of Diabetic Ketoacidosis at Type 1 Diabetes Diagnosis in Children Aged Less Than 16 Years from a Large Regional Center (Hangzhou, China). Front. Endocrinol. 2021, 27, 653519. [Google Scholar] [CrossRef]
- Cengiz, E.; Xing, D.; Wong, J.C.; Wolfsdorf, J.I.; Haymond, M.W.; Rewers, A.; Shanmugham, S.; Tamborlane, W.V.; Willi, S.M.; Seiple, D.L.; et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D exchange clinic registry. Pediatr. Diabetes 2013, 14, 447–454. [Google Scholar] [CrossRef]
- Maahs, D.M.; Hermann, J.M.; Holman, N.; Foster, N.C.; Kapellen, T.M.; Allgrove, J.; Schatz, D.A.; Hofer, S.E.; Campbell, F.; Steigleder-Schweiger, C.; et al. Rates of diabetic ketoacidosis: International comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care 2015, 38, 1876–1882. [Google Scholar] [CrossRef] [Green Version]
- Long, B.; Koyfman, A. Emergency Medicine Myths: Cerebral Edema in Pediatric Diabetic Ketoacidosis and Intravenous Fluids. J. Emerg. Med. 2017, 53, 212–221. [Google Scholar] [CrossRef]
- Tittel, S.R.; Rosenbauer, J.; Kamrath, C.; Ziegler, J.; Reschke, F.; Hammersen, J.; Mönkemöller, K.; Pappa, A.; Kapellen, T.; Holl, R.W.; et al. Did the COVID-19 Lockdown Affect the Incidence of Pediatric Type 1 Diabetes in Germany? Diabetes Care 2020, 43, e172–e173. [Google Scholar] [CrossRef]
- Ho, J.; Rosolowsky, E.; Pacaud, D.; Huang, C.; Lemay, J.A.; Brockman, N.; Rath, M.; Doulla, M. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr. Diabetes 2021, 22, 552–557. [Google Scholar] [CrossRef]
- McGlacken-Byrne, S.M.; Drew, S.E.V.; Turner, K.; Peters, C.; Amin, R. The SARS-CoV-2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus: A multi-centre study of the first COVID-19 wave. Diabet Med. 2021, 38, e14640. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.; Gorton, S.; Vangaveti, V.N.; Yates, J. Diabetic ketoacidosis incidence in children at first presentation of type 1 diabetes at an Australian regional hospital: The effect of health professional education. Pediatr. Diabetes 2018, 19, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Al Shaikh, A.; Farahat, F.; Saeedi, M.; Bakar, A.; Al Gahtani, A.; Al-Zahrani, N.; Jaha, L.; Aseeri, M.A.; Al-Jifree, H.M.; Al Zahrani, A. Incidence of diabetic ketoacidosis in newly diagnosed type 1 diabetes children in western Saudi Arabia: 11-year experience. J. Pediatric Endocrinol. Metab. 2019, 32, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, R.; Jesic, M.D.; Vorgucin, I.; Stankovic, S.; Folic, N.; Milenkovic, T.; Sajic, S.; Katanic, D.; Zivic, S.; Markovic, S.; et al. First report on the nationwide incidence of type 1 diabetes and ketoacidosis at onset in children in Serbia: A multicenter study. Eur. J. Pediatr. 2018, 177, 1155–1162. [Google Scholar] [CrossRef]
- Große, J.; Hornstein, H.; Manuwald, U.; Kugler, J.; Glauche, I.; Rothe, U. Incidence of Diabetic Ketoacidosis of New-Onset Type 1 Diabetes in Children and Adolescents in Different Countries Correlates with Human Development Index (HDI): An Updated Systematic Review, Meta-Analysis, and Meta-Regression. Horm. Metab. Res. 2018, 50, 209–222. [Google Scholar]
- Usher-Smith, J.A.; Thompson, M.; Ercole, A.; Walter, F.M. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: A systematic review. Diabetologia 2012, 55, 2878–2894. [Google Scholar] [CrossRef] [Green Version]
- Stipancić, G.; La Grasta Sabolić, L.; Pozgaj Sepec, M.; Radica, A.; Skrabić, V.; Severinski, S.; Kujundzić Tiljak, M. Regional differences in incidence and clinical presentation of type 1 diabetes in children aged under 15 years in Croatia. Croat. Med. J. 2012, 53, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Sharif, K.; Watad, A.; Coplan, L.; Amital, H.; Shoenfeld, Y.; Afek, A. Psychological stress and type 1 diabetes mellitus: What is the link? Expert Rev. Clin. Immunol. 2018, 14, 1081–1088. [Google Scholar] [CrossRef]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in Covid-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef]
- Kamrath, C.; Mönkemöller, K.; Biester, T.; Rohrer, T.R.; Warncke, K.; Hammersen, J.; Holl, R.W. Ketoacidosis in Children and Adolescents With Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA 2020, 324, 801–804. [Google Scholar] [CrossRef]
- Rabbone, I.; Schiaffini, R.; Cherubini, V.; Maffeis, C.; Scaramuzza, A. Diabetes Study Group of the Italian Society for Pediatric E. Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care 2020, 43, 2870–2872. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.; Seckold, R.; Smart, C.; King, B.R.; Howley, P.; Feltrin, R.; Smith, T.A.; Roy, R.; Lopez, P. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary Centre during the COVID-19 pandemic. Diabet. Med. 2020, 38, e14417. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.M.; Meusers, M.; Bachran, R.; Kuhnle-Krahl, U.; Jorch, N.; Hofer, S.E.; Holl, R.W.; DPV Initiative. Self-reported regular alcohol consumption in adolescents and emerging adults with type 1 diabetes: A neglected risk factor for diabetic ketoacidosis? Multicenter analysis of 29 630 patients from the DPV registry. Pediatr. Diabetes 2017, 18, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Pala, L.; Dicembrini, I.; Mannucci, E. Continuous subcutaneous insulin infusion vs. modern multiple injection regimens in type 1 diabetes: An updated meta-analysis of randomized clinical trials. Acta Diabetol. 2019, 56, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Burcul, I.; Arambasic, N.; Polic, B.; Kovacevic, T.; Bartulovic, I.; Catipovic Ardalic, T.; Markic, J. Characteristics of Children with Diabetic Ketoacidosis Treated in Pediatric Intensive Care Unit: Two-Center Cross-Sectional Study in Croatia. Medicina 2019, 10, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, F.; Günöz, H.; Saka, N.; Darendeliler, F.; Bundak, R.; Baş, F.; Neyzi, O. Epidemiologic Features of Type 1 Diabetic Patients between 0 and 18 Years of Age in İstanbul City. J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 49–56. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Thompson, M.J.; Zhu, H.; Sharp, S.J.; Walter, F.M. The pathway to diagnosis of type 1 diabetes in children: A questionnaire study. BMJ Open 2015, 5, e006470. [Google Scholar] [CrossRef] [Green Version]
- Iovane, B.; Cangelosi, A.M.; Bonaccini, I.; Di Mauro, D.; Scarabello, C.; Panigari, A.; Tiri, A.; Mastrorilli, C.; Fainardi, V.; Dodi, I.; et al. Diabetic ketoacidosis at the onset of Type 1 diabetes in young children Is it time to launch a tailored campaign for DKA prevention in children <5 years? Acta Biomed. 2018, 89, 67–71. [Google Scholar]
- Gallo de Moraes, A.; Surani, S. Effects of diabetic ketoacidosis in the respiratory system. World J. Diabetes 2019, 10, 16–22. [Google Scholar] [CrossRef]
- Fiordalisi, I.; Novotny, W.E.; Holbert, D.; Finberg, L.; Harris, G.D.; Critical Care Management Group. An 18-yr prospective study of pediatric diabetic ketoacidosis: An approach to minimizing the risk of brain herniation during treatment. Pediatr. Diabetes 2007, 8, 142–149. [Google Scholar] [CrossRef]
- Wolfler, A.; Osello, R.; Gualino, J.; Calderini, E.; Vigna, G.; Santuz, P.; Amigoni, A.; Savron, F.; Caramelli, F.; Rossetti, E.; et al. The Importance of Mortality Risk Assessment: Validation of the Pediatric Index of Mortality 3 Score. Pediatr. Crit. Care Med. 2016, 17, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.; Barnett, P.; McCaslin, I.; Nelson, D.; Trainor, J.; Louie, J.; Kaufman, F.; Quayle, K.; Roback, M.; Malley, R.; et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N. Engl. J. Med. 2001, 344, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.S.; Marcin, J.P.; Wootton-Gorges, S.L.; Buonocore, M.H.; Rewers, A.; Strain, J.; DiCarlo, J.; Neely, E.K.; Barnes, P.; Kuppermann, N. Correlation of clinical and biochemical findings with diabetic ketoacidosis-related cerebral edema in children using magnetic resonance diffusion-weighted imaging. J. Pediatr. 2008, 153, 541–546. [Google Scholar] [CrossRef] [PubMed]
- González Pannia, P.; Balboa, R.; Navarro, R.; Nocita, M.F.; Ferraro, M.; Mannucci, C. Prevalence of cerebral edema among diabetic ketoacidosis patients. Arch Argent Pediatr. 2020, 118, 332–336. [Google Scholar]
- Hsia, D.S.; Tarai, S.G.; Alimi, A.; Coss-Bu, J.A.; Haymond, M.W. Fluid management in pediatric patients with DKA and rates of suspected clinical cerebral edema. Pediatr. Diabetes 2015, 16, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.L.; Pinheiro, P.P.; Barberena, L.S.; Eckert, G.U. Diabetic ketoacidosis in a pediatric intensive care unit. J. Pediatr. RIO J. 2017, 93, 179–184. [Google Scholar] [CrossRef] [Green Version]
| Patients with DKA a | |||
|---|---|---|---|
| All patients n (%) | 58 (100%) | ||
| Demographic data | Females | 29 (50%) | |
| Family history of T1D b | 3 (5.8%) | ||
| Family history of T2D c | 25 (48.1%) | ||
| Symptoms at admission | Polydipsia | 43 (74.1%) | |
| Polyuria | 41 (70.7%) | ||
| Kussmaul breathing | 33 (56.9%) | ||
| Weight loss | 29 (50%) | ||
| Fatigue | 29 (50%) | ||
| Vomiting | 25 (43.1%) | ||
| Acetone breath | 24 (41.4%) | ||
| Loss of appetite | 23 (39.7%) | ||
| Nocturia | 23 (39.7%) | ||
| Abdominal pain | 17 (29.3%) | ||
| Nausea | 9 (15.5%) | ||
| Level of consciousness | Alert | 45 (77.6%) | |
| Respond to Voice | 9 (15.5%) | ||
| Respond to Pain | 3 (5.2%) | ||
| Unconscious | 1 (1.7%) | ||
| SD d | Reference Range | ||
| Laboratory parameters | Glucose (mmol/L) | 25.05 ± 8.85 | 3.3–5.5 |
| pH | 7.11 ± 0.18 | 7.35–7.45 | |
| HCO3 e (mmol/L) | 4.7 ± 4.33 | 21–28 | |
| pCO2 f (kPa) | 2.68 ± 3.05 | 4.5–6.2 | |
| BE g | −20.67 ± 7.11 | (−4)–(+2) | |
| HbA1c h (%) | 12.1 ± 2.21 | <6 | |
| Na i (mmol/L) | 132.25 ± 4.28 | 134–144 | |
| K j (mmol/L) | 4.20 ± 0.67 | 3.3–4.6 | |
| BUN k (mmol/L) | 5.10 ± 2.93 | 1.8–6.4 | |
| Creatinine (umol/L) | 54 ± 27.7 | 35–104 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lah Tomulić, K.; Matko, L.; Verbić, A.; Milardović, A.; Severinski, S.; Kolić, I.; Baraba Dekanić, K.; Šerifi, S.; Butorac Ahel, I. Epidemiologic Characteristics of Children with Diabetic Ketoacidosis Treated in a Pediatric Intensive Care Unit in a 10-Year-Period: Single Centre Experience in Croatia. Medicina 2022, 58, 638. https://doi.org/10.3390/medicina58050638
Lah Tomulić K, Matko L, Verbić A, Milardović A, Severinski S, Kolić I, Baraba Dekanić K, Šerifi S, Butorac Ahel I. Epidemiologic Characteristics of Children with Diabetic Ketoacidosis Treated in a Pediatric Intensive Care Unit in a 10-Year-Period: Single Centre Experience in Croatia. Medicina. 2022; 58(5):638. https://doi.org/10.3390/medicina58050638
Chicago/Turabian StyleLah Tomulić, Kristina, Lucija Matko, Arijan Verbić, Ana Milardović, Srećko Severinski, Ivana Kolić, Kristina Baraba Dekanić, Senada Šerifi, and Ivona Butorac Ahel. 2022. "Epidemiologic Characteristics of Children with Diabetic Ketoacidosis Treated in a Pediatric Intensive Care Unit in a 10-Year-Period: Single Centre Experience in Croatia" Medicina 58, no. 5: 638. https://doi.org/10.3390/medicina58050638
APA StyleLah Tomulić, K., Matko, L., Verbić, A., Milardović, A., Severinski, S., Kolić, I., Baraba Dekanić, K., Šerifi, S., & Butorac Ahel, I. (2022). Epidemiologic Characteristics of Children with Diabetic Ketoacidosis Treated in a Pediatric Intensive Care Unit in a 10-Year-Period: Single Centre Experience in Croatia. Medicina, 58(5), 638. https://doi.org/10.3390/medicina58050638






