Bevacizumab with Chemotherapy as a First-Line Treatment for Advanced Ovarian Cancer in a Serbian Cohort
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OC | Ovarian cancer |
| PFS | Progression-free survival |
| OS | Overall survival |
| ECOG | Eastern Cooperative Oncology Group |
| FIGO | International Federation of Gynecology and Obstetrics |
| VEGF | Vascular endothelial growth factor |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Luvero, D.; Plotti, F.; Aloisia, A.; Montera, R.; Terranova, C.; Nardone, C.D.C.; Scaletta, G.; Lopez, S.; Miranda, A.; Capriglione, S.; et al. Ovarian cancer relapse: From the latest scientific evidence to the best practice. Crit. Rev. Oncol. Hematol. 2019, 140, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Mäenpää, J.; et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1842–1855. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Haunschild, C.E.; Tewari, K.S. Bevacizumab use in the frontline, maintenance and recurrent settings for ovarian cancer. Future Oncol. 2020, 16, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kasherman, L.; Fazelzad, R.; Wang, L.; Bouchard-Fortier, G.; Lheureux, S.; Krzyzanowska, M.K. The use of bevacizumab in the modern era of targeted therapy for ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2021, 161, 601–612. [Google Scholar] [CrossRef]
- Scully, R.E.; Scully, R.; Sobin, L.; Serov, S. Histological Typing of Ovarian Tumours; Springer Science & Business Media: Berlin, Germany, 1999. [Google Scholar]
- Matz, M.; Coleman, M.P.; Sant, M.; Chirlaque, M.D.; Visser, O.; Gore, M.; Allemani, C.; Bouzbid, S.; Hamdi-Chérif, M.; Zaidi, Z.; et al. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol. Oncol. 2017, 144, 405–413. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. Obstet. Gynecol. Surv. 2014, 69, 402–404. [Google Scholar] [CrossRef]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012, 30, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oza, A.M.; Selle, F.; Davidenko, I.; Korach, J.; Mendiola, C.; Pautier, P.; Chmielowska, E.; Bamias, A.; DeCensi, A.; Zvirbule, Z.; et al. Efficacy and safety of bevacizumab-containing therapy in newly diagnosed ovarian cancer: ROSiA single-arm phase 3B study. Int. J. Gynecol. Cancer 2017, 27, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev. Anticancer. Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef]
- Ishikura, N.; Yorozu, K.; Kurasawa, M.; Yanagisawa, M.; Sugimoto, M.; Yamamoto, K. Sustained effect of continuous treatment with bevacizumab following bevacizumab in combination with chemotherapy in a human ovarian clear cell carcinoma xenograft model. Oncol. Rep. 2019, 42, 1057–1065. [Google Scholar] [CrossRef]
- Masi, G.; Salvatore, L.; Boni, L.; Loupakis, F.; Cremolini, C.; Fornaro, L.; Schirripa, M.; Cupini, S.; Barbara, C.; Safina, V.; et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: Final results of the randomized BEBYP trial. Ann. Oncol. 2015, 26, 724–730. [Google Scholar] [CrossRef]
- Căinap, C.; Bochis, O.V.; Vlad, C.; Popita, R.; Achimas-Cadariu, P.; Havasi, A.; Vidrean, A.; Dranca, A.; Piciu, A.; Bota, M.; et al. Prognostic factors for the efficacy of bevacizumab beyond progression in metastatic colorectal cancer. Front. Pharmacol. 2021, 12, 173. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nagashima, K.; Kawakami, T.; Mitani, S.; Komoda, M.; Tsuji, Y.; Izawa, N.; Kawakami, K.; Yamamoto, Y.; Makiyama, A.; et al. Second-line chemotherapy after early disease progression during first-line chemotherapy containing bevacizumab for patients with metastatic colorectal cancer. BMC Cancer 2021, 21, 1159. [Google Scholar] [CrossRef]
- Naumann, R.W.; Coleman, R.L.; Brown, J.; Moore, K.N. Phase III trials in ovarian cancer: The evolving landscape of front line therapy. Gynecol. Oncol. 2019, 153, 436–444. [Google Scholar] [CrossRef]
- Harter, P.; Bidzin´ski, M.; Colombo, N.; Floquet, A.; Rubio Perez, M.J.; Kim, J.W.; Lheureux, S.; Marth, C.; Nyvang, G.B.; Okamoto, A.; et al. DUO-O: A randomized phase III trial of durvalumab (durva) in combination with chemotherapy and bevacizumab (bev), followed by maintenance durva, bev and olaparib (olap), in newly diagnosed advanced ovarian cancer patients. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Sjoquist, K.M.; Espinoza, D.; Mileshkin, L.; Ananda, S.; Shannon, C.; Yip, S.; Goh, J.; Bowtell, D.; Harrison, M.; Friedlander, M.L. REZOLVE (ANZGOG-1101): A phase 2 trial of intraperitoneal bevacizumab to treat symptomatic ascites in patients with chemotherapy-resistant, epithelial ovarian cancer. Gynecol. Oncol. 2021, 161, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Wichelmann, T.A.; Abdulmujeeb, S.; Ehrenpreis, E.D. Bevacizumab and gastrointestinal perforations: A review from the FDA Adverse Event Reporting System (FAERS) database. Aliment. Pharmacol. Ther. 2021, 54, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
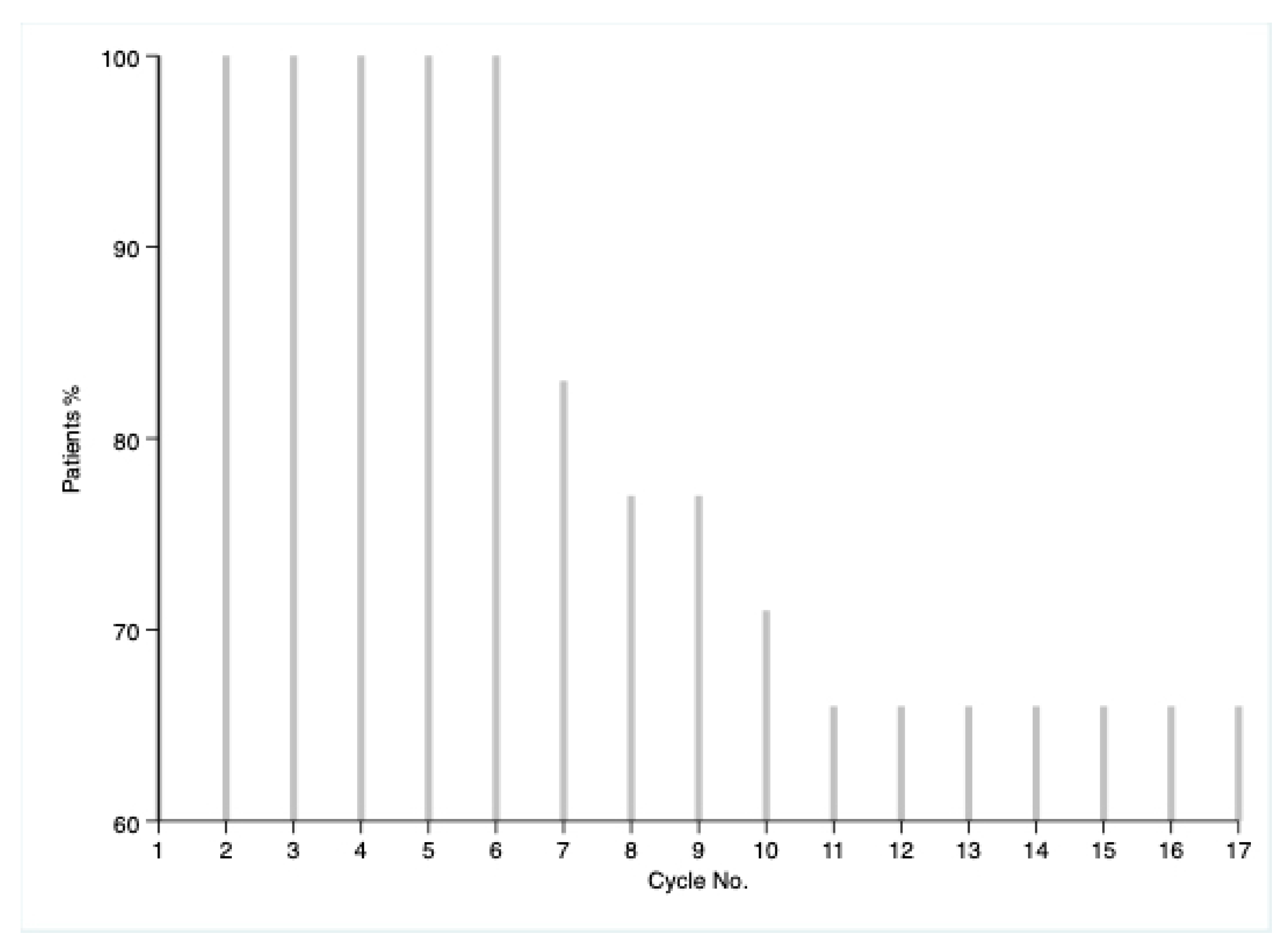
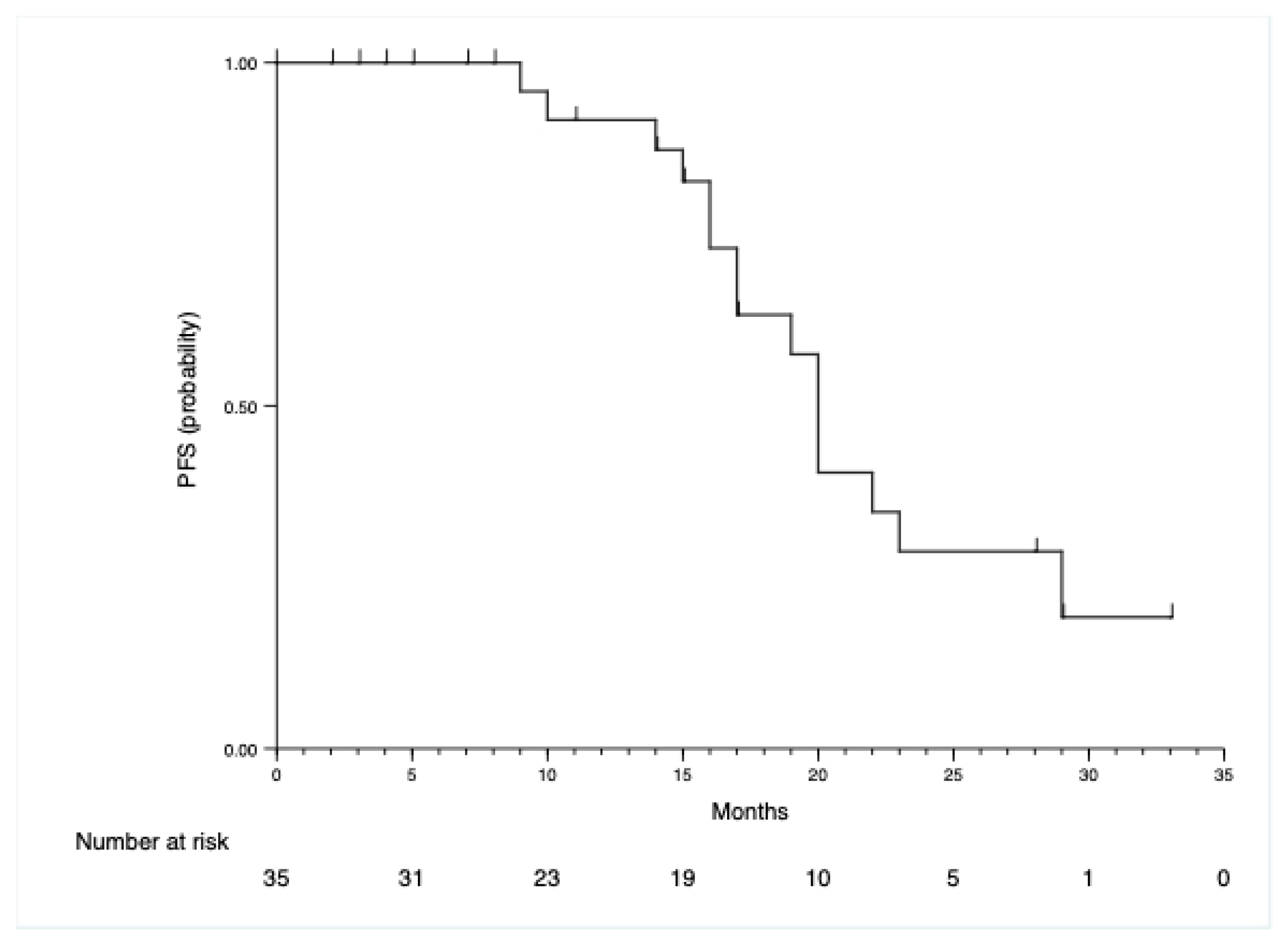
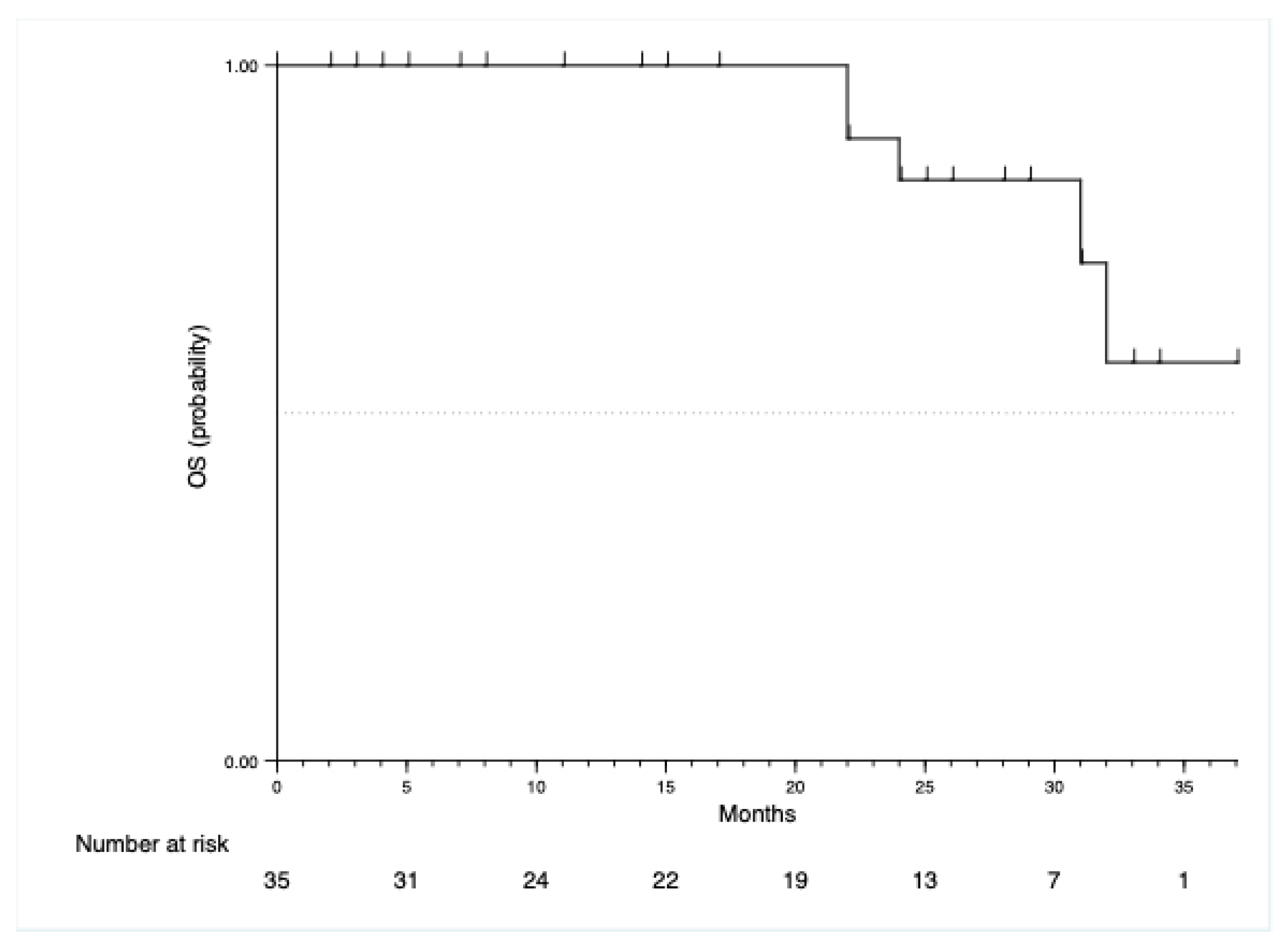
| Characteristic | N = 35 | Percentage |
|---|---|---|
| Age, years | ||
| Mean, SD | 61.8, 10.18 | |
| Histological subtype | ||
| Serous | 33 | 94.29 |
| Endometrioid | 0 | 0 |
| Mucinous | 1 | 2.86 |
| Clear cell | 1 | 2.86 |
| ECOG performance status | ||
| 0 | 10 | 28.57 |
| 1 | 25 | 71.43 |
| FIGO stage | ||
| IIIc | 19 | 54.29 |
| IVa | 12 | 34.29 |
| IVb | 4 | 11.43 |
| Ovarian cancer grade | ||
| Low grade | 4 | 11.43 |
| High grade | 31 | 88.57 |
| Ascites | ||
| Yes | 13 | 37.14 |
| No | 22 | 62.86 |
| Subgroup | PFS, No. of Events/No. of Patients | PFS, Median | OS, No. of Events/No. of Patients | OS, Median |
|---|---|---|---|---|
| Age, years | ||||
| <65 | 7/20 | 22 | 2/20 | NR |
| ≥65 | 8/15 | 20 | 3/15 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conic, I.; Nedovic, B.; Stojnev, S.; Todorovska, I.; Dimitrijevic, A.; Krstic, M.; Djordjevic, I.; Djordjevic, B. Bevacizumab with Chemotherapy as a First-Line Treatment for Advanced Ovarian Cancer in a Serbian Cohort. Medicina 2022, 58, 607. https://doi.org/10.3390/medicina58050607
Conic I, Nedovic B, Stojnev S, Todorovska I, Dimitrijevic A, Krstic M, Djordjevic I, Djordjevic B. Bevacizumab with Chemotherapy as a First-Line Treatment for Advanced Ovarian Cancer in a Serbian Cohort. Medicina. 2022; 58(5):607. https://doi.org/10.3390/medicina58050607
Chicago/Turabian StyleConic, Irena, Bojan Nedovic, Slavica Stojnev, Ilinka Todorovska, Aleksandra Dimitrijevic, Miljan Krstic, Ivana Djordjevic, and Biljana Djordjevic. 2022. "Bevacizumab with Chemotherapy as a First-Line Treatment for Advanced Ovarian Cancer in a Serbian Cohort" Medicina 58, no. 5: 607. https://doi.org/10.3390/medicina58050607
APA StyleConic, I., Nedovic, B., Stojnev, S., Todorovska, I., Dimitrijevic, A., Krstic, M., Djordjevic, I., & Djordjevic, B. (2022). Bevacizumab with Chemotherapy as a First-Line Treatment for Advanced Ovarian Cancer in a Serbian Cohort. Medicina, 58(5), 607. https://doi.org/10.3390/medicina58050607





