1. Introduction
Patellar tendinopathy is known to be a troublesome condition to treat [
1,
2]. When conservative treatments fail, surgery is indicated, and the traditional surgical treatment method is intra-tendinous revision surgery. However, the results with this tendon invasive method have been shown to be poor, with only 50% good clinical results [
2].
A new surgical treatment method, ultrasound-guided arthroscopic shaving, has shown good and reliable clinical results deleted word in clinical studies [
3,
4,
5,
6]. This method is based on the findings from ultrasound and Doppler-guided biopsies, showing that nerves were located outside the deep side of the tendon [
7]. The ultrasound-guided surgical treatment is targeting the regions with nerves outside the tendon, and no intra-tendinous revision surgery is performed. This allows for a more aggressive rehabilitation and a faster return to full tendon loading sport activities [
4,
5,
6].
In a sub-group of patients with patellar tendinopathy, athletes subjected to direct trauma to the anterior side of the patellar tendon, such as landing on a bent knee, we have observed a very distinct and disabling local tenderness on the superficial side of the proximal patellar tendon. In these patients, ultrasound and Doppler examination shows a localized thickening of the paratenon, including high blood flow, on the superficial side of the proximal patellar tendon. Ultrasound (US) and Doppler (DP)-guided arthroscopic shaving and additional open scraping of this superficial peritendinous tissue have resulted in good clinical outcomes [
6].
The aim with this study was to characterize this richly vascularized locally thickened paratenon, in a deleted word sub-group of patients with patellar tendinopathy by studying morphology and innervation patterns.
2. Materials and Methods
2.1. Patients and Clinical Procedures
The study was performed at the Department of Anatomy, Umeå University, Umeå, Sweden. Ethical approval to study tendon tissue samples was achieved from the Ethical Board Umeå University.
Six patients (4 men and 2 women; mean age 23 years, range 17–31 years) were included. All patients were sports active and had suffered from ultrasound and Doppler verified patellar tendinopathy in the proximal part of the patellar tendon for more than 3 months [
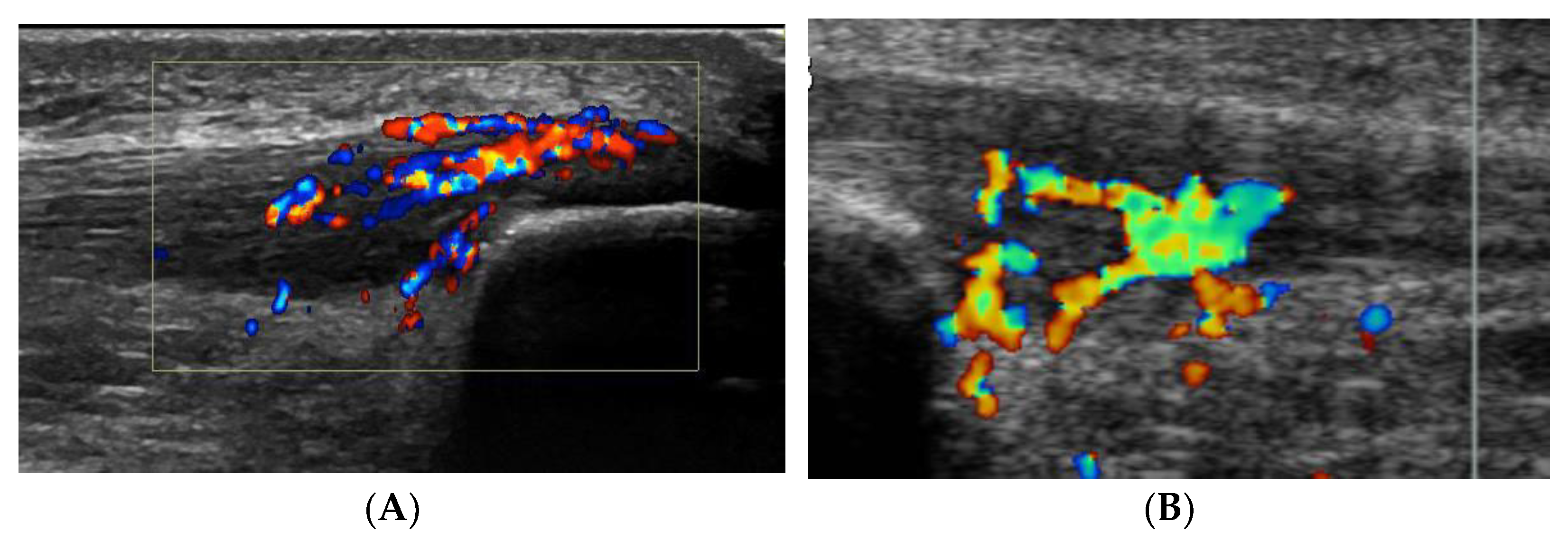
5]. In all deleted word these patients, there was also severe tenderness localized on the superficial side of the proximal patellar tendon, and ultrasound+Doppler examination showed a thickened paratenon including high blood flow in that region (
Figure 1A).
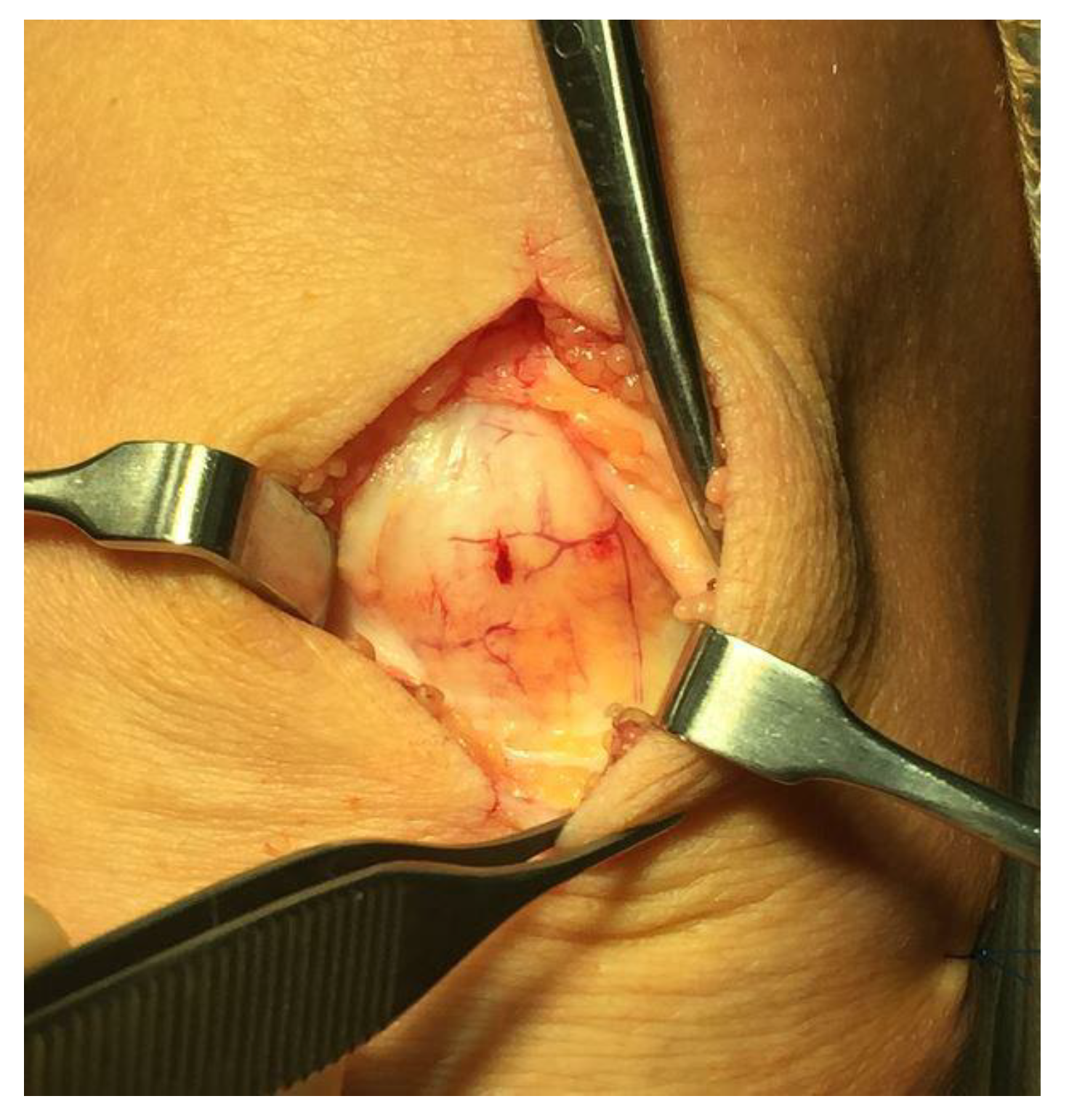
All patients underwent deleted words ultrasound (US) and Doppler (DP)-guided arthroscopic shaving and also open superficial scraping deleted words (
Figure 2) [
6]. Tissue sections from the superficial peritendinous tissue of the patellar tendon were analyzed for morphology and innervation patterns.
2.2. Sampling, Fixation and Sectioning
Immediately after surgery, the tissue specimens were put in fixative solution (4% formaldehyde in 0.1 M phosphate buffer, pH 7.0) at 4 °C overnight. Then the samples were washed three times in Tyrode’s solution containing 10% sucrose (pH 7.2), the first washing step being performed at 4 °C overnight. Before freezing, samples were divided into smaller pieces and then placed on a thin cardboard surrounded by OCT embedding medium (TissueTek, Miles Laboratories, Naperville, IL, USA). Eventually, the cardboard with the specimen was dipped in liquid propane chilled with liquid nitrogen and then stored at −80 °C until use. For immunohistochemical analyses, specimens were cryosectioned with a thickness of 7 μm (Leica Microsystem CM 300, Heidelberg, Germany) and mounted on superfrost plus slides (Thermo Scientific, Braunschweig, Germany).
2.3. Immunohistochemistry
Parallel sections were stained for morphology (hematoxylin and eosin (H&E) staining) [
8] and immunohistochemically for markers for general nerve (axonal) fibers βIII-tubulin (Sigma-Aldrich, St. Louis, MO, USA; code: T8660; mouse monoclonal), sympathetic nerve fibers (tyrosine hydroxylase, TH; Pel-Freez Arkansas; code: P40101; rabbit polyclonal) and sensory nerve fibers (calcitonin related gene peptide, CRGP; Santa Cruz Biotechnologies, code: sc-8856; goat polyclonal). The staining procedure was performed according to an established, previously described, protocol [
9,
10].
For staining for monoclonals (TH), rabbit normal serum (code X0902, DakoCytomation) and TRITC-conjugated rabbit anti-mouse (code R0276, DakoCytomation) was applied. Donkey normal serum (code: 017-000-121; Jackson Immune Research Laboratories Inc., West Grove, PA, USA) and FITC-conjugated donkey anti-goat secondary antibody (code: 705-095-147; Jackson Immune Research Inc.) were used for stainings for CGRP. For sections stained for TH, swine normal serum (code: 014-000-121; Jackson Immune Research Inc.) and TRITC-conjugated swine anti-rabbit secondary antibody (code: R0156, DakoCytomation) were used. Furthermore, normal serum and primary and secondary antibodies were diluted in 0.1% bovine serum albumin (BSA) in 0.01 M PBS (pH 7.4). When using goat antibodies (CGRP), all dilutions were made without BSA.
For all antibodies, control stainings using PBS instead of primary antibody were performed. The specificity of these antibodies in human tendon tissue has been shown in previous studies [
9,
10]. The microscopical evaluation was carried out using a Zeiss Axioscope 2 plus microscope equipped with epifluorescent technique and an Olympus DP70 digital camera. Figure montages were created using Adobe Photoshop CS5.
3. Results
As described above, all ultrasound+Doppler examinations showed a thickened para-tenon including high blood flow in the superficial peritendinous region (
Figure 1A). This was in contrast to the majority of patients with patellar tendinopathy where there is a normal paratenon and normal blood flow (
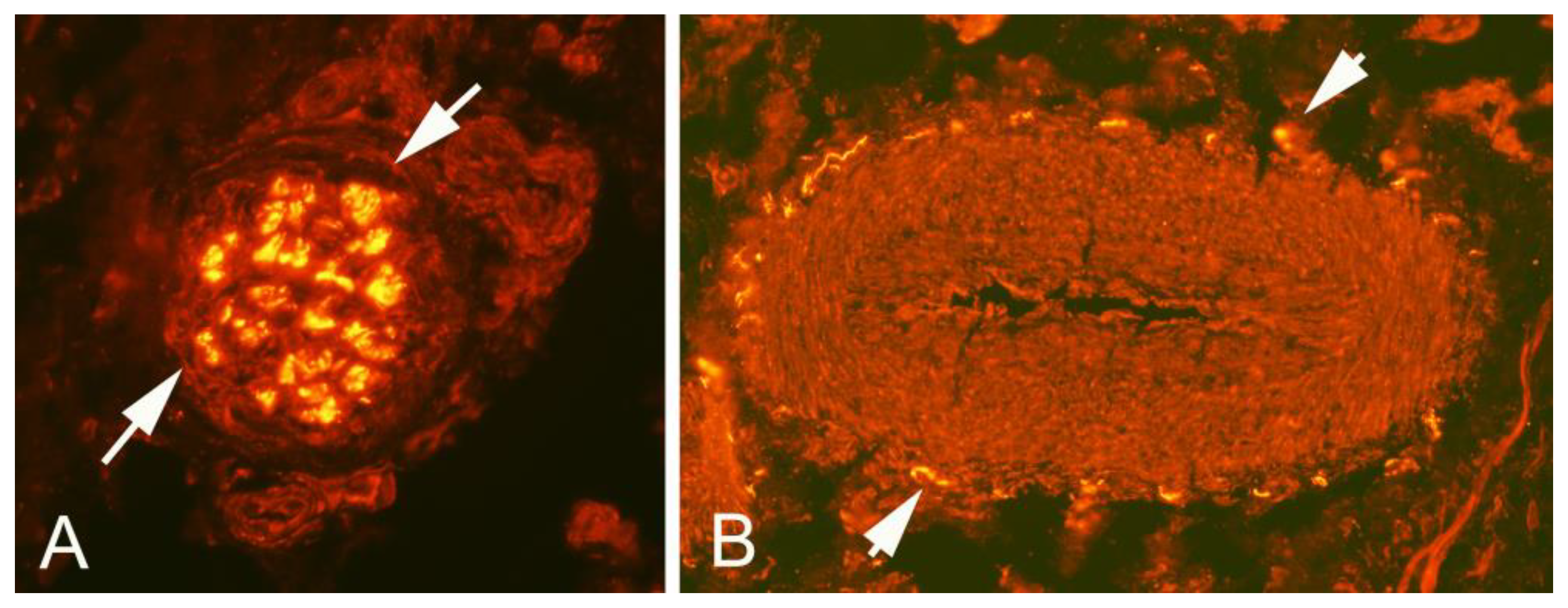
Figure 1B). All tissue specimens contained large portions of loose connective tissue with high numbers of blood vessels and also fat tissue. There were multiple nerve fascicles and sprouting nerve fibers as evidenced by evaluation for HTX and β-tubulin reactions (
Figure 3 and
Figure 4). Often those nerves were found in close vicinity to blood vessels and perivascular in blood vessel walls (
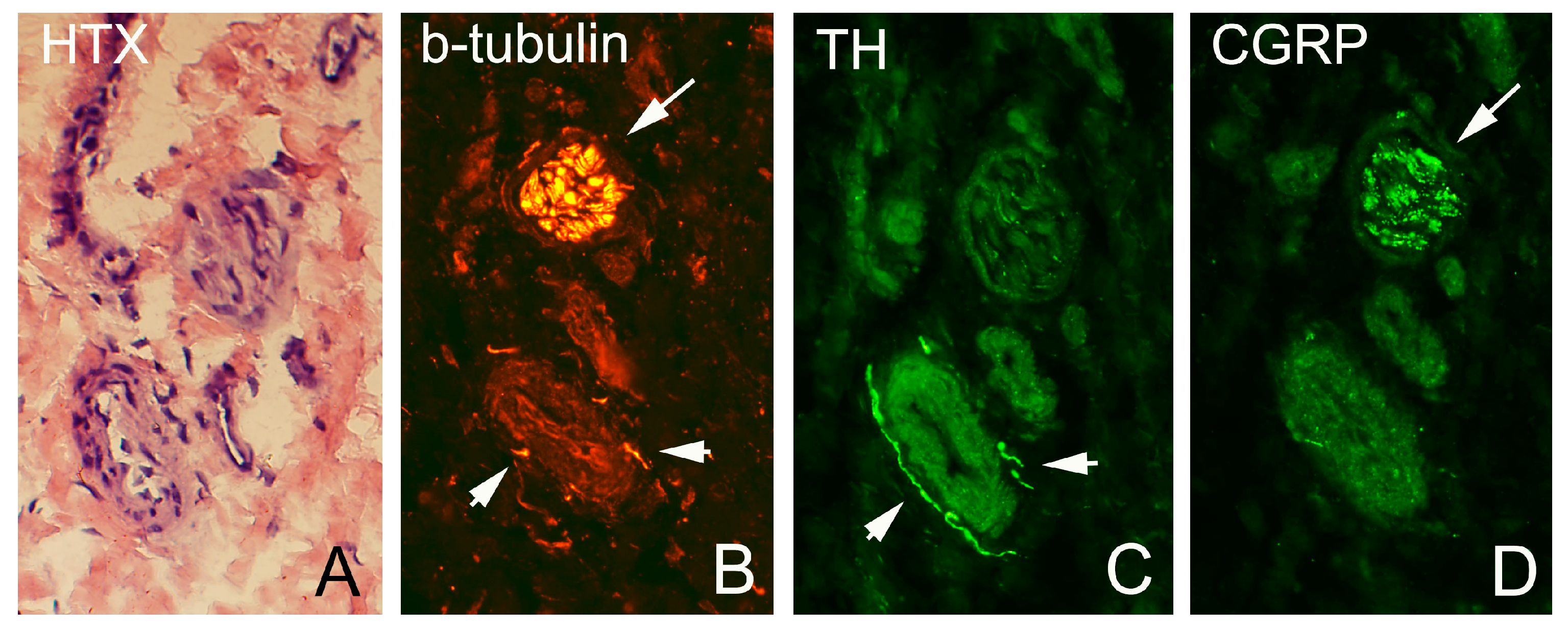
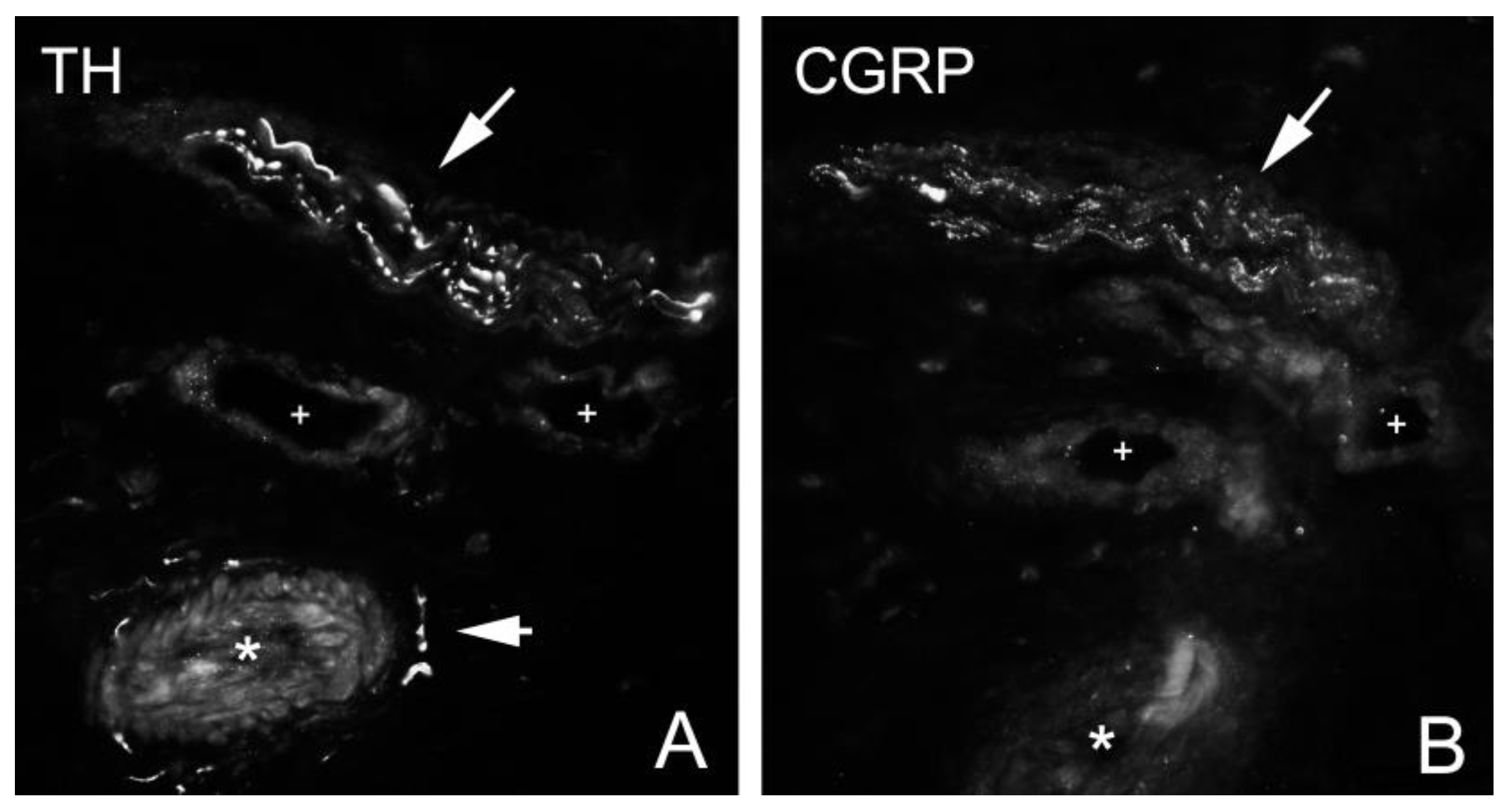
Figure 3). Sensory innervation (CGRP positive nerve fibers) was mostly found in axons of nerve fascicles (
Figure 4 and
Figure 5). Sympathetic innervation (TH positive nerve fibers) was found both in nerve fascicles and perivascular in big and small blood vessels.
4. Discussion
This study showed that there was a marked sympathetic and sensory innervation in the superficial peritendinous tissue in a sub-group of patients with patellar tendinopathy and additional severe tenderness on the superficial side of the proximal patellar tendon. Ultrasound and Doppler examination showed in all these patients a thickened paratenon including high blood flow.
The proximal patellar tendon in patients with chronic painful patellar tendinopathy has been scientifically evaluated, and traditionally the tendon changes are located dorsal and central in the tendon. Biopsies have shown that the nerves are located outside the dorsal side of the tendon [
7]. These new findings show that in a sub-group of patients there are sensory nerves also on the superficial side which highlights this tissue as a potential pain driver. All patients in the current study were involved in sports or recreational activities where falls with landing on bent knee were common. Multiple traumas to the superficial side of the tendon are likely causing these changes, but that needs to be further studied. Theoretically, it could also be part of the tendinopathy changes. Consequently, in a sub-group of patients with patellar tendinopathy pain might be coming from both the traditional dorsal side and the superficial side of the proximal patellar tendon. The findings are of clinical importance when trying to relieve pain from the patellar tendon. Our experience with patients suffering from patellar tendinopathy and having this type of change is that if only the dorsal side is focused for treatment (via the ultrasound and Doppler-guided shaving procedure), there are in some patients after surgery remaining severe tenderness on the superficial side. However, if the superficial side changes are also treated (via an open scraping procedure), there is a better pain relief. Of course, these observations need to be further studied in clinical studies.
A weakness in the current study is that the morphological results are descriptive, and that the sample size is relatively small. The findings need to be verified also in a larger material. In addition, normal control tissue needs to be evaluated. However, despite these weaknesses, we believe the findings add new information by highlighting the superficial peritendinous tissue as a potential pain driver in a sub-group of patients with deleted word patellar tendinopathy and also superficial tenderness.
5. Conclusions
In conclusion, for patients with chronic painful patellar tendinopathy, in a sub-group of patients suffering from severe tenderness also on the superficial side of the proximal patellar tendon, there are sensory nerves not only on the dorsal side but also on the superficial side of the tendon. The findings can be of importance for pain treatment in patients with chronic painful patellar tendinopathy.
Author Contributions
Conceptualization, C.S. and H.A.; methodology, C.S., L.M. and H.A.; software, C.S.; formal analysis, C.S.; investigation, C.S., L.M. and H.A.; writing—original draft preparation, H.A. and C.S.; writing—review and editing, L.M.; visualization, C.S. and L.M.; supervision, H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the University of Umeå for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Sture Forsgren for his excellent help and knowledge with the immunostainings and microscopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, K.M.; Maffuli, N.; Coleman, B.D.; Cook, J.L.; Taunton, J.E. Patellar tendinopathy: Some aspects of basic science and clinical management. Br. J. Sports Med. 1998, 32, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Bahr, R.; Fossan, B.; Løken, S.; Engebretsen, L. Surgical treatment compared with eccentric training for patellar tendinopathy (Jumpers knee): A randomized controlled trial. J. Bone Jt. Surg. Am. 2006, 88, 1689–1698. [Google Scholar] [CrossRef]
- Willberg, L.; Sunding, K.; Ohberg, L.; Forssblad, M.; Alfredson, H. Treatment of Jumper’s knee: Promising short-term results in a pilot study using a new arthroscopic approach based on imaging findings. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Willberg, L.; Sunding, K.; Forssblad, M.; Fahlstrom, M.; Alfredson, H. Sclerosing polidocanol injections or arthroscopic shaving to treat patellar tendinopathy/jumper’s knee? A randomised controlled study. Br. J. Sports Med. 2011, 45, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Sunding, K.; Willberg, L.; Werner, S.; Alfredson, H.; Forssblad, M.; Fahlstrom, M. Sclerosing injections and ultrasound-guided arthroscopic shaving for patellar tendinopathy—good clinical results and decreased tendon thickness after surgery—A medium term follow-up study. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, H.; Masci, L.M. Ultrasound and Doppler-guided surgery for the treatment of Jumper’s knee in professional rugby players. PST Pain Stud. Treat. 2015, 3, 53069. [Google Scholar] [CrossRef][Green Version]
- Danielson, P.; Andersson, G.; Alfredson, H.; Forsgren, S. Marked sympathetic component in the perivascular innervation of the dorsal paratendinous tissue of the patellar tendon in arthroscopically treated tendinosis patients. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Spang, C.; Scott, A.; Danielson, P.; Lorentzon, R.; Forsgren, S. VGluT2 and NMDAR1 expression in cells in the inflammatory infiltrates in experimentally induced myositis: Evidence of local glutamate signaling suggests autocrine/paracrine effects in an overuse injury model. Inflammation 2012, 35, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Spang, C.; Alfredson, H. Richly innervated soft tissues covering the superficial aspect of the extensor origin in patients with chronic painful tennis elbow—Implication for treatment? J. Musculoskelet. Neuronal Interact. 2017, 17, 97–103. [Google Scholar] [PubMed]
- Spang, C.; Renström, L.; Alfredson, H.; Forsgren, S. Marked expression of TNF receptors in human peritendinous tissues including in nerve fascicles with axonal damage—Studies on tendinopathy and tennis elbow. J. Musculoskelet. Neuronal Interact. 2017, 17, 226–236. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).