Abstract
Background and Objectives: Diabetes mellitus (DM) can cause macrovascular and microvascular complications, potentially resulting in further life-threatening complications. In general, the global prevalence of type 2 DM is increasing. To date, the care of DM comprises three aspects: diet, medication and exercise; among them, exercise is the most economical. Albuminuria is associated with renal injury and the progress of chronic kidney disease (CKD). The effects of habitual exercise in patients with new onset of diabetic kidney disease (DKD) have not been generally recognized. Our aim was to conduct an observational study regarding the effects of regular exercise on proteinuria and associated metabolic indices in patients with newly diagnosed type 2 DM. To investigate the effects of an exercise habit on albuminuria and the metabolic indices including renal function, blood glucose, and plasma lipids among patients with newly diagnosed type 2 DM. Materials and Methods: A cross-sectional study was conducted on newly diagnosed DM patients in two teaching hospitals in Taiwan from 1 June to 31 December 2020. The DM patients participated in the Diabetes Shared Care Network. According to the DM care mode, the patients’ blood biochemical results were analysed. Based on exercise duration, the patients were divided into two groups, i.e., the exercise group (≥150 min per week) and the non-exercise group (<150 min per week). Clinical demographic features and laboratory examination including blood and urine biochemistries were determined. Results: A total of 229 patients including 99 males (43.2%) and 130 females (56.8%) participated in the study. The proportion of DM patients with normoalbuminuria was higher (p < 0.05) in the exercise group (69.8%) than in the non-exercise group (53.7%), and the proportion of DM patients with micro or macroalbuminuria was lower in the exercise group (30.2%) than in the non-exercise group (46.3%). Levels of glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), triglycerides (TG) and high-density lipoprotein (HDL) were significantly different in both groups. Compared with the non-exercise group, lower HbA1c (6.89 ± 0.69 vs. 7.16 ± 1.05%) (p < 0.05), lower FPG (121.9 ± 25.7 vs. 140.5 ± 42.4 mg/dL) (p < 0.05), lower TG (115.6 ± 53.6 vs. 150.2 ± 15.4 mg/dL) (p < 0.05), and higher HDL (50.3 ± 11.4 vs. 44.1 ± 9.26 mg/dL) (p < 0.05) levels were noted in the exercise group. Conclusions: Regular exercise remains imperative and may bear an impact on albuminuria, blood glucose, and plasma lipids among type 2 DM patients. Therefore, medical staff and healthcare providers should encourage patients to maintain an exercise duration ≥150 min per week for preventing and controlling DM progression.
1. Introduction
Diabetes mellitus (DM) represents one of the fastest increasing chronic diseases globally. The number of DM patients has quadrupled in the past three decades, and DM is the ninth major cause of death globally. DM can result in high complication and mortality rates, resulting in a heavy burden of medical expenses [1,2,3]. To date, about 1 in 11 adults worldwide have DM, 90% of whom have type 2 DM. Asia is a major area of the rapidly emerging type 2 DM global epidemic [2,3,4,5]. Even genetic predisposition could partly contribute toward individual susceptibility to type 2 DM. The unhealthy diet and sedentary lifestyle may play an important role in the current global epidemic [6,7,8]. DM is a metabolic disease and associated with chronic inflammation in a complex immunological process. Insulin resistance (IR) can also cause a series of immune responses that aggravate the inflammatory state, further resulting in hyperglycemia [9]. Diabetic kidney disease (DKD) is one of the most common complications of DM. The pathological mechanism behind DM is insufficient insulin secretion (damage to the number and/or function of β cells) and IR in liver, muscle and fat cells, which can increase blood glucose levels and affect lipid metabolism, resulting in increases in cholesterol and triglycerides (TG) [10,11], which further leads to arteriosclerosis and cardiovascular disease [12]. Increases in blood glucose can also cause thickening or nodular sclerosis of the glomerular basement membrane (GBM), resulting in an increase in creatinine (Cr) and the production of proteinuria, which affects renal function [13]. At present, the three main measures for the treatment and care of DM are medication, diet and exercise; of these, exercise is the most economical. Regular exercise can reduce IR, increase insulin sensitivity and improve the efficiency of glucose entry into cells during exercise to reduce the concentration of glycated hemoglobin (HbA1c) and improve blood glucose levels [14]. In addition, exercise can improve plasma lipid levels, especially by reducing TG and increasing high-density lipoprotein (HDL) [15]. The studies of Stensvold et al. [16] and Larose et al. [17] also found that after interventions of aerobic, resistance and combined aerobic-resistance exercise, HbA1c, TG and HDL were significantly improved in the intervention group compared with the control group. Exercise could make an improvement in physical functioning, prevention of cardiovascular complications, delay the progression of renal dysfunction and the occurrence of proteinuria [18,19].
Overall, regular exercise was planned and repeated to improve or maintain physical health parameters in study participants. Exercising habitually and frequently helps in the prevention of serious illness such as cardiovascular disease, heart disease, metabolic disease, type 2 DM, and obesity. Many cases of type 2 DM are preventable with lifestyle modifications, such as maintaining a healthy body weight, adhering to a healthy diet, maintaining physical activity, and abstaining from smoking and alcohol consumption. The aim of our present work is to explore the association between habitual exercise and albuminuria and metabolic indices in patients with newly diagnosed type 2 DM.
2. Materials and Methods
2.1. Study Subjects and Locations
A cross-sectional study was conducted by the sampling of newly diagnosed type 2 DM patients from two teaching hospitals (Taoyuan Armed Forces General Hospital and Hsin Chu Armed Hospital) in Taiwan from 1 June to 31 December 2020. All patients were participants in the Diabetes Shared Care Network in Taiwan. Demographic features, biochemical data including available blood and spot urine sample results were investigated. Nephrologists, dietitians, and nurses were involved in this study. Only newly diagnosed type 2 DM patients in the educational program for DM and CKD with a fixed diet regimen of at least one month were included. Patients with confirmed diagnosis or clinical history of periodic paralysis, thyroid or adrenal disorders, inherited kidney disease, and renal tubular acidosis were excluded from the study. These comorbidity factors and associated diseases may have potential effects on proteinuria or albuminuria. To avoid other potential confounders and to focus on the target population of stable newly diagnosed type 2 DM patients, we additionally excluded patients with a history of hypertension, acute kidney injury, massive hematuria, renal transplant, dialysis treatment, bladder irrigation, prior creation of a neobladder, pregnancy, obstructive uropathy, and patients under 18 years of age. To avoid any confounding effects of medication and to solely investigate the original effects from the exercise habit, patients who had used any drugs including traditional Chinese medicine were also excluded in this study. For optimal health, it is recommended that adults perform at least 150 min per week of moderate-intensity exercise. In this study, our nurses asked all the participating patients to complete a self-report of exercise habits according to the US Physical Activity Guidelines. Based on the exercise habits, the patients were divided into two groups, i.e., the exercise group (≥150 min per week for more than 6 months) and the non-exercise group (<150 min per week for more than 6 months) [6,10,14]. The detailed report of exercise type revealed jogging as the most common among all subjects.
2.2. Research Tools
The analysis of medical records was conducted on a database of patients who underwent follow-up. The analysis examined the following factors: (1) Physiological data: sex, age, blood pressure (BP) and renal function stage for diabetic nephropathy (DN). (2) Test items: (1) DM control indicators: HbA1c and fasting plasma glucose (FPG); (2) plasma lipids: total cholesterol, TG, HDL and low-density lipoprotein (LDL); and (3) renal function: Cr and eGFR. In addition, urine albumin levels were determined based on regular urine collection. Urinary albumin excretion < 20 mg/L defines normoalbuminuria, 20–200 mg/L indicates microalbuminuria and >200 mg/L indicates macroalbuminuria. The urine protein-creatinine ratio (UPCR) and urine albumin-to-creatinine ratio (UACR) were then calculated. (3) To provide patients with self-management guidance and record patients’ daily physical activities. Only newly diagnosed stable DM patients who were not on medication with a fixed diet regimen in the educational program were included. To avoid other potential confounders and to focus on the target population, we also excluded patients with acute kidney injury, massive hematuria, renal transplant, pregnancy, obstructive uropathy, and under the age of 18.
2.3. Sample Size and Statistical Methods
Patients satisfying the inclusion criteria participated in the study, and the basic characteristics of demographic variables were analysed according to category. We designed a sample size of at least 100 for each group, calculated to provide 85.4%, 80% and 99.9%, 99.5% and 97.1% of power (alpha = 0.05, two-tail) on age, HbA1c and FPG, triglyceride, and HDL to detect statistically significant differences between the two groups [20]. Using the Kolmogorov–Smirnov test, no significant p values (0.200 and 0.052) were noted in both exercise and non-exercise groups, respectively, which confirmed the normality of distribution of HbA1c.
2.4. Data Analysis
After completion of data collection, SPSS for Windows 20.0 software was used for the statistical analysis. Descriptive statistics were used to describe the sex, age, renal function stage for DN and exercise distribution of the patients. HbA1c, FPG, total cholesterol, TG, HDL, LDL, Cr, eGFR, UPCR, UACR, urine albumin, BP and other indicators are expressed as mean ± standard deviation (SD), and independent t-test was used to determine the differences between the exercise and non-exercise groups.
3. Results
A total of 240 patients were initially included in the study. To avoid potential confounding effects of drugs, we subsequently excluded four and seven patients due to the use of anti-hypertensive drugs and oral hypoglycemic agents from the study. A total of 229 patients completed the study, including 99 males (43.2%) and 130 females (56.8%) with an average age of 63.41 ± 11.81 years. A total of 106 patients comprised the exercise group (46.3%), and 123 patients comprised the non-exercise group (53.7%) (Table 1).

Table 1.
Demographic and biochemical variables in both groups.
The levels of HbA1c, FPG, TG and HDL were all significantly different in both groups. Compared with the patients in the non-exercise group, the patients in the exercise group had lower HbA1c (6.89 ± 0.69 vs. 7.16 ± 1.05%) (p < 0.05), lower FPG (121.9 ± 25.7 vs. 140.5 ± 42.4 mg/dL) (p < 0.05), lower TG (115.6 ± 53.6 vs. 150.2 ± 15.4 mg/dL) (p < 0.05), and higher HDL (50.3 ± 11.4 vs. 44.1 ± 9.26 mg/dL) (p < 0.05) levels (Table 1).
This study found that patients with normoalbuminuria predominated in both groups, but a higher percentage of patients with normoalbuminuria in the exercise group than in the non-exercise group (69.8% vs. 53.7%) was noted. Lower percentages of patients with microalbuminuria and macroalbuminuria were included in the exercise group than in the non-exercise group (21.7% vs. 30.9% and 8.5% vs. 15.4%) (Table 2). Compared with those in the non-exercise group, lower percentages of patients with micro- or macroalbuminuria were observed in the exercise group (Figure 1).

Table 2.
Relation between albuminuria levels of both groups.
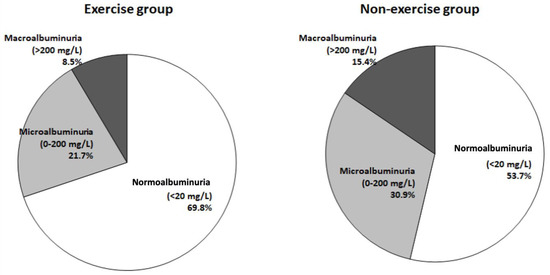
Figure 1.
Percentages and levels of albuminuria between the exercise and non-exercise groups.
In terms of the severity of albuminuria, the analysis of biochemical results showed that albuminuria was significantly correlated with HbA1c, FPG, TG and HDL (Table 3). After multiple logistic regression analysis using exercise as a dependent variable, the analysis results also demonstrated a trend where albuminuria was strongly correlated with HbA1c, TG and HDL (Table 4). Statistical analysis was conducted using the general linear model (GLM) with regression coefficients, with the results shown in the Supplementary Table S1.

Table 3.
The comparison of biochemical variables between exercise and non-exercise groups stratified by three levels of normo-, micro-, and macroalbuminuria.

Table 4.
Multiple logistic regression analysis using exercise as a dependent variable.
4. Discussion
Our study collected data from 229 newly diagnosed type 2 DM patients in Taiwan and found that HbA1c, TG and HDL levels were significantly different in the exercise group compared with the non-exercise group. Therefore, an exercise duration ≥ 150 min per week is speculated to delay the occurrence of albuminuria and reduce the risk of DN in DM patients.
Regular exercise is an important aspect of a healthy lifestyle as it can reduce cardiovascular disease, DM, and malignancy and is associated with decreasing hypertension, overweight, and obesity. In previous studies, Stensvold et al. [16] and Larose et al. [17] reported that the interventions of aerobic, resistance and combined aerobic-resistance exercise may significantly improve levels of HbA1c, TG and HDL in patients with metabolic syndrome. Research by Lin et al. [21] indicated that exercise could reduce total cholesterol and LDL, while in this study, total cholesterol and LDL were not significantly improved. According to the literature, DM patients should consider the type, intensity, frequency, and duration of exercise. Types of exercise include the following: (1) aerobic exercise, (2) muscular endurance exercise, (3) stretching exercises and (4) balance exercises. The exercise intensity should be low or moderate. Both the ratings of perceived exertion (RPE) and the targeted heart rate (THR) = maximum heart rate (220-age) × 50–70% recommended by the 2018 American Diabetes Association (ADA): Standards of Medical Care can be used as references, and the most suitable exercise intensity for DM patients is that at which the patient can talk freely but cannot sing freely (i.e., an intensity that causes them to breathe heavily) during exercise. The recommended exercise frequency is 150 min per week. Exercise should take place within 1–2 h after a meal and can be performed multiple times per day, and each exercise session should be longer than 20 min [22,23,24].
Although the exercise duration was more than 150 min per week, whether the exercise intensity met the RPE recommendations of the 2022 ADA Standards of Medical Care was not evaluated in our study [24]. In addition, muscular endurance exercises with dumbbells, extendible ropes and resistance bands, stretching exercises and balance exercises, such as walking in a straight line, can be implemented to reduce joint stiffness and the risk of falls. Patients with CKD could be advised to increase their physical activity when appropriate [18]. Previous studies recommended that exercise can improve BP [25,26], but our present study showed no significant difference in BP between the two groups. In addition to exercise, diet and medication also affect BP. The BP of the two groups was controlled at 130 ± 1.3/80 ± 1.1 mmHg, which is close to the ideal BP range.
Renal function indicators, such as Cr, the eGFR, UPCR and UACR, also showed no significant differences between the two groups. According to the literature, the following factors affect renal function: (A) Age: the eGFR declines with age; (B) BP: if BP is not controlled to below 130/80 mmHg, it can cause glomerulosclerosis and affect renal function; (C) Blood glucose: the advanced glycation end-products (AGEs) and cytokines produced by hyperglycemia (HbA1c above 7%) can cause mesangial expansion and reduce the eGFR; (D) Plasma lipids, especially LDL: if LDL is not controlled to below 100 mg/dL, renal atherosclerosis, which affects renal function, can easily occur [13,27].
Among the indicators of albuminuria, microalbuminuria is a diagnostic indicator and an early clinical manifestation of DN. DN is the most common chronic complication of DM and one of the major causes of end-stage renal failure. Therefore, prevention of the progression of DM to DN is an important clinical task. Urinary albumin excretion, i.e., albuminuria, is a commonly used diagnostic criterion for DN. Albuminuria is divided into normoalbuminuria (urine albumin < 20 mg/L), microalbuminuria (urine albumin 20–200 mg/L), and macroalbuminuria (urine albumin > 200 mg/L) [3,4,5]. Proteinuria or albuminuria is also an imperative marker of renal function estimation, which can help in the early detection of kidney disease progression [28]. As well as being a major indication of kidney disease, albuminuria constitutes a marker of cardiovascular disease and kidney disease progression in addition to a predictor of mortality. A reduction in the degree of albuminuria has been demonstrated to improve both cardiovascular and renal outcomes [29,30]. In our study, the patients were stratified by exercise duration.
Exercise training has been recommended for patients with CKD by the Kidney Disease Improving Global Outcomes [31]. A substantial number of meta-analyses have confirmed the positive impacts of regular exercise programs for CKD patients on physical performance, cardiopulmonary function, blood lipids, and quality of life [32,33,34]. Previous review suggested that high levels of physical activity appeared to be closely related to low proteinuria [35], and an observational study on non-diabetic women revealed similar results [36]. Afshinnia et al. [37] reported that exercise training can reduce proteinuria in obese individuals, though the long-term effects have not been evaluated with high-quality experimental studies. However, the sedentary time of CKD patients, especially those with severe renal function impairment, is still significantly higher than that of individuals without CKD. Glavinovic et al. [38] reported that the sedentary time of CKD was 10-times higher than that of individuals without CKD. Based on the results of our study, in clinical practice, health education provided by medical staff to DM patients should include the following: (1) The exercise duration should be ≥150 min; (2) A self-assessment of exercise intensity can be considered as bearing the ability to talk freely but not to sing freely (i.e., breathing heavily) during exercise. With exercise that meets these indicators, blood glucose, TG and LDL can be reduced, HDL can be increased, and renal function can be maintained.
These results of our study can serve as a basis for future research on the exercise habits of type 2 DM patients and as a reference for medical staff to educate patients in clinical practice to promote patients’ health and improve their quality of life. However, our study has several limitations: the study results may have been influenced by several physiologic factors including age, gender, nutrition, exercise type (walking, hiking, swimming, etc.), lifestyle, diet, and various genetic conditions. In addition, we did not collect the 24-h urine samples to assess urinary albumin excretion in this study. In addition to the cross-sectional design, the need for further experimental study regarding the proposed evaluation, the lack of large sample size calculation and restrictions related to the statistical analysis represent the limitations in this study.
5. Conclusions
This study investigated the effects of exercise duration on the albuminuria and blood biochemistry results among newly diagnosed type 2 DM patients. The results showed that DM patients with exercise duration ≥150 min per week may have lower albuminuria, FPG, HbA1c, TG levels and higher HDL levels compared with the patients with exercise duration <150 min per week. Regular exercise remains vital for controlling DM. In clinical practice, healthcare professionals and physicians would encourage patients to maintain a habit of exercising ≥150 min per week during the coronavirus disease 2019 (COVID-19) pandemic.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/medicina58050577/s1, Table S1: GLM regression coefficients- albuminuria (mg/L).
Author Contributions
Conceptualization, P.-J.H. and H.-Y.K.; data curation, P.-J.H. and Y.-H.H.; writing—original draft preparation, H.-Y.K. and P.-J.H.; writing—review and editing, P.-J.H. and Y.-H.H.; supervision, P.-J.H., S.-W.W., F.-H.C., H.-C.F., W.-F.C. and Y.-W.T.; project administration, P.-J.H. and W.-F.C.; funding acquisition, P.-J.H. and W.-F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Research Fund of the Taoyuan Armed Forces General Hospital (TYAFGH-D-111038) and (TYAFGH-D-111028).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGH), National Defense Medical Center. The approval number is TSGHIRB: C202105071.
Informed Consent Statement
Informed consent was obtained from the patients.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Acknowledgments
We thank the patients for consenting to the publication of their clinical information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.-A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Ley, S.-H.; Hu, F.-B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Prasad, R.-B.; Groop, L. 100 years of insulin: Towards improved precision and a new classification of diabetes mellitus. J. Endocrinol. 2021, 252, R59–R70. [Google Scholar] [CrossRef] [PubMed]
- Lee-Ødegård, S.; Olsen, T.; Norheim, F.; Drevon, C.A.; Birkeland, K.I. Potential mechanisms for how long-term physical activity may reduce insulin resistance. Metabolites 2022, 12, 208. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 17. Diabetes advocacy: Standards of medical care in diabetes-2022. Diabetes Care 2022, 45 (Suppl. 1), S254–S255. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tu, S.-T.; Sheu, W.-H. 2019 Diabetes Atlas: Achievements and challenges in diabetes care in Taiwan. J Formos Med Assoc. 2019, 118 (Suppl. 2), S130–S134. [Google Scholar] [CrossRef]
- Mooradian, A.-D. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009, 5, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Du, R. Study of the physiological effects of bicycle exercise on the body’s metabolism. J. Des. Environ. 2014, 15, 1–14. [Google Scholar]
- Varghese, R.-T.; Jialal, I. Diabetic nephropathy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Colberg, S.-R.; Sigal, R.-J.; Yardley, J.-E.; Riddell, M.-C.; Dunstan, D.-W.; Dempsey, P.-C.; Horton, E.-S.; Castorino, K.; Tate, D.-F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanuso, S.; Jimenez, A.; Pugliese, G.; Corigliano, G.; Balducci, S. Exercise for the management of type 2 diabetes: A review of the evidence. Acta Diabetol. 2010, 47, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Stensvold, D.; Tjønna, A.E.; Skaug, E.A.; Aspenes, S.; Stølen, T.; Wisløff, U.; Slørdahl, S.A. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J. Appl. Physiol. 2010, 108, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Larose, J.; Sigal, R.-J.; Khanwala, F.; Prud’homme, D.; Boulé, N.-G.; Kenny, G.-P. Associations between physical fitness and HbA1C in chronic diabetes mellitus. Diabetologia 2011, 54, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Johansen, K.-L.; Painter, P. Exercise in individuals with CKD. Am. J. Kidney Dis. 2012, 59, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Y.; Xiong, L.; Luo, Y.; Huang, Z.; Yi, B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: Evidence from a meta-analysis. BMC Nephrol. 2019, 20, 398. [Google Scholar] [CrossRef]
- Calculate Sample Size Needed to Compare 2 Means: 2-Sample Equivalence. Available online: http://powerandsamplesize.com/Calculators/Compare-2-Means/2-Sample-Non-Inferiority-or-Superiority (accessed on 16 February 2022).
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: UK prospective diabetes study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Huang, P.; Qu, H.; Liu, C.; Chen, B.; Gao, M. The effect of healthy eating courses and voluntary exercise offered in the community on hyperlipidaemia. Taipei City Med. J. 2011, 8, 36–44. [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes. Available online: http://care.diabetesjournals.org/content/41/Supplement_1/S38 (accessed on 16 February 2022).
- American Diabetes Association Professional Practice Committee; Draznin, B.; Aroda, V.-R.; Bakris, G.; Benson, G.; Brown, F.-M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; et al. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care 2022, 45 (Suppl. 1), S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.-J.; Bennett, P.-N.; Fraser, S.-F.; Warmington, S.-A. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: A systematic review and meta-analysis. Am. J. Physiol. Renal Physiol. 2019, 316, F856–F872. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sasaki, T.; Yamamoto, S.; Hayashi, H.; Ako, S.; Tanaka, Y. Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 18195. [Google Scholar] [CrossRef] [PubMed]
- Collister, D.; Ferguson, T.; Komenda, P.; Tangri, N. The Patterns, Risk Factors, and Prediction of Progression in Chronic Kidney Disease: A Narrative Review. Semin Nephrol. 2016, 36, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.-J.; Lin, H.-C.; Chang, S.-T.; Hsu, J.-T.; Lin, W.-S.; Chung, C.-M.; Chang, J.-J.; Hung, K.-C.; Shih, Y.-W.; Chen, F.-C.; et al. Albuminuria and neck circumference are determinate factors of successful accurate estimation of glomerular filtration rate in high cardiovascular risk patients. PLoS ONE 2018, 13, e0185693. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Liao, M.-T.; Hsiao, P.-J.; Lu, C.-L.; Hsu, Y.-J.; Lu, K.-C.; Chu, P. Antiproteinuria effect of calcitriol in patients with chronic kidney disease and vitamin D deficiency: A randomized controlled study. J. Ren. Nutr. 2020, 30, 200–207. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 2012, 23, 1917–1928. [Google Scholar] [CrossRef] [Green Version]
- Stevens, P.-E.; Levin, A. Kidney disease: Improving global outcomes chronic kidney disease guideline development work group members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Heiwe, S.; Jacobson, S.-H. Exercise training for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2011, 10, CD003236. [Google Scholar] [CrossRef]
- Heiwe, S.; Jacobson, S.-H. Exercise training in adults with CKD: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 64, 383–393. [Google Scholar] [CrossRef]
- Pei, G.; Tang, Y.; Tan, L.; Tan, J.; Ge, L.; Qin, W. Aerobic exercise in adults with chronic kidney disease (CKD): A meta-analysis. Int. Urol. Nephrol. 2019, 51, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.; Joshi, A.; Hise, M.-K. Association of physical activity and renal function in subjects with and without metabolic syndrome: A review of the third National Health and nutrition examination survey (NHANES III). Am. J. Kidney Dis. 2006, 48, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.-S.; Fisher, N.-D.; Forman, J.-P.; Curhan, G.-C. Physical activity and albuminuria. Am. J. Epidemiol. 2010, 171, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshinnia, F.; Wilt, T.-J.; Duval, S.; Esmaeili, A.; Ibrahim, H.-N. Weight loss and proteinuria: Systematic review of clinical trials and comparative cohorts. Nephrol. Dial. Transplant. 2010, 25, 1173–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glavinovic, T.; Ferguson, T.; Komenda, P.; Rigatto, C.; Duhamel, T.-A.; Tangri, N.; Bohm, C. CKD and sedentary time: Results from the Canadian health measures survey. Am. J. Kidney Dis. 2018, 72, 529–537. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).