Late-Onset Hypogonadism in a Male Patient with Long COVID Diagnosed by Exclusion of ME/CFS
Abstract
1. Introduction
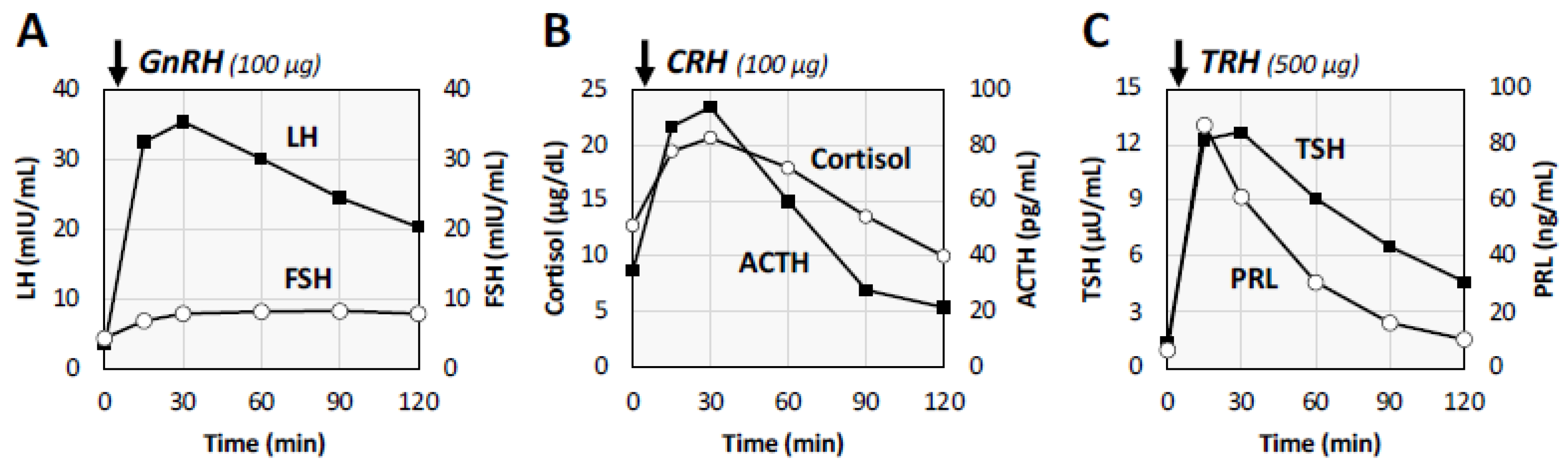
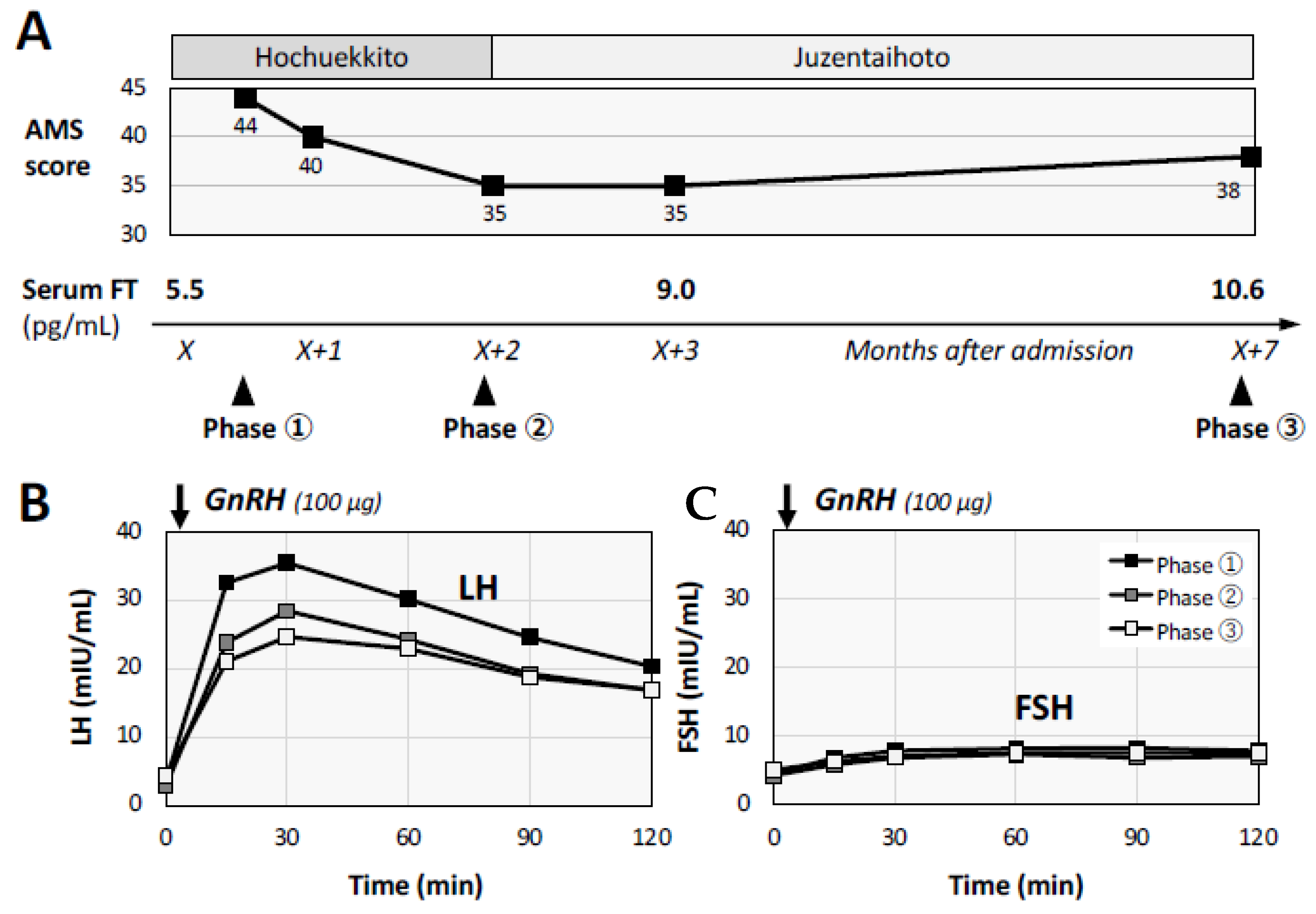
2. Case Presentation of a Male Patient with Post-COVID Conditions
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021, 22, e102–e107. [Google Scholar] [CrossRef]
- Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Honda, H.; Sakurada, Y.; Sunada, N.; Omura, D.; Hasegawa, K.; Hagiya, H.; Obika, M.; et al. Clinical Characteristics of Japanese Patients Who Visited a COVID-19 Aftercare Clinic for Post-Acute Sequelae of COVID-19/Long COVID. Cureus 2021, 13, e18568. [Google Scholar] [CrossRef]
- Sakurada, Y.; Sunada, N.; Honda, H.; Tokumasu, K.; Otsuka, Y.; Nakano, Y.; Hanayama, Y.; Furukawa, M.; Hagiya, H.; Otsuka, F. Serial Changes of Long COVID Symptoms and Clinical Utility of Serum Antibody Titers for Evaluation of Long COVID. J. Clin. Med. 2022, 11, 1309. [Google Scholar] [CrossRef]
- Bansal, R.; Gubbi, S.; Koch, C.A. COVID-19 and chronic fatigue syndrome: An endocrine perspective. J. Clin. Transl. Endocrinol. 2022, 27, 100284. [Google Scholar] [CrossRef]
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”—A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol. Res. Perspect. 2022, 10, e00911. [Google Scholar] [CrossRef]
- Zung, W.W. A SELF-RATING DEPRESSION SCALE. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Daig, I.; Heinemann, L.A.; Kim, S.; Leungwattanakij, S.; Badia, X.; Myon, E.; Moore, C.; Saad, F.; Potthoff, P.; Thai do, M. The Aging Males’ Symptoms (AMS) scale: Review of its methodological characteristics. Health Qual. Life Outcomes 2003, 1, 77. [Google Scholar] [CrossRef] [PubMed]
- Namiki, M.; Akaza, H.; Shimazui, T.; Ito, N.; Iwamoto, T.; Baba, K.; Kumano, H.; Koh, E.; Tsujimura, A.; Matsumiya, K.; et al. Clinical practice manual for late-onset hypogonadism syndrome. Int. J. Urol. 2008, 15, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Corona, G.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men. Aging Male 2021, 24, 119–138. [Google Scholar] [CrossRef]
- Kadihasanoglu, M.; Aktas, S.; Yardimci, E.; Aral, H.; Kadioglu, A. SARS-CoV-2 Pneumonia Affects Male Reproductive Hormone Levels: A Prospective, Cohort Study. J. Sex. Med. 2021, 18, 256–264. [Google Scholar] [CrossRef]
- Salonia, A.; Pontillo, M.; Capogrosso, P.; Gregori, S.; Tassara, M.; Boeri, L.; Carenzi, C.; Abbate, C.; Cignoli, D.; Ferrara, A.M.; et al. Severely low testosterone in males with COVID-19: A case-control study. Andrology 2021, 9, 1043–1052. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hulme, J.; Tran, H.D.; Vo, T.K.; Vo, G.V. The potential impact of COVID-19 on male reproductive health. J. Endocrinol. Investig. 2022, 1–13. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. SARS-CoV-2 and Male Infertility: Possible Multifaceted Pathology. Reprod. Sci. 2021, 28, 23–26. [Google Scholar] [CrossRef]
- Ma, X.; Guan, C.; Chen, R.; Wang, Y.; Feng, S.; Wang, R.; Qu, G.; Zhao, S.; Wang, F.; Wang, X.; et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell. Mol. Immunol. 2021, 18, 487–489. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S. COVID-19 and hypogonadism: Secondary immune responses rule-over endocrine mechanisms. Hum. Fertil. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; De Marinis, L. Empty sella syndrome: Multiple endocrine disorders. Handb. Clin. Neurol. 2021, 181, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef]
- Yong, S.J.; Liu, S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 2021, e2315. [Google Scholar] [CrossRef]
- Simani, L.; Ramezani, M.; Darazam, I.A.; Sagharichi, M.; Aalipour, M.A.; Ghorbani, F.; Pakdaman, H. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J. Neurovirol. 2021, 27, 154–159. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- González-Hermosillo, J.A.; Martínez-López, J.P.; Carrillo-Lampón, S.A.; Ruiz-Ojeda, D.; Herrera-Ramírez, S.; Amezcua-Guerra, L.M.; Martínez-Alvarado, M.D.R. Post-Acute COVID-19 Symptoms, a Potential Link with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A 6-Month Survey in a Mexican Cohort. Brain Sci. 2021, 11, 760. [Google Scholar] [CrossRef]
- Takeuchi, H.; Okubo, H. Clinical efficiency of combination therapy using testosterone replacement therapy, phosphodiesterase 5 inhibitors and Kampo herbal medicine for eugonadal patients with late-onset hypogonadism syndrome. Exp. Ther. Med. 2021, 22, 1173. [Google Scholar] [CrossRef]
- Kumamoto, T.; Hisasue, S.; Yasuda, M.; Ide, H.; China, T.; Inoue, M.; Saito, K.; Isotani, S.; Yamaguchi, R.; Muto, S.; et al. Hochuekkito Efficacy in Late-Onset Hypogonadism (LOH) patients. Kampo Med. 2013, 64, 160–165. [Google Scholar] [CrossRef][Green Version]
- Ito, S.; Manabe, E.; Dai, Y.; Ishihara, M.; Tsujino, T. Juzentaihoto improves adenine-induced chronic renal failure in BALB/c mice via suppression of renal fibrosis and inflammation. J. Pharmacol. Sci. 2022, 148, 172–178. [Google Scholar] [CrossRef]
- Harada, K.; Hanayama, Y.; Yasuda, M.; Hasegawa, K.; Obika, M.; Kataoka, H.; Itoshima, K.; Okada, K.; Otsuka, F. Clinical relevance of low androgen to gastroesophageal reflux symptoms. Endocr. J. 2018, 65, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Namiki, T.; Odaguchi, H.; Arita, R.; Hisanaga, A.; Mitani, K.; Ito, T. Prevention and Recovery of COVID-19 Patients With Kampo Medicine: Review of Case Reports and Ongoing Clinical Trials. Front. Pharmacol. 2021, 12, 656246. [Google Scholar] [CrossRef] [PubMed]
- Kaswa, R.; Govender, I. Novel coronavirus pandemic: A clinical overview. S. Afr. Fam. Pract. 2020, 62, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef] [PubMed]

| Complete Blood Count | Biochemistry | |||||
|---|---|---|---|---|---|---|
| White blood cells | 7610 | /μL | Total protein | 7.6 | g/dL | |
| Neutrophils | 73.7 | % | Albumin | 5.0 | g/dL | |
| Lymphocytes | 20.8 | % | Total bilirubin | 1.17 | mg/dL | |
| Eosinophils | 0.6 | % | Aspartate transaminase | 16 | U/L | |
| Red blood cells | 550 × 104 | /μL | Alanine transaminase | 16 | U/L | |
| Hemoglobin | 15.7 | g/dL | Alkaline phosphatase | 68 | U/L | |
| Platelets | 28 × 104 | /μL | γ-glutamyl transpeptidase | 21 | U/L | |
| Endocrine data [Normal Range] | Lactate dehydrogenase | 178 | U/L | |||
| Cortisol | 12.9 | [4.5–21.1] | μg/dL | Sodium | 141 | mmol/L |
| Adrenocorticotropin | 44.1 | [7.2–63.3] | pg/mL | Potassium | 3.6 | mmol/L |
| Free thyroxine | 1.48 | [0.97–1.69] | ng/dL | Chloride | 106 | mmol/L |
| Thyroid-stimulating hormone | 1.40 | [0.33–4.05] | μIU/mL | Calcium | 9.7 | mg/dL |
| Follicle-stimulating hormone | 4.2 | [1.3–17.0] | μIU/mL | Phosphate | 3.2 | mg/dL |
| Lutenizing hormone | 3.0 | [0.52–7.8] | μIU/mL | Magnesium | 2.1 | mg/dL |
| Prolactin | 13.7 | [3.0–17.3] | ng/mL | Zinc | 89 | μg/dL |
| Growth hormone | 2.54 | [0–2.47] | ng/mL | Blood urea nitrogen | 8.0 | mg/dL |
| Insulin-like growth factor 1 | 258 | [99–275] | ng/mL | Creatinine | 0.80 | mg/dL |
| Free testosterone | 5.5 | [6.5–17.7] | pg/mL | Uric acid | 5.6 | mg/dL |
| vitamin B1 | 51 | [24–66] | ng/mL | Triglyceride | 102 | mg/dL |
| vitamin B12 | 547 | [197–771] | pg/mL | Low-density lipoprotein cholesterol | 123 | mg/dL |
| Fasting plasma glucose | 104 | [73–109] | mg/dL | C-reactive protein | 0.02 | mg/dL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soejima, Y.; Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Harada, K.; Nakamoto, K.; Sunada, N.; Sakurada, Y.; Hasegawa, K.; Hagiya, H.; et al. Late-Onset Hypogonadism in a Male Patient with Long COVID Diagnosed by Exclusion of ME/CFS. Medicina 2022, 58, 536. https://doi.org/10.3390/medicina58040536
Soejima Y, Otsuka Y, Tokumasu K, Nakano Y, Harada K, Nakamoto K, Sunada N, Sakurada Y, Hasegawa K, Hagiya H, et al. Late-Onset Hypogonadism in a Male Patient with Long COVID Diagnosed by Exclusion of ME/CFS. Medicina. 2022; 58(4):536. https://doi.org/10.3390/medicina58040536
Chicago/Turabian StyleSoejima, Yoshiaki, Yuki Otsuka, Kazuki Tokumasu, Yasuhiro Nakano, Ko Harada, Kenta Nakamoto, Naruhiko Sunada, Yasue Sakurada, Kou Hasegawa, Hideharu Hagiya, and et al. 2022. "Late-Onset Hypogonadism in a Male Patient with Long COVID Diagnosed by Exclusion of ME/CFS" Medicina 58, no. 4: 536. https://doi.org/10.3390/medicina58040536
APA StyleSoejima, Y., Otsuka, Y., Tokumasu, K., Nakano, Y., Harada, K., Nakamoto, K., Sunada, N., Sakurada, Y., Hasegawa, K., Hagiya, H., Ueda, K., & Otsuka, F. (2022). Late-Onset Hypogonadism in a Male Patient with Long COVID Diagnosed by Exclusion of ME/CFS. Medicina, 58(4), 536. https://doi.org/10.3390/medicina58040536