Radiocontrast Media Hypersensitivity Reactions in Children
Abstract
:1. Introduction
2. Adverse Reactions to Iodinated Contrast Media
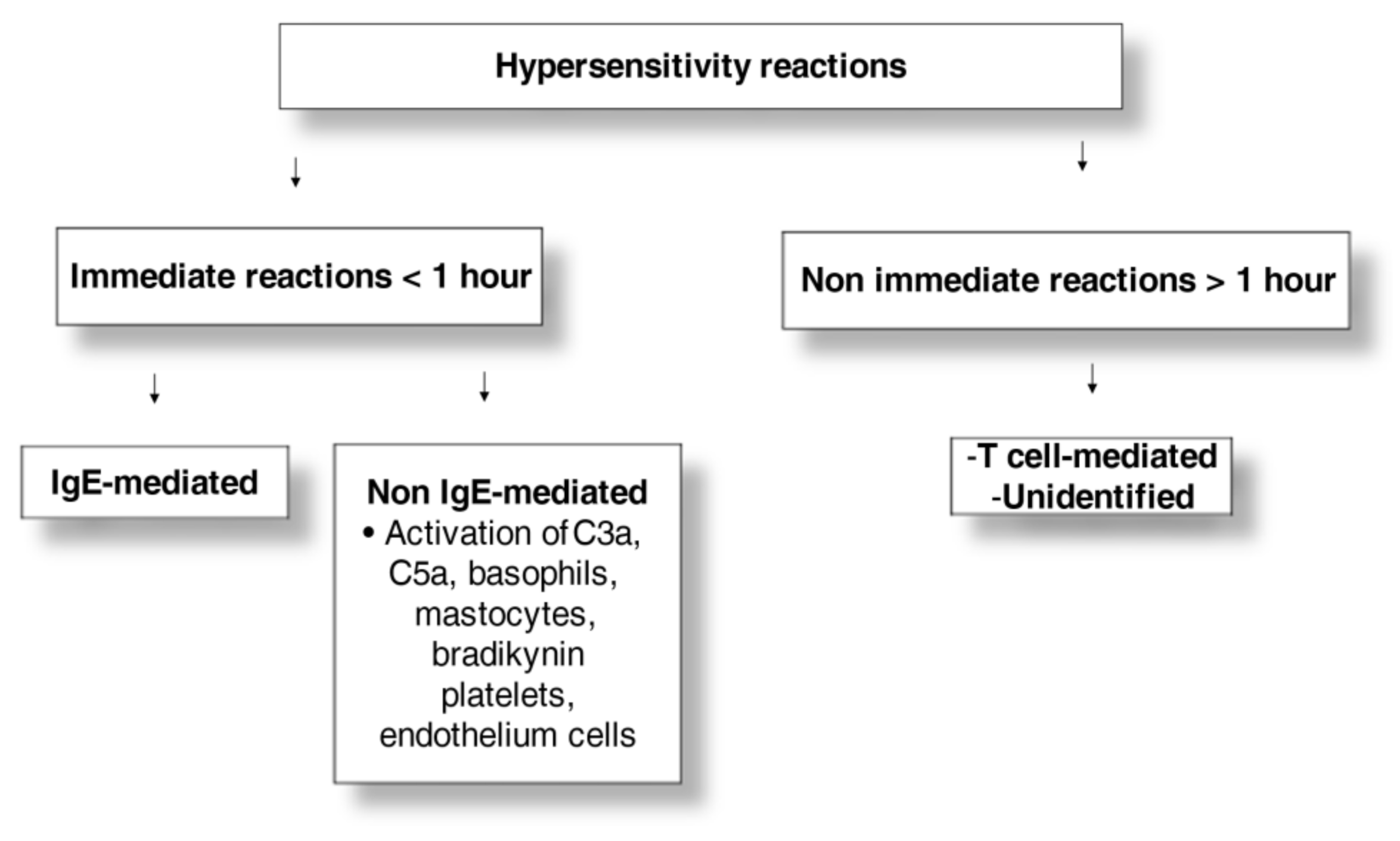
3. Clinical Presentation and Pathophysiology
4. Diagnostic Tests
4.1. Skin Tests
4.2. Tryptase
4.3. Basophil Activation Test
4.4. Other “In Vitro” Tests
5. Management
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Meaning | Meaning | ||
| BAT | basophil activation test | IDT | intradermal test |
| CM | contrast media | IM | intramuscular |
| CT | computer tomography | RCM | radiocontrast media |
| DPT | drug provocation test | SCARs | severe cutaneous adverse reaction |
| HRs | hypersensitivity reactions | SPT | skin prick test |
| ICM | iodinated contrast media | TAr | type A reactions |
References
- ACR Committee on Drugs and Contrast Media. Manual of Contrast Media; American College of Radiology: Reston, VA, USA, 2021; pp. 1–128. [Google Scholar]
- Rawlins, M.D.; Thompson, J.W. Mechanisms of adverse drug reactions. In Davies’s Textbook of Adverse Drug Reactions; Davies, D.M., Ferner, R.E., de Glanville, H., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 18–45. [Google Scholar]
- Costantino, M.T.; Romanini, L.; Gaeta, F.; Stacul, F.; Valluzzi, R.L.; Passamonti, M.; Bonadonna, P.; Cerri, G.; Pucci, S.; Ricci, P.; et al. SIRM-SIAAIC consensus, an Italian document on management of patients at risk of hypersensitivity reactions to contrast media. Clin. Mol. Allergy 2020, 31, 13. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, P.; Boehm, I. Physiological reaction following contrast medium administration: What kind of reaction is this? Eur. J. Intern. Med. 2019, 62, e15. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Lee, H.; Suh, J.; Yang, M.; Kang, W.; Kim, E. Differences between drug-induced and contrast media-induced adverse reactions based on spontaneously reported adverse drug reactions. PLoS ONE 2015, 10, e0142418. [Google Scholar] [CrossRef] [PubMed]
- Bansie, R.D.; Karim, A.F.; van Maaren, M.S.; Hermans, M.A.; van Daele, P.; van Wijk, R.G.; Rombach, S.M. Assessment of immediate and non-immediate hypersensitivity contrast reactions by skin tests and provocation tests: A review. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211015061. [Google Scholar] [CrossRef]
- Katayama, H.; Yamaguchi, K.; Kozuka, T.; Takashima, T.; Seez, P.; Matsuura, K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990, 175, 621–628. [Google Scholar] [CrossRef]
- Brockow, K.; Christiansen, C.; Kanny, G.; Clément, O.; Barbaud, A.; Bircher, A.; Dewachter, P.; Guéant, J.L.; Rodriguez Guéant, R.M.; Mouton-Faivre, C.; et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005, 60, 150–158. [Google Scholar] [CrossRef]
- Callahan, M.J.; Poznauskis, L.; Zurakowski, D.; Taylor, G.A. Nonionic iodinated intravenous contrast material-related reactions: Incidence in large urban children’s hospital retrospective analysis of data in 12,494 patients. Radiology 2009, 250, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Fjelldal, A.; Nordshus, T.; Eriksson, J. Experiences with iohexol (Omnipaque) at urography. Pediatr. Radiol. 1987, 17, 491–492. [Google Scholar] [CrossRef]
- Mikkonen, R.; Kontkanen, T.; Kivisaari, L. Late and acute adverse reactions to iohexol in a pediatric population. Pediatr. Radiol. 1995, 25, 350–352. [Google Scholar] [CrossRef]
- Ansell, G. Adverse reactions to contrast agents. Investig. Radiol. 1970, 5, 374–391. [Google Scholar] [CrossRef]
- Dillman, J.R.; Strouse, P.J.; Ellis, J.H.; Cohan, R.H.; Jan, S.C. Incidence and severity of acute allergic-like reactions to i.v. nonionic iodinated contrast material in children. Am. J. Roentgenol. 2007, 188, 1643–1647. [Google Scholar] [CrossRef]
- Wang, C.L.; Cohan, R.H.; Ellis, J.H.; Caoili, E.M.; Wang, G.; Francis, I.R. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. Am. J. Roentgenol. 2008, 191, 409–415. [Google Scholar] [CrossRef]
- Dewachter, P.; Laroche, D.; Mouton-Faivre, C.; Bloch-Morot, E.; Cercueil, J.P.; Metge, L.; Carette, M.F.; Vergnaud, M.C.; Clément, O. Immediate reactions following iodinated contrast media injection: A study of 38 cases. Eur. J. Radiol. 2011, 77, 495–501. [Google Scholar] [CrossRef]
- Rosado Ingelmo, A.; Doña Diaz, I.; Cabañas Moreno, R.; Moya Quesada, M.C.; García-Avilés, C.; García Nuñez, I.; Martínez Tadeo, J.I.; Mielgo Ballesteros, R.; Ortega-Rodríguez, N.; Padial Vilchez, M.A.; et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J. Investig. Allergol. Clin. Immunol. 2016, 26, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Dillman, J.R.; Ellis, J.H.; Cohan, R.H.; Strouse, P.J.; Jan, S.C. Frequency and severity of acute allergic-like reactions to gadolinium- containing i.v. contrast media in children and adults. Am. J. Roentgenol. 2007, 189, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Brockow, K.; Romano, A.; Aberer, W.; Bircher, A.J.; Barbaud, A.; Bonadonna, P.; Faria, E.; Kanny, G.; Lerch, M.; Pichler, W.J.; et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media—A European multicenter study. Allergy 2009, 64, 234–241. [Google Scholar] [CrossRef]
- Franceschini, F.; Bottau, P.; Caimmi, S.; Cardinale, F.; Crisafulli, G.; Liotti, L.; Saretta, F.; Bernardini, R.; Mori, F.; Caffarelli, C. Mechanisms of hypersensitivity reactions induced by drugs. Acta Biomed. 2019, 28, 44–51. [Google Scholar] [CrossRef]
- Torres, M.J.; Trautmann, A.; Böhm, I.; Scherer, K.; Barbaud, A.; Bavbek, S.; Bonadonna, P.; Cernadas, J.R.; Chiriac, A.M.; Gaeta, F.; et al. Practice parameters for diagnosing and managing iodinated contrast media hypersensitivity. Allergy 2021, 76, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Clement, O.; Dewachter, P.; Mouton-Faivre, C.; Nevoret, C.; Guilloux, L.; Bloch Morot, E.; Katsahian, S.; Laroche, D.; Investigators of the CIRTACI Study; Audebert, M.; et al. Immediate hypersensitivity to contrast agents: The French 5-year CIRTACI Study. eClin. Med. 2018, 1, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasker, F.; Fleming, H.; McNeill, G.; Creamer, D.; Walsh, S. Contrast media and cutaneous reactions. Part 2: Delayed hypersensitivity reactions to iodinated contrast media. Clin. Exp. Dermatol. 2019, 44, 844–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egbert, R.E.; De Cecco, C.N.; Schoepf, U.J.; McQuiston, A.D.; Meinel, F.G.; Katzberg, R.W. Delayed adverse reactions to the parenteral administration of iodinated contrast media. Am. J. Roentgenol. 2014, 203, 1163–1170. [Google Scholar] [CrossRef]
- Kanny, G.; Pichler, W.; Morisset, M.; Franck, P.; Marie, B.; Kohler, C.; Renaudin, J.M.; Beaudouin, E.; Laudy, J.S.; Moneret-Vautrin, D.A. T-cell mediated reactions to iodinated contrast media: Evaluation by skin and lymphocyte activations tests. J. Allergy Clin. Immunol. 2005, 115, 179–185. [Google Scholar] [CrossRef]
- Cohan, R.H.; Dunnick, N.R. Intravascular contrast media: Adverse reactions. Am. J. Roentgenol. 1987, 149, 665–670. [Google Scholar] [CrossRef]
- Bush, W.H.; Swanson, D.P. Acute reactions to intravascular contrast media: Types, risk factors, recognition, and specific treatment. Am. J. Roentgenol. 1991, 157, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Dunnick, N.R.; Cohan, R.H. Cost, corticosteroids, and contrast media. Am. J. Roentgenol. 1994, 162, 527–529. [Google Scholar] [CrossRef] [Green Version]
- Almen, T. The etiology of contrast medium reactions. Investig. Radiol. 1994, 29, S37–S45. [Google Scholar] [CrossRef]
- Lieberman, P.L.; Seigle, R.L. Reactions to radiocontrast material. Anaphylactoid events in radiology. Clin. Rev. Allergy Immunol. 1999, 17, 469–496. [Google Scholar] [CrossRef]
- Lieberman, P.L.; Seigle, R.L.; Taylor, W.W. Anaphylactoid reactions to iodinated contrast material. J. Allergy Clin. Immunol. 1978, 62, 174–180. [Google Scholar] [CrossRef]
- Laroche, D.; Namour, F.; Lefrancois, C.; Aimone-Gastin, I.; Romano, A.; Sainte-Laudy, J.; Laxenaire, M.C.; Guéant, J.L. Anaphylactoid and anaphylactic reactions to iodinated contrast material. Allergy 1999, 54, 13–16. [Google Scholar] [CrossRef]
- Standen, J.R.; Nogrady, M.B.; Dunbar, J.S.; Goldblum, R.B. The osmotic effects of methylglucamine diatrizoate (renografin 60) in intravenous urography in infants. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1965, 93, 473–479. [Google Scholar]
- Morris, T.W.; Harnish, P.P.; Reece, K.; Katzberg, R.W. Tissue fluid shifts during renal arteriography with conventional and low osmolality agents. Investig. Radiol. 1983, 18, 335–340. [Google Scholar] [CrossRef]
- Sánchez-Borges, M.; Aberer, W.; Brockow, K.; Celik, G.E.; Cernadas, J.; Greenberger, P.A.; Masse, M.S.; Schrijvers, R.; Trautmann, A. Controversies in drug allergy: Radiographic contrast media. J. Allergy Clin. Immunol. Pract. 2019, 7, 61–65. [Google Scholar] [CrossRef]
- Schrijvers, R.; Breynaert, C.; Ahmedali, Y.; Bourrain, J.L.; Demoly, P.; Chiriac, A.M. Skin testing for suspected iodinated contrast media hypersensitivity. J. Allergy Clin. Immunol. 2018, 6, 1246–1254. [Google Scholar] [CrossRef]
- Lerondeau, B.; Trechot, P.; Waton, J.; Poreaux, C.; Luc, A.; Schmutz, J.L.; Paris, C.; Barbaud, A. Analysis of cross-reactivity among radiocontrast media in 97 hypersensitivity reactions. J. Allergy Clin. Immunol. 2016, 137, 633–635. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Lee, S.Y.; Kang, H.R.; Kim, J.Y.; Hahn, S.; Park, C.M.; Chang, Y.S.; Goo, J.M.; Cho, S.H. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: A meta-analysis. Allergy 2015, 70, 625–637. [Google Scholar] [CrossRef]
- Goksel, O.; Aydin, O.; Atasoy, C.; Akyar, S.; Demirel, Y.S.; Misirligil, Z.; Bavbek, S. Hypersensitivity reactions to contrast media: Prevalence, risk factors and the role of skin tests in diagnosis–a cross-sectional survey. Int. Arch. Allergy Immunol. 2011, 155, 297–305. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Koh, Y.I.; Kim, J.H.; Ban, G.Y.; Lee, Y.K.; Hong, G.N.; Jin, U.R.; Choi, B.J.; Shin, Y.S.; Park, H.S.; et al. The potential utility of Iodinated Contrast Media (ICM) skin testing in patients with ICM hypersensitivity. J. Korean Med. Sci. 2015, 30, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.Y.; Lee, J.H.; Park, S.Y.; Seo, B.; Won, H.K.; Kang, Y.; An, J.; Kwon, H.S.; Song, W.J.; Cho, Y.S.; et al. Novel strategy for the prevention of recurrent hypersensitivity reactions to radiocontrast media based on skin testing. J. Allergy Clin. Immunol. Pract. 2019, 7, 2707–2713. [Google Scholar] [CrossRef]
- Caglayan Sozmen, S.; Povesi Dascola, C.; Mastrorilli, C.; Gioia, E.; Rizzuti, L.; Caffarelli, C. Diagnostic accuracy of patch test in children with food allergy. Pediatr. Allergy Immunol. 2015, 26, 416–422. [Google Scholar] [CrossRef]
- Gómez, E.; Ariza, A.; Blanca-López, N.; Torres, M.J. Nonimmediate hypersensitivity reactions to iodinated contrast media. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 345–353. [Google Scholar] [CrossRef]
- Torres, M.J.; Gomez, F.; Dona, I.; Rosado, A.; Mayorga, L.; Garcia, I.; Blanca-Lopez, N.; Canto, G.; Blanca, M. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy 2012, 67, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Böhm, I.; Medina, J.; Prieto, P.; Block, W.; Schild, H.H. Fixed drug eruption induced by an iodinated non-ionic X-ray contrast medium: A practical approach to identify the causative agent and to prevent its recurrence. Eur. Radiol. 2007, 17, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Laroche, D.; Vergnaud, M.-C.; Sillard, B.; Soufarapis, H.; Bricard, H. Biochemical markers of anaphylactoid reactions to drugs. Comparison of plasma histamine and tryptase. Anesthesiology 1991, 75, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Calcinai, E.; Rinaldi, L.; Povesi Dascola, C.; Terracciano, L.; Corradi, M. Hydrogen peroxide in exhaled breath condensate in asthmatic children during acute exacerbation and after treatment. Respiration 2012, 84, 291–298. [Google Scholar] [CrossRef]
- Manna, A.; Caffarelli, C.; Varini, M.; Povesi Dascola, C.; Montella, S.; Maglione, M.; Sperlì, F.; Santamaria, F. Clinical application of exhaled nitric oxide measurement in pediatric lung diseases. J. Pediatr. 2012, 38, 74. [Google Scholar] [CrossRef] [Green Version]
- Caffarelli, C.; Dascola, C.P.; Peroni, D.; Ricò, S.; Stringari, G.; Varini, M.; Folesani, G.; Corradi, M. Airway acidification in childhood asthma exacerbations. Allergy Asthma Proc. 2014, 35, e51–e56. [Google Scholar] [CrossRef]
- Pinnobphun, P.; Buranapraditkun, S.; Kampitak, T.; Hirankarn, N.; Klaewsongkram, J. The diagnostic value of basophil activation test in patients with an immediate hypersensitivity reaction to radiocontrast media. Ann. Allergy Asthma Immunol. 2011, 106, 387–393. [Google Scholar] [CrossRef]
- Salas, M.; Gomez, F.; Fernandez, T.D.; Dona, I.; Aranda, A.; Ariza, A.; Blanca-López, N.; Mayorga, C.; Blanca, M.; Torres, M.J. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy 2013, 68, 1203–1206. [Google Scholar] [CrossRef]
- Hari, Y.; Frutig-Schnyder, K.; Hurni, M.; Yawalkar, N.; Zanni, M.P.; Schnyder, B.; Kappeler, A.; Von Greyerz, S.; Braathen, L.R.; Pichler, W.J. T cell involvement in cutaneous drug eruptions. Clin. Exp. Allergy 2001, 31, 1398–1408. [Google Scholar] [CrossRef]
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
- Laroche, D.; Aimone-Gastin, I.; Dubois, F.; Huet, H.; Gerard, P.; Vergnaud, M.C.; Mouton-Faivre, C.; Guéant, J.L.; Laxenaire, M.C.; Bricard, H. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology 1998, 209, 183–190. [Google Scholar] [CrossRef]
- Mita, H.; Tadokoro, K.; Akiyama, K. Detection of IgE antibody to a radiocontrast medium. Allergy 1998, 53, 1133–1140. [Google Scholar] [CrossRef]
- Caffarelli, C.; Franceschini, F.; Caimmi, D.; Mori, F.; Diaferio, L.; Di Mauro, D.; Mastrorilli, C.; Arasi, S.; Barni, S.; Bottau, P.; et al. SIAIP position paper: Provocation challenge to antibiotics and non-steroidal anti-inflammatory drugs in children. J. Pediatr. 2018, 44, 147. [Google Scholar] [CrossRef]
- Caffarelli, C.; Ricò, S.; Rinaldi, L.; Povesi Dascola, C.; Terzi, C.; Bernasconi, S. Blood pressure monitoring in children undergoing food challenge: Association with anaphylaxis. Ann. Allergy Asthma Immunol. 2012, 108, 285–286. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Katayama, H.; Takashima, T.; Kozuka, T.; Seez, P.; Matsuura, K. Prediction of severe adverse reactions to ionic and nonionic contrast media in Japan: Evaluation of pretesting. A report from the Japanese Committee on the safety of contrast media. Radiology 1991, 178, 363–367. [Google Scholar] [CrossRef]
- Alnaes, M.B. Include desensitization to radiocontrast media in the diagnostic algorithm of radio contrast media hypersensitivity. Allergy 2021, 76, 1302–1303. [Google Scholar] [CrossRef]
- Uppal, S.; DeCicco, A.E.; Intini, A.; Josephson, R.A. Rapid desensitization to overcome contrast allergy prior to urgent coronary angiography. Int. Heart J. 2018, 59, 622–625. [Google Scholar] [CrossRef] [Green Version]
- Lasser, E.C.; Berry, C.C.; Talner, L.B.; Santini, L.C.; Lang, E.K.; Gerber, F.H.; Stolberg, H.O. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N. Engl. J. Med. 1987, 317, 845–849. [Google Scholar] [CrossRef]
- Worthley, D.L.; Gillis, D.; Kette, F.; Smith, W. Radiocontrast anaphylaxis with failure of premedication. Intern. Med. J. 2005, 35, e58–e60. [Google Scholar] [CrossRef]
- Lindsay, R.; Paterson, A.; Edgar, D. Preparing for severe contrast media reactions in children, results of a national survey, a literature review and a suggested protocol. Clin. Radiol. 2011, 66, 340–348. [Google Scholar] [CrossRef]
- Schmid, A.; Morelli, J.; Hungerbuhler, M.; Boehm, I. Cross- reactivity among iodinated contrast agents: Should we be concerned? Quant. Imaging Med. Surg. 2021, 11, 4028–4041. [Google Scholar] [CrossRef]
| Class | Combination | Iodine Content (mg/mL) | Osmolality (mOsm/kg) | |
|---|---|---|---|---|
| Ionic monomers with high osmolality | Meglumine iothalamate Na | Conray® | 325 | 1843 |
| Meglumine diatrizoate Na | Gastrografin® | 306 | 1530 | |
| Metrizoate Na | Isopaque® | 370 | 2100 | |
| Ionic dimers with low osmolality | Ioxaglate acid | Hexabrix® | 320 | 580 |
| Iodipamide | Cholografin® Meglumine® | 260 | ||
| Iotroxate | Biliscopin® | 105 | 600 | |
| Nonionic monomers | Iopamidol | Iopamiro® | 300 | 616 |
| Iohexol | Omnipaque® | 300 | 640 | |
| Ioversol | Optiray® | 320 | 702 | |
| Iopentol | Imagopaque® | 250 | 350 | |
| Iomeprol | Iomeron® | 400 | 726 | |
| Iopromide | Ultravist® | 300 | 590 | |
| Iobitridol | Xenetix® | 350 | 915 | |
| Ioxilan | Oxilan® | 350 | 721 | |
| Non ionic dimers | Iotrolan | Isovist® | 300 | 320 |
| Iosimenol * | 340 | 290 | ||
| Iodixanol (isoosmolal) | Visipaque® | 320 | 290 |
| Test | Concentration |
|---|---|
| Skin prick test | 1:1 |
| Intradermal test | 1:10 1:1 only in nonimmediate reaction |
| Patch test | 1:1 only in nonimmediate reaction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saretta, F.; Caimmi, S.; Mori, F.; Bianchi, A.; Bottau, P.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Paglialunga, C.; Ricci, G.; et al. Radiocontrast Media Hypersensitivity Reactions in Children. Medicina 2022, 58, 517. https://doi.org/10.3390/medicina58040517
Saretta F, Caimmi S, Mori F, Bianchi A, Bottau P, Crisafulli G, Franceschini F, Liotti L, Paglialunga C, Ricci G, et al. Radiocontrast Media Hypersensitivity Reactions in Children. Medicina. 2022; 58(4):517. https://doi.org/10.3390/medicina58040517
Chicago/Turabian StyleSaretta, Francesca, Silvia Caimmi, Francesca Mori, Annamaria Bianchi, Paolo Bottau, Giuseppe Crisafulli, Fabrizio Franceschini, Lucia Liotti, Claudia Paglialunga, Giampaolo Ricci, and et al. 2022. "Radiocontrast Media Hypersensitivity Reactions in Children" Medicina 58, no. 4: 517. https://doi.org/10.3390/medicina58040517
APA StyleSaretta, F., Caimmi, S., Mori, F., Bianchi, A., Bottau, P., Crisafulli, G., Franceschini, F., Liotti, L., Paglialunga, C., Ricci, G., & Caffarelli, C. (2022). Radiocontrast Media Hypersensitivity Reactions in Children. Medicina, 58(4), 517. https://doi.org/10.3390/medicina58040517








