Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19
Abstract
:1. Introduction
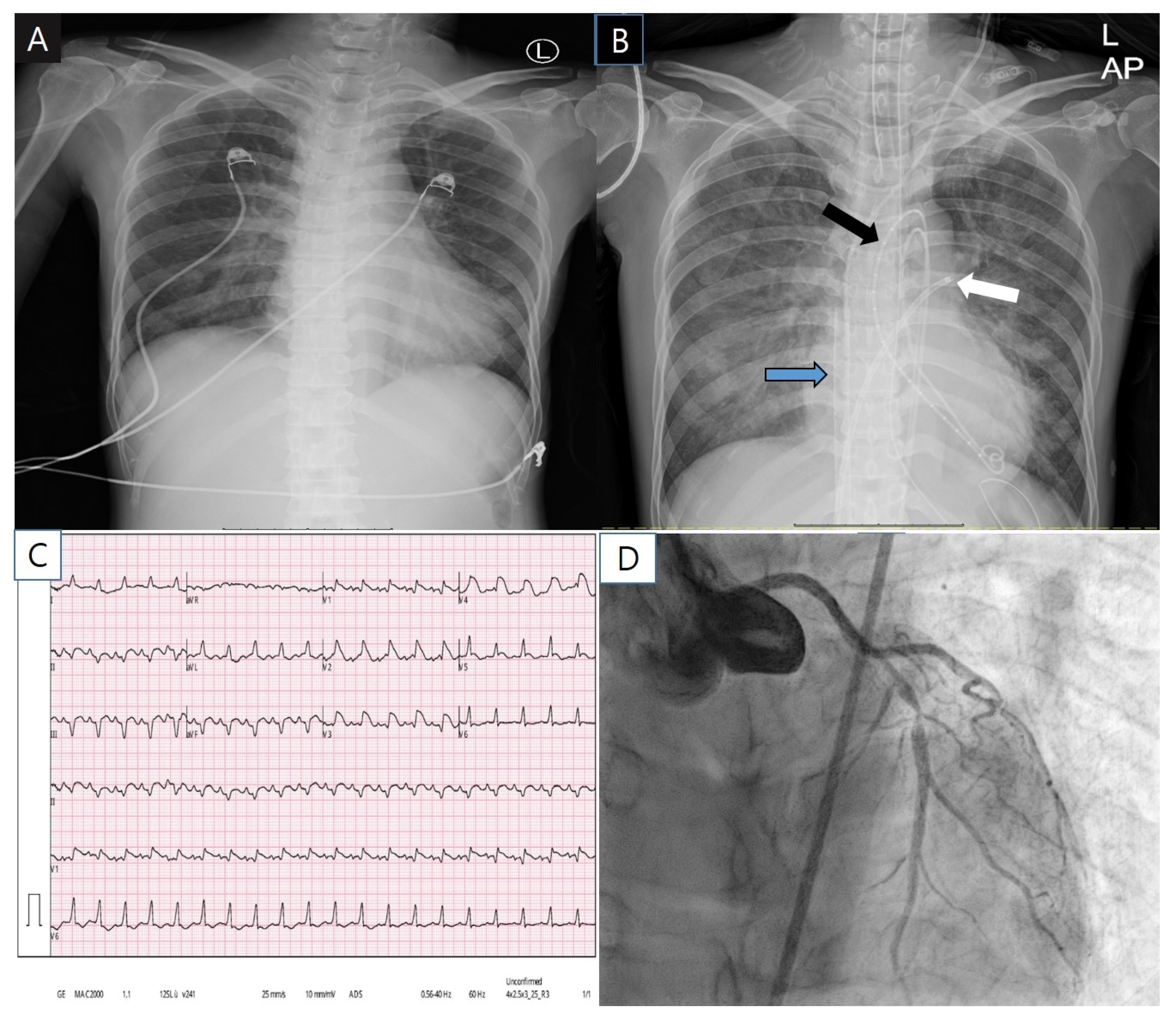
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Patient Consent
Abbreviation List
| COVID-19 | coronavirus disease 2019 |
| GCM | giant cell myocarditis |
| SARS-CoV-2 | severe acute respiratory syndrome-coronavirus-2 |
| HAV | hepatitis A virus |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| RPR | rapid plasma reagin test |
References
- Folegatti, P.M.; Bittaye, M.; Flaxman, A.; Lopez, F.R.; Bellamy, D.; Kupke, A.; Mair, C.; Makinson, R.; Sheridan, J.; Rohde, C.; et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: A dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 816–826. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Rukasin, C.R.F.; Beachkofsky, T.M.; Phillips, E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019, 85, 2694–2706. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.J.M.; Nelson, M.R.; Collins, L.C., Jr.; Spooner, C.; Hemann, B.A.; Gibbs, B.T.; Atwood, J.E.; Howard, R.S.; Chang, A.S.; Cruser, D.L.; et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS ONE 2015, 10, e0118283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after Covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; de Lemos, J.A.; Desai, S.S.; Emaminia, A.; et al. Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation 2021, 144, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Shay, D.K.; Shimabukuro, T.T.; DeStefano, F. Myocarditis occurring after immunization with mRNA- based COVID-19 vaccines. JAMA Cardiol. 2021, 6, 1115. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, S.E.; Falgout, L.; Heide, R.S.V. COVID-19 myocarditis: Quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc. Pathol. 2021, 54, 107361. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, B.H.; Hare, J.M. Inflammatory cardiomyopathic syndromes. Circ. Res. 2017, 121, 803–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Costa, L.; Ruscitti, P.; Navarini, L.; Del Puente, A.; Giacomelli, R.; Scarpa, R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subject? Autoimmun. Rev. 2020, 19, 102524. [Google Scholar] [CrossRef] [PubMed]
- Bobbio, E.; Björkenstam, M.; Nwaru, B.I.; Giallauria, F.; Hessman, E.; Bergh, N.; Polte, C.L.; Lehtonen, J.; Karason, K.; Bollano, E. Short- and long-term outcomes after heart transplantation in cardiac sarcoidosis and giant-cell myocarditis: A systematic review and meta-analysis. Clin. Res. Cardiol. 2022, 111, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Kandolin, R.; Lehtonen, J.; Salmenkivi, K.; Raisanen-Sokolowski, A.; Lommi, J.; Kupari, M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ. Heart Fail. 2013, 6, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Viral Study | |
|---|---|
| Respiratory Syncytial virus | Negative |
| Parainfluenza 1,2,3,4 | All negatives |
| Influenza A | Negative |
| Influenza B, A-H3, A-H1-pan, A-H1-2009 | All negatives |
| Human Rhinovirus | Negative |
| Human Enterovirus | Negative |
| Human Metapneumovirus | Negative |
| Adenovirus | Negative |
| Coronavirus OC43, NL63, HKU1, 229E | All negatives |
| HAV, HBV, HCV | All negatives |
| HIV | Negative |
| RPR | Negative |
| SARS-CoV-2 | Negative |
| Bacterial study | |
| Blood culture | Negative |
| Mycoplasma pneumonia | Negative |
| Chlamydophila pneumonia | Negative |
| Bordetella pertussis | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.-H.; Na, J.-Y.; Yang, J.-H.; Moon, S.-H.; Kim, S.-H.; Jung, J.-J.; Cha, H.-J.; Ahn, J.-H.; Park, Y.-W.; Cho, S.-Y.; et al. Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19. Medicina 2022, 58, 449. https://doi.org/10.3390/medicina58030449
Kang D-H, Na J-Y, Yang J-H, Moon S-H, Kim S-H, Jung J-J, Cha H-J, Ahn J-H, Park Y-W, Cho S-Y, et al. Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19. Medicina. 2022; 58(3):449. https://doi.org/10.3390/medicina58030449
Chicago/Turabian StyleKang, Dong-Hoon, Joo-Young Na, Jun-Ho Yang, Seong-Ho Moon, Sung-Hwan Kim, Jae-Jun Jung, Ho-Jeong Cha, Jong-Hwa Ahn, Yong-Whi Park, Sang-Yeong Cho, and et al. 2022. "Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19" Medicina 58, no. 3: 449. https://doi.org/10.3390/medicina58030449
APA StyleKang, D.-H., Na, J.-Y., Yang, J.-H., Moon, S.-H., Kim, S.-H., Jung, J.-J., Cha, H.-J., Ahn, J.-H., Park, Y.-W., Cho, S.-Y., Yu, H.-K., Lee, S.-H., Park, M.-Y., Kim, J.-W., & Byun, J.-H. (2022). Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19. Medicina, 58(3), 449. https://doi.org/10.3390/medicina58030449






