Ankle Dorsiflexor Function after Gastrocsoleus Lengthening in Children with Cerebral Palsy: A Literature Review
Abstract
1. Introduction
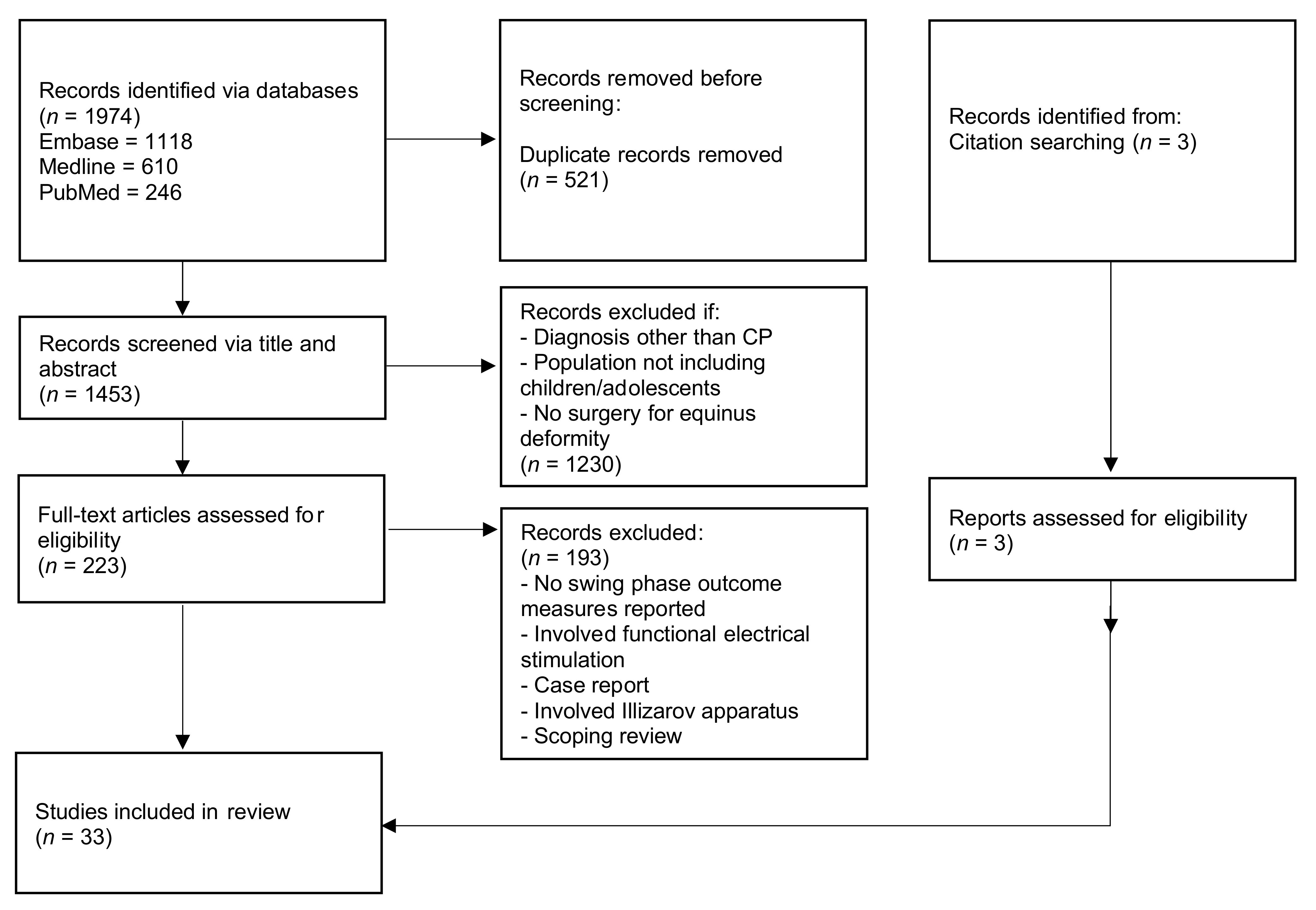
2. Materials and Methods
3. Results
3.1. Swing Phase Ankle Kinematics
3.2. Electromyography
3.3. Physical Examination Measures
3.4. Ankle–Foot Orthosis Use
3.5. Tibialis Anterior Tendon Shortening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bax, M.; Goldstein, M.; Rosenbaum, P.; Leviton, A.; Paneth, N.; Dan, B.; Jacobsson, B.; Damiano, D. Proposed definition and classification of cerebral palsy, April 2005. Dev. Med. Child Neurol. 2005, 47, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.H.; Selber, P. Musculoskeletal aspects of cerebral palsy. J. Bone Jt. Surg. Br. 2003, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Horsch, A.; Götze, M.; Geisbüsch, A.; Beckmann, N.; Tsitlakidis, S.; Berrsche, G.; Klotz, M. Prevalence and classification of equinus foot in bilateral spastic cerebral palsy. World J. Pediatrics 2019, 15, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, S.A.; Blumstein, G.; Kay, R.M.; Dorey, F.; Wren, T.A. Prevalence of specific gait abnormalities in children with cerebral palsy revisited: Influence of age, prior surgery, and Gross Motor Function Classification System level. Dev. Med. Child Neurol. 2017, 59, 79–88. [Google Scholar] [CrossRef]
- Rodda, J.M.; Graham, H.K.; Carson, L.; Galea, M.P.; Wolfe, R. Sagittal gait patterns in spastic diplegia. J. Bone Jt. Surg. Br. 2004, 86, 251–258. [Google Scholar] [CrossRef]
- Graham, K.H.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 1–24. [Google Scholar] [CrossRef]
- Rutz, E.; McCarthy, J.; Shore, B.J.; Shrader, M.W.; Veerkamp, M.; Chambers, H.; Davids, J.R.; Kay, R.M.; Narayanan, U.; Novacheck, T.F.; et al. Indications for gastrocsoleus lengthening in ambulatory children with cerebral palsy: A Delphi consensus study. J. Child. Orthop. 2020, 14, 405–414. [Google Scholar] [CrossRef]
- Firth, G.B.; McMullan, M.; Chin, T.; Ma, F.; Selber, P.; Eizenberg, N.; Wolfe, R.; Graham, H.K. Lengthening of the gastrocnemius-soleus complex: An anatomical and biomechanical study in human cadavers. J. Bone Jt. Surg. Am. 2013, 95, 1489–1496. [Google Scholar] [CrossRef]
- Tinney, A.; Khot, A.; Eizenberg, N.; Wolfe, R.; Graham, H.K. Gastrocsoleus recession techniques: An anatomical and biomechanical study in human cadavers. Bone Jt. J. 2014, 96, 778–782. [Google Scholar] [CrossRef]
- Rutz, E.; Thomason, P.; Willoughby, K.; Graham, H.K. Integrated Management in Cerebral Palsy: Musculoskeletal Surgery and Rehabilitation in Ambulatory Patients. In Cerebral Palsy: A Mutlidisciplinary Approach, 3rd ed.; Panteliadis, C.P., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 229–251. [Google Scholar]
- Saraph, V.; Zwick, E.B.; Zwick, G.; Steinwender, C.; Steinwender, G.; Linhart, W. Multilevel surgery in spastic diplegia: Evaluation by physical examination and gait analysis in 25 children. J. Pediatric Orthop. 2002, 22, 150–157. [Google Scholar] [CrossRef]
- Zwick, E.B.; Saraph, V.; Linhart, W.E.; Steinwender, G. Propulsive function during gait in diplegic children: Evaluation after surgery for gait improvement. J. Pediatric Orthop. Part B 2001, 10, 226–233. [Google Scholar] [CrossRef]
- Thomason, P.; Baker, R.; Dodd, K.; Taylor, N.; Selber, P.; Wolfe, R.; Graham, H.K. Single-event multilevel surgery in children with spastic diplegia: A pilot randomized controlled trial. J. Bone Jt. Surg. Ser. A 2011, 93, 451–460. [Google Scholar] [CrossRef]
- Ma, N.; Sclavos, N.; Passmore, E.; Thomason, P.; Graham, K.; Rutz, E. Three-Dimensional Gait Analysis in Children Undergoing Gastrocsoleus Lengthening for Equinus Secondary to Cerebral Palsy. Medicina 2021, 57, 98. [Google Scholar] [CrossRef]
- Borton, D.C.; Walker, K.; Pirpiris, M.; Nattrass, G.R.; Graham, H.K. Isolated calf lengthening in cerebral palsy. Outcome analysis of risk factors. J. Bone Jt. Surg. Br. 2001, 83, 364–370. [Google Scholar] [CrossRef]
- Shore, B.J.; White, N.; Kerr Graham, H. Surgical correction of equinus deformity in children with cerebral palsy: A systematic review. J. Child Orthop. 2010, 4, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Esquenazi, A.; Benedetti, M.G.; Desloovere, K. Gait analysis: Clinical facts. Eur. J. Phys. Rehabil. Med. 2016, 52, 560–574. [Google Scholar] [PubMed]
- Wren, T.A.; Gorton, G.E., 3rd; Ounpuu, S.; Tucker, C.A. Efficacy of clinical gait analysis: A systematic review. Gait Posture 2011, 34, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.; Buccoliero, T.; Wolf, S.I.; Heitzmann, D.; Gantz, S.; Braatz, F.; Wenz, W. Long-term results after gastrocnemius-soleus intramuscular aponeurotic recession as a part of multilevel surgery in spastic diplegic cerebral palsy. J. Bone Jt. Surg. Ser. A 2012, 94, 627–637. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; Crivellini, M.; Albertini, G. Long-term evaluation of isolated gastrocnemius fascia lengthening in children with cerebral palsy using gait analysis. J. Pediatric Orthop. Part B 2009, 18, 228–233. [Google Scholar] [CrossRef]
- Svehlik, M.; Kraus, T.; Steinwender, G.; Zwick, E.B.; Saraph, V.; Linhart, W.E. The Baumann procedure to correct equinus gait in children with diplegic cerebral palsy: Long-term results. J. Bone Jt. Surg. Ser. B 2012, 94, 1143–1147. [Google Scholar] [CrossRef]
- Lofterød, B.; Fosdahl, M.A.; Terjesen, T. Can Persistent Drop Foot After Calf Muscle Lengthening Be Predicted Preoperatively? J. Foot Ankle Surg. 2009, 48, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Davids, J.R.; Rogozinski, B.M.; Hardin, J.W.; Davis, R.B. Ankle dorsiflexor function after plantar flexor surgery in children with cerebral palsy. J. Bone Jt. Surg. Ser. A 2011, 93, e138. [Google Scholar] [CrossRef]
- Perry, J.; Burnfield, J. Ankle Foot Complex. In Gait Analysis: Normal and Pathological Function, 2nd ed.; SLACK Incorporated: West Deptford, NJ, USA, 2010. [Google Scholar]
- Reimers, J. Functional changes in the antagonists after lengthening the agonists in cerebral palsy. I. Triceps surae lengthening. Clin. Orthop. Relat. Res. 1990, 253, 30–34. [Google Scholar]
- Hullin, M.G.; Robb, J.E.; Loudon, I.R. Gait patterns in children with hemiplegic spastic cerebral palsy. J. Pediatric Orthop. B 1996, 5, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Skaaret, I.; Steen, H.; Huse, A.B.; Holm, I. Comparison of gait with and without ankle-foot orthoses after lower limb surgery in children with unilateral cerebral palsy. J. Child. Orthop. 2019, 13, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Rutz, E.; Baker, R.; Tirosh, O.; Romkes, J.; Haase, C.; Brunner, R. Tibialis anterior tendon shortening in combination with Achilles tendon lengthening in spastic equinus in cerebral palsy. Gait Posture 2011, 33, 152–157. [Google Scholar] [CrossRef]
- Dussa, C.U.; Bohm, H.; Doderlein, L.; Fujak, A. Is shortening of Tibialis Anterior in addition to calf muscle lengthening required to improve the active dorsal extension of the ankle joint in patients with Cerebral Palsy? Gait Posture 2021, 83, 210–216. [Google Scholar] [CrossRef]
- Tsang, S.T.J.; McMorran, D.; Robinson, L.; Herman, J.; Robb, J.E.; Gaston, M.S. A cohort study of tibialis anterior tendon shortening in combination with calf muscle lengthening in spastic equinus in cerebral palsy. Gait Posture 2016, 50, 23–27. [Google Scholar] [CrossRef]
- Klausler, M.; Speth, B.M.; Brunner, R.; Tirosh, O.; Camathias, C.; Rutz, E. Long-term follow-up after tibialis anterior tendon shortening in combination with Achilles tendon lengthening in spastic equinus in cerebral palsy. Gait Posture 2017, 58, 457–462. [Google Scholar] [CrossRef]
- Adolfsen, S.E.; Ounpuu, S.; Bell, K.J.; DeLuca, P.A. Kinematic and kinetic outcomes after identical multilevel soft tissue surgery in children with cerebral palsy. J. Pediatric Orthop. 2007, 27, 658–667. [Google Scholar] [CrossRef]
- Baddar, A.; Granata, K.; Damiano, D.L.; Carmines, D.V.; Blanco, J.S.; Abel, M.F. Ankle and knee coupling in patients with spastic diplegia: Effects of gastrocnemius-soleus lengthening. J. Bone Jt. Surg. Ser. A 2002, 84, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Sung, K.H.; Lee, K.M.; Lee, S.Y.; Choi, I.H.; Cho, T.J.; Yoo, W.J.; Park, M.S. Recurrence of Equinus Foot Deformity after Tendo-Achilles Lengthening in Patients with Cerebral Palsy. J. Pediatric Orthop. 2015, 35, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.; Brunner, R.; Vegvari, D.; Heitzmann, D.; Gantz, S.; Maier, M.W.; Braatz, F.; Wolf, S.I. The effects of muscle-tendon surgery on dynamic electromyographic patterns and muscle tone in children with cerebral palsy. Gait Posture 2013, 38, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Cimolin, V.; Crivellini, M.; Albertini, G. Gait analysis before and after gastrocnemius fascia lengthening in children with cerebral palsy. J. Appl. Biomater. Biomech. 2005, 3, 98–105. [Google Scholar] [PubMed]
- Granata, K.P.; Abel, M.F.; Damiano, D.L. Joint angular velocity in spastic gait and the influence of muscle- tendon lengthening. J. Bone Jt. Surg. Ser. A 2000, 82, 174–186. [Google Scholar] [CrossRef]
- Kay, R.M.; Rethlefsen, S.A.; Ryan, J.A.; Wren, T.A.L. Outcome of gastrocnemius recession and tendo-achilles lengthening in ambulatory children with cerebral palsy. J. Pediatric Orthop. Part B 2004, 13, 92–98. [Google Scholar] [CrossRef]
- Lofterød, B.; Terjesen, T. Local and distant effects of isolated calf muscle lengthening in children with cerebral palsy and equinus gait. J. Child Orthop. 2008, 2, 55–61. [Google Scholar] [CrossRef]
- Lyon, R.; Liu, X.; Schwab, J.; Harris, G. Kinematic and kinetic evaluation of the ankle joint before and after tendo Achilles lengthening in patients with spastic diplegia. J. Pediatric Orthop. 2005, 25, 479–483. [Google Scholar] [CrossRef]
- Park, C.I.; Park, E.S.; Kim, H.W.; Rha, D.-w. Soft Tissue Surgery for Equinus Deformity in Spastic Hemiplegic Cerebral Palsy: Effects on Kinematic and Kinetic Parameters. Yonsei Med. J. 2006, 47, 657–666. [Google Scholar] [CrossRef][Green Version]
- Patikas, D.; Wolf, S.I.; Schuster, W.; Armbrust, P.; Dreher, T.; Doderlein, L. Electromyographic patterns in children with cerebral palsy: Do they change after surgery? Gait Posture 2007, 26, 362–371. [Google Scholar] [CrossRef]
- Rose, S.A.; DeLuca, P.A.; Davis, I.R.B.; Ounpuu, S.; Gage, J.R. Kinematic and kinetic evaluation of the ankle after lengthening of the gastrocnemius fascia in children with cerebral palsy. J. Pediatric Orthop. 1993, 13, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Saraph, V.; Zwick, E.B.; Auner, C.; Schneider, F.; Steinwender, G.; Linhart, W. Gait improvement surgery in diplegic children: How long do the improvements last? J. Pediatric Orthop. 2005, 25, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Saraph, V.; Zwick, E.B.; Uitz, C.; Linhart, W.; Steinwender, G. The Baumann procedure for fixed contracture of the gastrosoleus in cerebral palsy: Evaluation of function of the ankle after multilevel surgery. J. Bone Jt. Surg. Ser. B 2000, 82, 535–540. [Google Scholar] [CrossRef]
- Steinwender, G.; Saraph, V.; Zwick, E.B.; Uitz, C.; Linhart, W. Fixed and dynamic equinus in cerebral palsy: Evaluation of ankle function after multilevel surgery. J. Pediatric Orthop. 2001, 21, 102–107. [Google Scholar] [CrossRef]
- Sung, K.H.; Chung, C.Y.; Lee, K.M.; Akhmedov, B.; Lee, S.Y.; Choi, I.H.; Cho, T.J.; Yoo, W.J.; Park, M.S. Long term outcome of single event multilevel surgery in spastic diplegia with flexed knee gait. Gait Posture 2013, 37, 536–541. [Google Scholar] [CrossRef]
- Svehlik, M.; Slaby, K.; Soumar, L.; Smetana, P.; Kobesova, A.; Trc, T. Evolution of walking ability after soft tissue surgery in cerebral palsy patients: What can we expect? J. Pediatric Orthop. Part B 2008, 17, 107–113. [Google Scholar] [CrossRef]
- Terjesen, T.; Lofterød, B.; Skaaret, I. Gait improvement surgery in ambulatory children with diplegic cerebral palsy. Acta Orthop. 2015, 86, 511–517. [Google Scholar] [CrossRef]
- Thompson, N.; Stebbins, J.; Seniorou, M.; Wainwright, A.M.; Newham, D.J.; Theologis, T.N. The use of minimally invasive techniques in multi-level surgery for children with cerebral palsy: Preliminary results. J. Bone Jt. Surg. Ser. B 2010, 92, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Tylkowski, C.M.; Horan, M.; Oeffinger, D.J. Outcomes of gastrocnemius-soleus complex lengthening for isolated equinus contracture in children with cerebral palsy. J. Pediatric Orthop. 2009, 29, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Yngve, D.A.; Chambers, C. Vulpius and Z-lengthening. J. Pediatric Orthop. 1996, 16, 759–764. [Google Scholar] [CrossRef]
- Davis, R.B.; Davids, J.R.; Gorton, G.E.; Aiona, M.; Scarborough, N.; Oeffinger, D.; Tylkowski, C.; Bagley, A. A minimum standardized gait analysis protocol: Development and implementation by the Shriners Motion Analysis Laboratory network (SMALnet). In Proceedings of the Pediatric Gait: A New Millennium in Clinical Care and Motion Analysis Technology, Chicago, IL, USA, 22 July 2000; pp. 1–7. [Google Scholar]
- Boyd, R.; Graham, H.K. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur. J. Neurol. 1999, 6, S23–S35. [Google Scholar] [CrossRef]
- Baker, R.; McGinley, J.L.; Schwartz, M.H.; Beynon, S.; Rozumalski, A.; Graham, H.K.; Tirosh, O. The Gait Profile Score and Movement Analysis Profile. Gait Posture 2009, 30, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J. Functional changes in the antagonists after lengthening the agonists in cerebral palsy. II. Quadriceps strength before and after distal hamstring lengthening. Clin. Orthop. Relat. Res. 1990, 253, 35–37. [Google Scholar]
- Cash, D.J.W.; Jones, J.W.M. The role of tenodesis in surgery of the upper limb. J. Bone Jt. Surg. Br. Vol. 2011, 93, 285–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sclavos, N.; Ma, N.; Passmore, E.; Thomason, P.; Graham, H.K.; Rutz, E. Ankle Dorsiflexor Function after Gastrocsoleus Lengthening in Children with Cerebral Palsy: A Literature Review. Medicina 2022, 58, 375. https://doi.org/10.3390/medicina58030375
Sclavos N, Ma N, Passmore E, Thomason P, Graham HK, Rutz E. Ankle Dorsiflexor Function after Gastrocsoleus Lengthening in Children with Cerebral Palsy: A Literature Review. Medicina. 2022; 58(3):375. https://doi.org/10.3390/medicina58030375
Chicago/Turabian StyleSclavos, Nicholas, Norine Ma, Elyse Passmore, Pam Thomason, H. Kerr Graham, and Erich Rutz. 2022. "Ankle Dorsiflexor Function after Gastrocsoleus Lengthening in Children with Cerebral Palsy: A Literature Review" Medicina 58, no. 3: 375. https://doi.org/10.3390/medicina58030375
APA StyleSclavos, N., Ma, N., Passmore, E., Thomason, P., Graham, H. K., & Rutz, E. (2022). Ankle Dorsiflexor Function after Gastrocsoleus Lengthening in Children with Cerebral Palsy: A Literature Review. Medicina, 58(3), 375. https://doi.org/10.3390/medicina58030375






