Effect of Two Different Rehabilitation Approaches on Pulmonary Functional Tests, Neuromuscular Functions and Quality of Life in Juvenile Myasthenia Gravis: A Randomized Controlled Trial Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
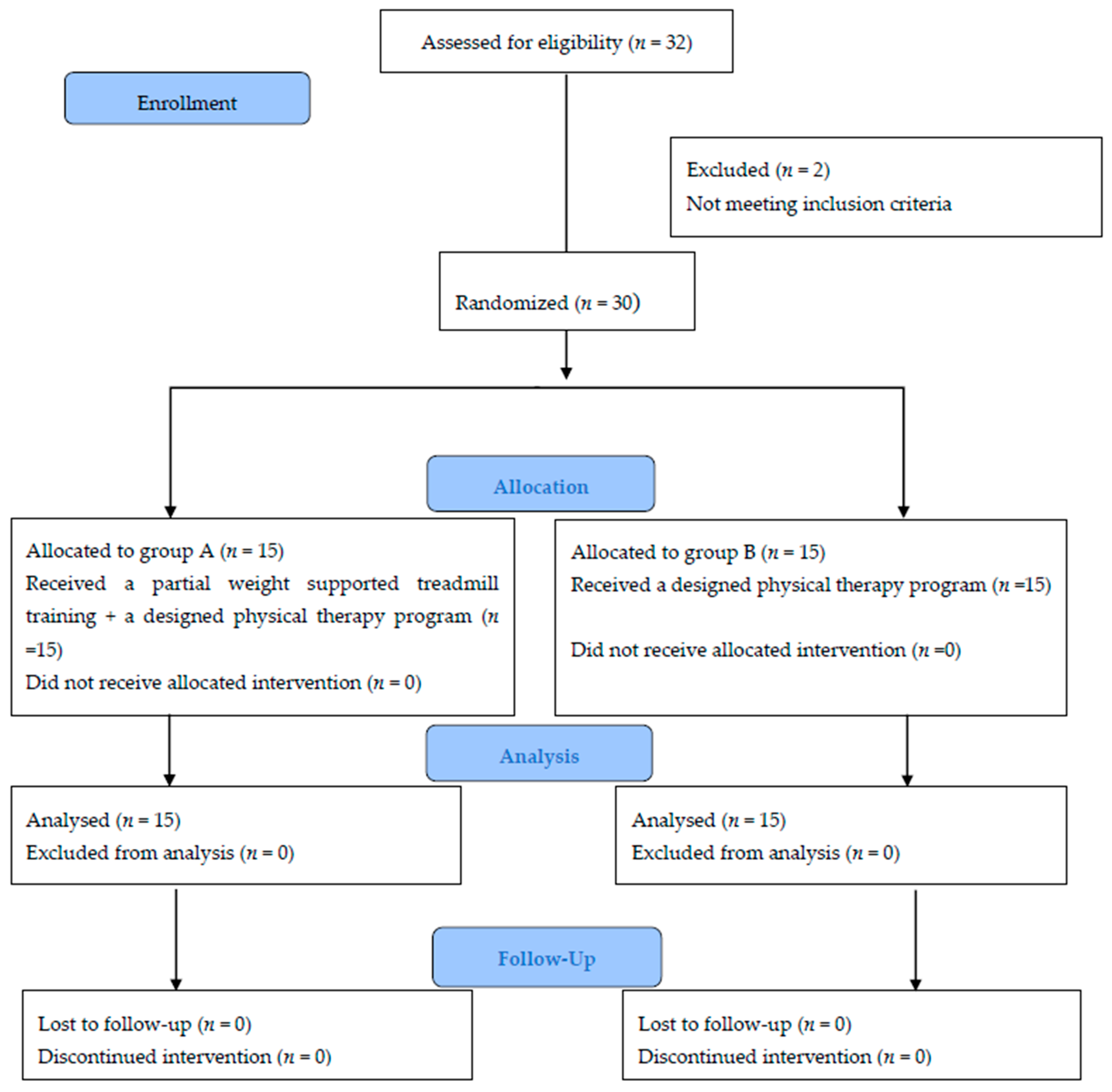
2.4. Randomisation, Allocation and Blinding
2.5. Outcome Measures
2.5.1. Primary Outcome Measures
Pulmonary Functional Tests
Neurophysiological Measurements
Isometric Muscle Force
2.5.2. Secondary Outcome Measures
Balance Assessment
Walking Endurance
Fatigue and Health-Related Quality of Life
2.6. Interventions
2.6.1. Criteria for Discontinuation
2.6.2. A Specialized Exercise Program
Breathing Exercises
Physical Exercises
- -
- Resistance exercises: latissimus dorsi pull down, biceps curl, triceps pushdown, leg curl, and leg extension were carried out. Each exercise was performed for 2 sets for 10 repetitions maximum. Individual adjustment of weight was conducted for each patient.
- -
- Sit-to-stand while holding a small Swiss ball, stepping up and down, stepping up sideways and down, throwing and catching ball, heel-toe walking, stand on one foot (goal = 30 s), and tandem stance exercise. Each exercise was repeated 10 times.
- -
- Stretching of hamstring, calf muscles, lower back, piriformis, pectoralis major (held for 20 s, relation for 20 s, and repeated 3 times).
2.6.3. Partial Body Weight Supported Treadmill Training
2.7. Data Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Effect of Treatment on Both Groups
3.2.1. Within-Group Comparison
3.2.2. Between-Group Comparisons
4. Discussion
Implications and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwiatkowska, K.; Lamtych, M.; Kubiak, K.; Badiuk, N. Physiotherapy in myasthenia gravis. J. Phys. Educ. Sport 2018, 8, 1027–1038. [Google Scholar]
- Mishra, S. Juvenile Myasthenia Gravis: A Short Review. Progress. Asp. Pediatr. Neonatol. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Finnis, M.F.; Jayawant, S. Juvenile Myasthenia Gravis: A Paediatric Perspective. Autoimmune Dis. 2011, 2011, 404101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, A.; Kassardjian, C.D.; Katzberg, H.D.; Bril, V.; Breiner, A. Selective or predominant triceps muscle weakness in African–American patients with myasthenia gravis. Neuromuscul. Disord. 2017, 27, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Twork, S.; Wiesmeth, S.; Klewer, J.; Pöhlau, D.; Kugler, J. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual. Life Outcomes 2010, 8, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiter, A.M.; Verschuuren, J.J.G.M.; Tannemaat, M.R. Fatigue in patients with myasthenia gravis. A systematic review of the literature. Neuromuscul. Disord. 2020, 30, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Heliopoulos, I.; Patlakas, G.; Vadikolias, K.; Artemis, N.; Kleopa, K.A.; Maltezos, E.; Piperidou, H. Maximal voluntary ventilation in myasthenia gravis. Muscle Nerve 2003, 27, 715–719. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Myasthenic crisis. QJM 2009, 102, 97–107. [Google Scholar] [CrossRef]
- Hess, J.; Woollacott, M.; Shivitz, N. Aging process. Clin. Exp. Res. 2006, 18, 107–115. [Google Scholar]
- Horlings, C.G.; Van Engelen, B.G.; Allum, J.H.; Bloem, B.R. A weak balance: The contribution of muscle weakness to postural instability and falls. Nat. Clin. Pract. Neurol. 2008, 4, 504–515. [Google Scholar] [CrossRef]
- Ben Kibler, W.; Press, J.; Sciascia, A. The Role of Core Stability in Athletic Function. Sports Med. 2006, 36, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Corrado, B.; Di Luise, C.; Iammarrone, C.S. Management of Muscle Spasticity in Children with Cerebral Palsy by Means of Extracorporeal Shockwave Therapy: A Systematic Review of the Literature. Dev. Neurorehabilit. 2021, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Corrado, B.; Giardulli, B.; Costa, M. Evidence-Based Practice in Rehabilitation of Myasthenia Gravis. A Systematic Review of the Literature. J. Funct. Morphol. Kinesiol. 2020, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Fonzo, M.; Sirico, F.; Corrado, B. Evidence-Based Physical Therapy for Individuals with Rett Syndrome: A Systematic Review. Brain Sci. 2020, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Hochhergger, B.; Meireless, G.S.; Irion, K.; Zanetti, G.; Garcia, E.; Moreira, J.; Marchiori, E. The chest and ageing: Radiological findings. J. Bras. Pneumol. 2012, 38, 656–665. [Google Scholar]
- Freitag, S.; Hallebach, S.; Baumann, I.; Kalischewski, P.; Rassler, B. Effects of long-term respiratory muscle endurance training on respiratory and functional outcomes in patients with Myasthenia gravis. Respir. Med. 2018, 144, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Lohi, E.L.; Lindberg, C.; Andersen, O. Physical training effects in myasthenia gravis. Arch. Phys. Med. Rehabil. 1993, 74, 1178–1180. [Google Scholar]
- Rahbek, M.A.; Mikkelsen, E.E.; Overgaard, K.; Vinge, L.; Andersen, H.; Dalgas, U. Exercise in myasthenia gravis: A feasibility study of aerobic and resistance training. Muscle Nerve 2017, 56, 700–709. [Google Scholar] [CrossRef]
- Yun, N.S.; Lee, K.Y.; Kim, J.Y. The kinematic and kinematic analysis of treadmill gait with various inclination and speed. J Korean Soc. Aerobic Exerc. 2001, 5, 49–68. [Google Scholar]
- Brouwer, B.; Parvataneni, K.; Olney, S.J. A comparison of gait biomechanics and metabolic requirements of overground and treadmill walking in people with stroke. Clin. Biomech. 2009, 24, 729–734. [Google Scholar] [CrossRef]
- Ganesan, M.; Sathyaprabha, T.N.; Gupta, A.; Pal, P.K. Effect of Partial Weight-Supported Treadmill Gait Training on Balance in Patients with Parkinson Disease. PM&R 2014, 6, 22–33. [Google Scholar] [CrossRef]
- Cup, E.H.; Pieterse, A.J.; Broek-Pastoor, J.M.T.; Munneke, M.; van Engelen, B.G.; Hendricks, H.T.; van der Wilt, G.J.; Oostendorp, R.A. Exercise Therapy and Other Types of Physical Therapy for Patients with Neuromuscular Diseases: A Systematic Review. Arch. Phys. Med. Rehabil. 2007, 88, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Ulrich, B. Coordination of pelvis-HAT (head, arms and trunk) in anterior-posterior and medio-lateral directions during treadmill gait in preadolescents with/without Down syndrome. Gait Posture 2006, 23, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Damiano, D.L.; DeJong, S.L. A Systematic Review of the Effectiveness of Treadmill Training and Body Weight Support in Pediatric Rehabilitation. J. Neurol. Phys. Ther. 2009, 33, 27–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattern-Baxter, K.; Bellamy, S.; Mansoor, J.K. Effects of Intensive Locomotor Treadmill Training on Young Children with Cerebral Palsy. Pediatr. Phys. Ther. 2009, 21, 308–318. [Google Scholar] [CrossRef]
- Mutlu, A.; Krosschell, K.; Gaebler-Spira, D. Treadmill training with partial body-weight support in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2009, 51, 268–275. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.M.; Darras, B.T.; Kang, P.B. Juvenile myasthenia gravis. Muscle Nerve 2009, 39, 423–431. [Google Scholar] [CrossRef]
- Jaretzki, A.; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia gravis: Recommendations for clinical research standards. Neurology 2000, 55, 16–23. [Google Scholar]
- Mottram, C.D. Ruppel’s Manual of Pulmonary Function Testing, 11th ed.; Mosby: St. Louis, MO, USA, 2017; pp. 110–174. [Google Scholar]
- Molin, C.J.; Punga, A.R. Compound motor action potential: Electrophysiological marker for muscle training. J. Clin. Neurophysiol. 2016, 33, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.B. Pediatric Nerve conduction studies and EMG. In The Clinical Neurophysiology Primer; Blum, A.S., Rutkove, S.B., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 369–389. [Google Scholar]
- Duez, L.; Qerama, E.; Fuglsang-Frederiksen, A.; Bangsbo, J.; Jensen, T.S. Electrophysiological characteristics of motor units and muscle fibers in trained and untrained young male subjects. Muscle Nerve 2010, 42, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kierkegaard, M.; Tollbäck, A. Reliability and feasibility of the six minute walk test in subjects with myotonic dystrophy. Neuromuscul. Disord. 2007, 17, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and Gender-Related Test Performance in Community-Dwelling Elderly People: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and Gait Speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar] [CrossRef] [Green Version]
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Varni, J.W.; Limbers, C.A.; Burwinkle, T.M. Impaired health-related quality of life in children and adolescents with chronic conditions: A comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual. Life Outcomes 2007, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Crichton, A.; Knight, S.; Oakley, E.; Babl, F.E.; Anderson, V. Fatigue in Child Chronic Health Conditions: A Systematic Review of Assessment Instruments. Pediatrics 2015, 135, e1015–e1031. [Google Scholar] [CrossRef] [Green Version]
- Kohler, M.; Clarenbach, C.F.; Böni, L.; Brack, T.; Russi, E.W.; Bloch, K.E. Quality of Life, Physical Disability, and Respiratory Impairment in Duchenne Muscular Dystrophy. Am. J. Respir. Crit. Care Med. 2005, 172, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [Green Version]
- Elsais, A.; Johansen, B.; Kerty, E. Airway limitation and exercise intolerance in well-regulated myasthenia gravis patients. Acta Neurol. Scand. Suppl. 2010, 190, 12–17. [Google Scholar] [CrossRef]
- McDonald, C.M. Physical Activity, Health Impairments, and Disability in Neuromuscular Disease. Am. J. Phys. Med. Rehabil. 2002, 81, S108–S120. [Google Scholar] [CrossRef] [PubMed]
- Dany, A.; Rapin, A.; Réveillère, C.; Calmus, A.; Tiffreau, V.; Morrone, I.; Novella, J.; Jolly, D.; Boyer, F.C. Exploring quality of life in people with slowly-progressive neuromuscular disease. Disabil. Rehabil. 2017, 39, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- LeMura, L.; Von Duvillard, S.P. Clinical Exercise Physiology: Application and Physiological Principles, 1st ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; pp. 235–238. [Google Scholar]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Amital, H.; Shoenfeld, Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun. Rev. 2018, 17, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.N.; Da Costa, C.S.N.; Golineleo, M.T.B.; Rocha, N.A.C.F. Functional strength training in child with cerebral palsy GMFCS IV: Case report. Dev. Neurorehabilit. 2013, 16, 308–314. [Google Scholar] [CrossRef]
- Krivickas, L.S. Exercise in Neuromuscular Disease. J. Clin. Neuromuscul. Dis. 2003, 5, 29–39. [Google Scholar] [CrossRef]
- Westerberg, E.; Molin, C.J.; Lindblad, I.; Emtner, M.; Punga, A.R. Physical exercise in myasthenia gravis is safe and improves neuromuscular parameters and physical performance-based measures: A pilot study. Muscle Nerve 2017, 56, 207–214. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2014, 1, CD002840. [Google Scholar] [CrossRef]
- Thom, J.; Morse, C.; Birch, K.M.; Narici, M.V. Influence of muscle architecture on the torque and power–velocity characteristics of young and elderly men. Eur. J. Appl. Physiol. 2007, 100, 613–619. [Google Scholar] [CrossRef]
- Hsu, C.; Lin, H.; Tsai, W.; Lai, Y.; Huang, C.; Su, Y.; Cheng, B.; Su, M.; Lin, W.; Chang, C.; et al. Respiratory muscle training improves functional outcomes and reduces fatigue in patients with myasthenia gravis: A single center hospital-based prospective stud. BioMed Res. Int. 2020, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Braith, R.W.; Beck, D.T. Resistance exercise: Training adaptations and developing a safe exercise prescription. Hear. Fail. Rev. 2008, 13, 69–79. [Google Scholar] [CrossRef]
- Mehndiratta, M.M.; Pandey, S.; Kuntzer, T. Acetylcholinesterase inhibitor treatment for myasthenia gravis. Cochrane Database Syst. Rev. 2014, 10, CD006986. [Google Scholar] [CrossRef] [PubMed]
- Shei, R.-J. Recent Advancements in Our Understanding of the Ergogenic Effect of Respiratory Muscle Training in Healthy Humans: A Systematic Review. J. Strength Cond. Res. 2018, 32, 2665–2676. [Google Scholar] [CrossRef]
- Areas, G.P.T.; Borghi-Silva, A.; Lobato, A.N.; Silva, A.A.; Freire, R.C., Jr.; Areas, F.Z.S. Effect of upper extremity proprioceptive neuromuscular facilitation combined with elastic resistance bands on respiratory muscle strength: A randomized controlled trial. Braz. J. Phys. Ther. 2013, 17, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Held, H.E.; Pendergast, D.R. The effects of respiratory muscle training on respiratory mechanics and energy cost. Respir. Physiol. Neurobiol. 2014, 200, 7–17. [Google Scholar] [CrossRef]
- Fregonezi, G.A.; Resqueti, V.R.; Güell Rous, R. Pursed lips breathing. Arch. Bronconeumol. 2004, 40, 279–282. [Google Scholar] [CrossRef]
- Park, J.; Han, D. Effects of high intensity aerobic exercise on treadmill on maximum-expiratory lung capacity of elderly women. J. Phys. Ther. Sci. 2017, 29, 1454–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnozhe, F.M.; Mokhtar, M.M.; Halim, M.N.; Atteya, M. Effect of treadmill-walking training with deep breathing exercises on pulmonary functions in patients with Parkinson’s disease. J. Adv. Pharm. Edu. Res. 2019, 9, 41–45. [Google Scholar]
- Korean Academy of Cardiorespiratory Physical Therapy. Cardiovascular and Pulmonary: Physical Therapy Intervention; Panmun Education Publication Company: Seoul, Korea, 2014. [Google Scholar]
- Westerberg, E.; Molin, C.J.; Spörndly Nees, S.; Widenfalk, J.; Punga, A.R. The impact of physical exercise on neuromuscular function in Myasthenia gravis patients: A single-subject design study. Medicine 2018, 97, e11510. [Google Scholar] [CrossRef]
- Wong, S.H.; Nitz, J.C.; Williams, K.; Brauer, S.G. Effects of balance strategy training in myasthenia gravis: A case study series. Muscle Nerve 2014, 49, 654–660. [Google Scholar] [CrossRef]
- Mioxham, J.; Jolley, C. Breathlessness, fatigue and the respiratory muscles. Clin. Med. 2009, 9, 448–452. [Google Scholar] [CrossRef]
- Alekseeva, T.M.; Gavrilov, Y.V.; Kreis, O.A.; Valko, P.O.; Weber, K.P.; Valko, Y. Fatigue in patients with myasthenia gravis. J. Neurol. 2018, 265, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Day, J.A.; Fox, E.J.; Lowe, J.; Swales, H.B.; Behrman, A.L. Locomotor Training with Partial Body Weight Support on a Treadmill in a Nonambulatory Child with Spastic Tetraplegic Cerebral Palsy: A Case Report. Pediatr. Phys. Ther. 2004, 16, 106–113. [Google Scholar] [CrossRef] [PubMed]
| Group A | Group B | p-Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 14.20 ± 1.01 | 14.46 ± 1.06 | 0.48 |
| Weight (kg) | 54.73 ± 3.34 | 55.02 ± 3.02 | 0.81 |
| Height (cm) | 157.13 ± 2.82 | 158.46 ± 2.74 | 0.20 |
| BMI (kg/m2) | 22.16 ± 1.07 | 21.89 ± 0.83 | 0.46 |
| Sex, n (%) | |||
| Females | 12 (80%) | 13 (87%) | 0.62 |
| Males | 3 (20%) | 2 (13%) | |
| Severity, n (%) | |||
| Grade IIA | 11 (73%) | 12 (80%) | 0.66 |
| Grade IIB | 4 (27%) | 3 (20%) |
| Group A | Group B | |||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | MD | p-Value | |
| FVC (L) | ||||
| Pre-treatment | 1.39 ± 0.08 | 1.38 ± 0.07 | 0.01 | 0.64 |
| Post-treatment | 1.54 ± 0.11 | 1.46 ± 0.07 | 0.08 | 0.02 * |
| MD (% of change) | 0.15 (10.8%) | 0.08 (5.8%) | ||
| p = 0.001 * | p = 0.001 * | |||
| FEV1 (L) | ||||
| Pre-treatment | 1.28 ± 0.06 | 1.29 ± 0.07 | -0.01 | 0.60 |
| Post-treatment | 1.41 ± 0.08 | 1.34 ± 0.05 | 0.07 | 0.01 * |
| MD (% of change) | 0.13 (10.16) | 0.05 (3.88) | ||
| p = 0.001 * | p = 0.001 * | |||
| PEFR(L/min) | ||||
| Pre-treatment | 166.40 ± 1.84 | 165.46 ± 1.51 | 0.94 | 0.14 |
| Post-treatment | 179.53 ± 1.30 | 171.80 ± 1.08 | 7.73 | 0.001 * |
| MD (% of change) | 13.13 (7.89%) | 6.34 (3.83%) | ||
| p = 0.001 * | p = 0.001 * | |||
| MVV (L/min) | ||||
| Pre-treatment | 43.26 ± 1.27 | 42.66 ± 1.23 | 0.60 | 0.20 |
| Post-treatment | 49.20 ± 1.32 | 47.13 ± 1.06 | 2.07 | 0.001 * |
| MD (% of change) | 5.94 (13.73%) | 4.47 (10.48%) | ||
| p = 0.001 * | p = 0.001 * |
| Group A | Group B | |||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | MD | p-Value | |
| CMAP amplitude of rectus femoris(mV) | ||||
| Pre-treatment | 4.13 ± 0.35 | 4.20 ± 0.41 | −0.07 | 0.63 |
| Post-treatment | 7.33 ± 0.72 | 5.93 ± 0.61 | 1.40 | 0.001 * |
| MD (% of change) | 3.2 (77.48%) | 1.73 (41.19%) | ||
| p = 0.001 * | p = 0.001 * | |||
| CMAP amplitude of biceps(mV) | ||||
| Pre-treatment | 5.33 ± 1.11 | 5.20 ± 0.94 | 0.13 | 0.72 |
| Post-treatment | 7.46 ± 0.83 | 6.93 ± 1.09 | 0.53 | 0.14 |
| MD (% of change) | 2.13 (39.96) | 1.73 (33.27) | ||
| p = 0.001 * | p = 0.001 * | |||
| Isometric muscle force of biceps brachii (kg) | ||||
| Pre-treatment | 12.20 ± 0.86 | 11.80 ± 0.77 | 0.40 | 0.19 |
| Post-treatment | 18.73 ± 1.22 | 18.20 ± 1.08 | 0.53 | 0.21 |
| MD (% of change) | 6.53 (53.52%) | 6.40 (54.24%) | ||
| p = 0.001 * | p = 0.001 * | |||
| Isometric muscle force of rectus femoris(kg) | ||||
| Pre-treatment | 22.93 ± 0.96 | 22.73 ± 0.79 | 0.2 | 0.54 |
| Post-treatment | 30. 60 ± 1.05 | 26.60 ± 1.29 | 4 | 0.001 * |
| MD (% of change) | 7.67 (33.45%) | 3.87 (17.03%) | ||
| p = 0.001 * | p = 0.001 * | |||
| PedsQL TM MFS | ||||
| Pre-treatment | 70.86 ± 0.91 | 70.40 ± 0.73 | 0.46 | 0.13 |
| Post-treatment | 77.80 ± 0.86 | 74.33 ± 0.72 | 3.47 | 0.001 * |
| MD (% of change) | 6.94 (9.79%) | 3.93 (5.58%) | ||
| p = 0.001 * | p = 0.001 * | |||
| PedsQLtotal composite | ||||
| Pre-treatment | 66.86 ± 0.91 | 66.53 ± 0.51 | 0.33 | 0.23 |
| Post-treatment | 77.33 ± 0.48 | 71.60 ± 0.51 | 5.73 | 0.001 * |
| MD (% of change) | 10.47 (15.66%) | 5.07 (7.62%) | ||
| p = 0.001 * | p = 0.001 * | |||
| 6MWD (m) | ||||
| Pre-treatment | 311.46 ± 2.35 | 310.66 ± 2.58 | 0.80 | 0.38 |
| Post-treatment | 350 ± 2.36 | 330.73 ± 2.65 | 19.27 | 0.001 * |
| MD (% of change) | 38.54 (12.37%) | 20.07 (6.46%) | ||
| p = 0.001 * | p = 0.001 * | |||
| Group A | Group B | |||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | MD | p-Value | |
| APSI | ||||
| Pre-treatment | 1.57 ± 0.08 | 1.54 ± 0.09 | 0.03 | 0.31 |
| Post-treatment | 1.04 ± 0.05 | 1.40 ± 0.09 | −0.36 | 0.001 * |
| MD (% of change) | 0.53 (33.76%) | 0.14 (9.09%) | ||
| p = 0.001 * | p = 0.001 * | |||
| MLSI | ||||
| Pre-treatment | 1.80 ± 0.07 | 1.76 ± 0.09 | 0.04 | 0.31 |
| Post-treatment | 1.17 ± 0.11 | 1.56 ± 0.06 | −0.39 | 0.001 * |
| MD (% of change) | 0.63 (35%) | 0.20 (11.36%) | ||
| p = 0.001 * | p = 0.001 * | |||
| OSI | ||||
| Pre-treatment | 1.97 ± 0.09 | 1.96 ± 0.12 | 0.01 | 0.87 |
| Post-treatment | 1.37 ± 0.07 | 1.64 ± 0.09 | −0.27 | 0.001 * |
| MD (% of change) | 0.60 (30.46%) | 0.32 (16.33%) | ||
| p = 0.001 * | p = 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, R.A.; Mohamed, E.S.H.; Basiouny, M.A.; Hamoda, I.M.; Hanoura, E.S.M.; Elhenedi, E.I.; Sherief, A.E.A.A. Effect of Two Different Rehabilitation Approaches on Pulmonary Functional Tests, Neuromuscular Functions and Quality of Life in Juvenile Myasthenia Gravis: A Randomized Controlled Trial Study. Medicina 2022, 58, 374. https://doi.org/10.3390/medicina58030374
Mohamed RA, Mohamed ESH, Basiouny MA, Hamoda IM, Hanoura ESM, Elhenedi EI, Sherief AEAA. Effect of Two Different Rehabilitation Approaches on Pulmonary Functional Tests, Neuromuscular Functions and Quality of Life in Juvenile Myasthenia Gravis: A Randomized Controlled Trial Study. Medicina. 2022; 58(3):374. https://doi.org/10.3390/medicina58030374
Chicago/Turabian StyleMohamed, Rasha A., El Sayed H. Mohamed, Mohamed A. Basiouny, Ibrahim M. Hamoda, El Sayed M. Hanoura, Elbadawy I. Elhenedi, and Abd El Aziz A. Sherief. 2022. "Effect of Two Different Rehabilitation Approaches on Pulmonary Functional Tests, Neuromuscular Functions and Quality of Life in Juvenile Myasthenia Gravis: A Randomized Controlled Trial Study" Medicina 58, no. 3: 374. https://doi.org/10.3390/medicina58030374
APA StyleMohamed, R. A., Mohamed, E. S. H., Basiouny, M. A., Hamoda, I. M., Hanoura, E. S. M., Elhenedi, E. I., & Sherief, A. E. A. A. (2022). Effect of Two Different Rehabilitation Approaches on Pulmonary Functional Tests, Neuromuscular Functions and Quality of Life in Juvenile Myasthenia Gravis: A Randomized Controlled Trial Study. Medicina, 58(3), 374. https://doi.org/10.3390/medicina58030374





