Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report
Abstract
1. Introduction
2. Patients and Materials
- Male or female subjects between 30 years and 65 years;
- The presence of deep wrinkles in the facial area, e.g., around the eyes, around the mouth and cheeks, including wrinkles and spots on the face;
- Skin free of diseases that could interfere with the evaluation of the results;
- Willingness to abstain from any cosmetic or surgical procedures in the treatment area for theduration of the clinical investigation;
- Willingness to abstain from any facial surgical procedures for the duration of the clinical;
- investigation including application of botulinum toxin;
- Willingness to abstain from excessive weight gain or weight loss (±10% of body weight), and/or drastic dietary changes for the duration of the clinical investigation;
- Written informed consent.
2.1. Exclusion Criteria
- For females: pregnancy, lactating, planned pregnancy;
- History of mental disorders or emotional instability;
- History of allergic reaction to HA products;
- Facial surgery or implantation of dermal fillers in the nasolabial region within the last 24 months;
- Skin of the to be treated region affected by cosmetic treatments (e.g., laser therapy within the last 12 months, chemical peeling within the last 3 months, and dermabrasion within the last 12 months, and botulinum toxin within the last 12 months);
- Connective tissue diseases;
- Diabetes mellitus or uncontrolled systemic diseases;
- Known human immune deficiency virus-positive individuals;
- Presence of silicone implants or implants of another non-absorbable substance (permanent fillers) in the area of product application;
- Cutaneous lesions in the evaluated area;
- Tendency to keloid formation and/or hypertrophic scars;
- Autoimmune disease;
- Any medical condition prohibiting inclusion in the study according to the judgment of the investigator;
- Subjects for whom due to a mental disorder or a mental disability a custodian has been appointed or who are legally or magisterially arrested or housed;
- Current or previous (within 30 days of enrollment) treatment with an investigational drug and/or medical device or participation in another clinical study;
- History of allergies to cosmetic filling products and recurrent herpes simplex virus;
- Heavy smokers (>20 cigarettes per day).
2.2. Procedure
2.3. Detailed Procedure
2.4. Follow-Ups
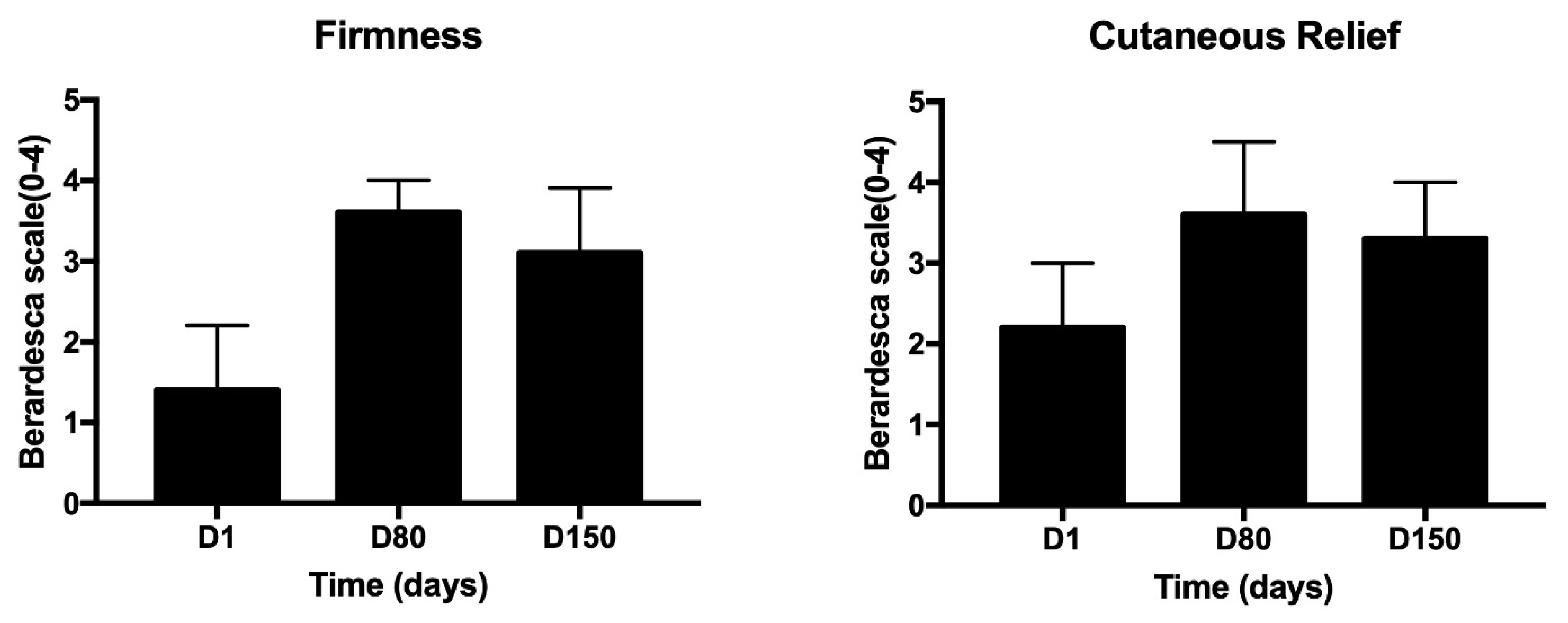
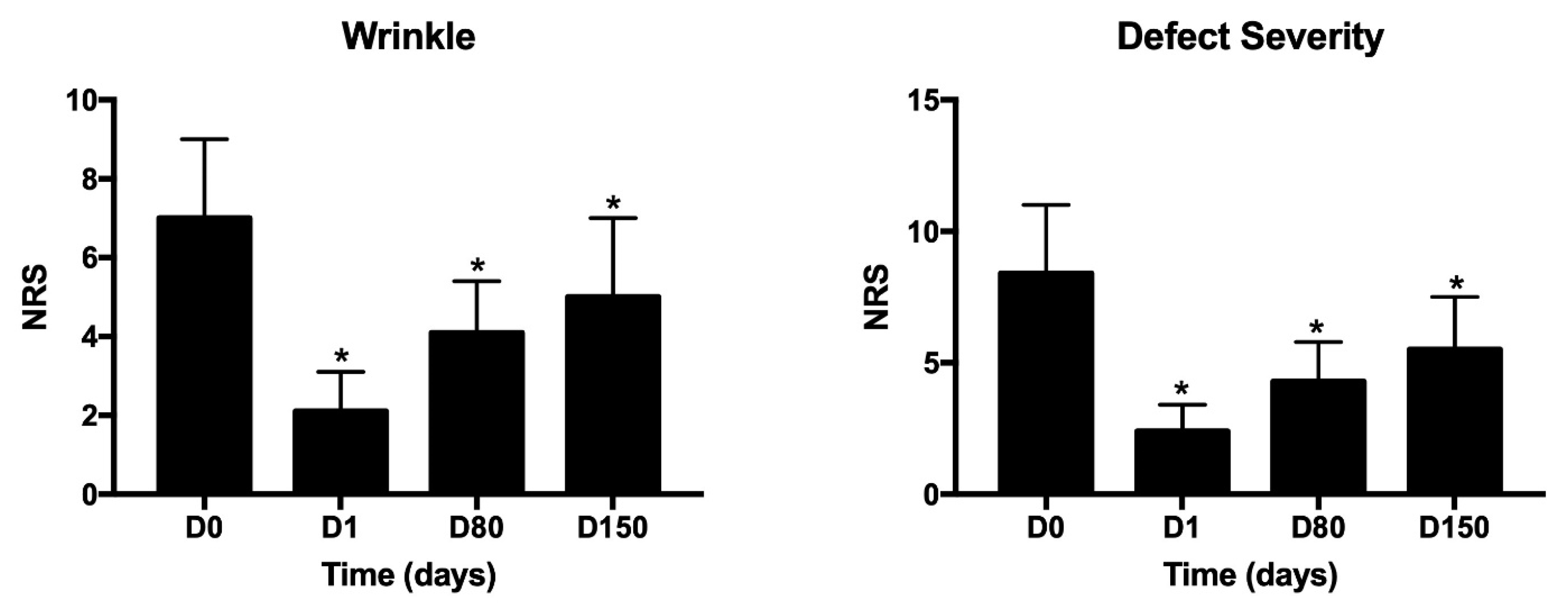
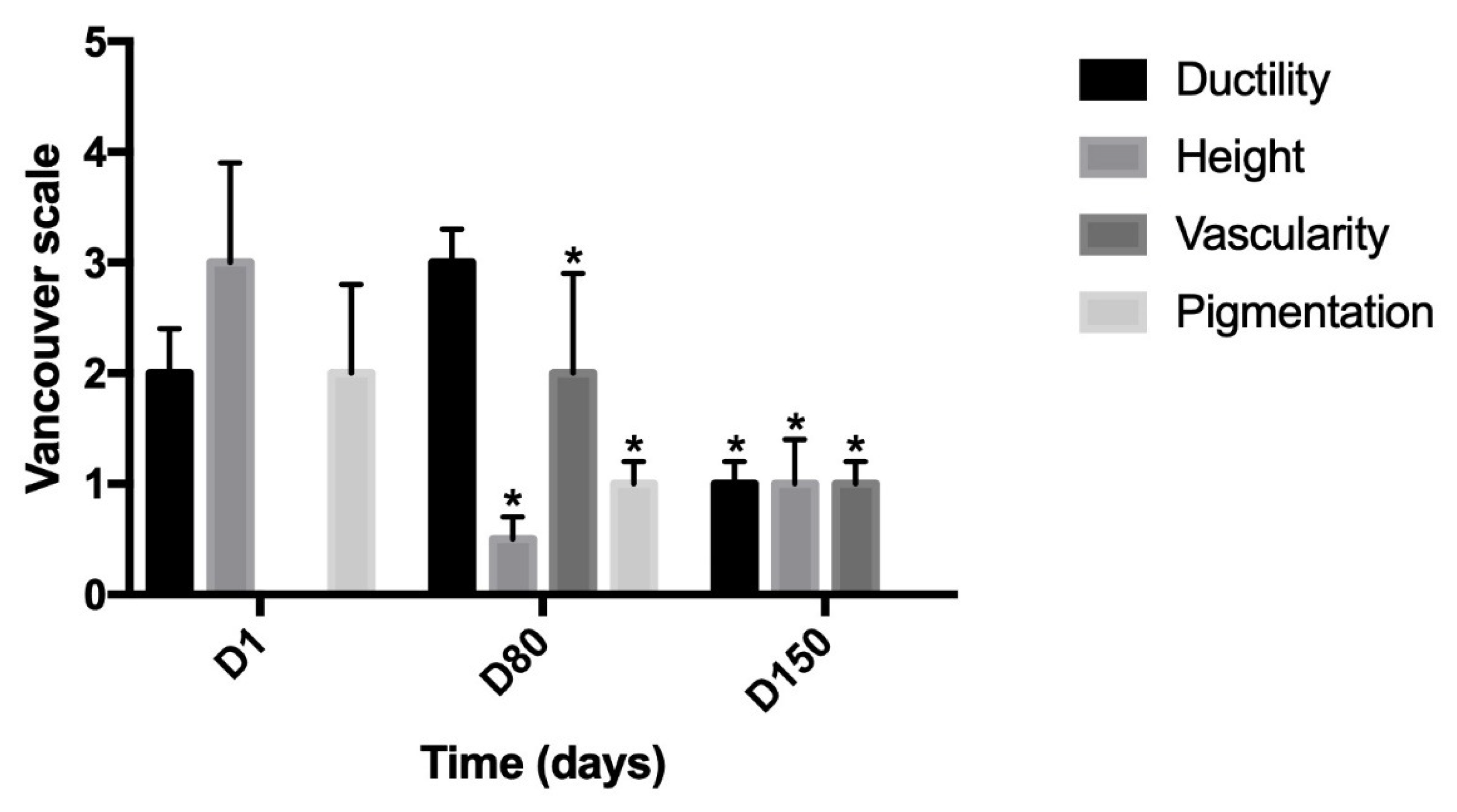
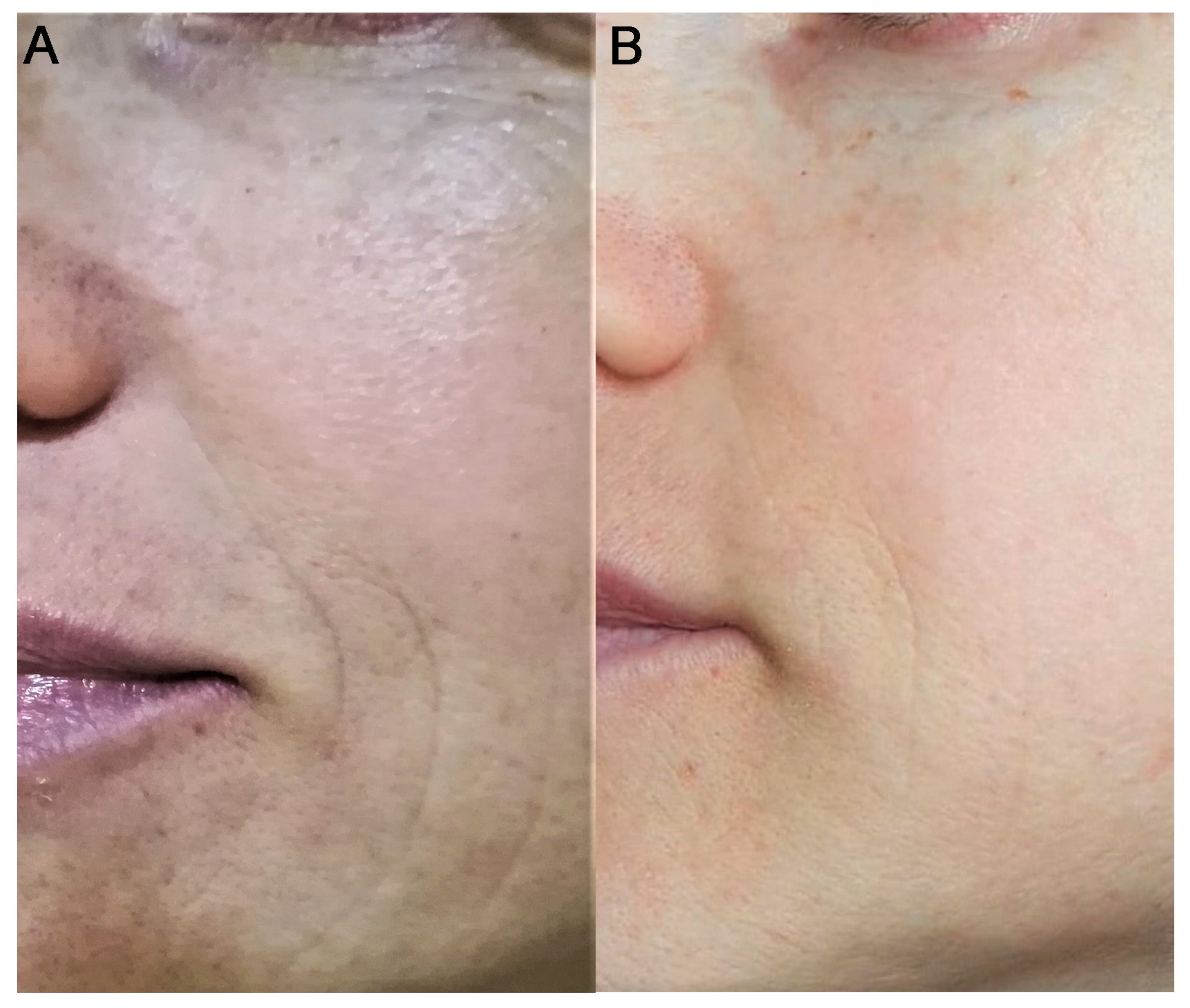
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.K.; Balendra, V.; Esposto, J.; Obaid, A.A.; Maccioni, R.B.; Jha, N.K.; Perry, G.; Moustafa, M.; Al-Shehri, M.; Singh, M.P.; et al. Therapeutic Antiaging Strategies. Biomedicines 2022, 10, 2515. [Google Scholar] [CrossRef]
- De Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; Feitosa, M.S.D.A.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J. Adipose-Derived Stem Cells for Facial Rejuvenation. J. Pers. Med. 2022, 12, 117. [Google Scholar] [CrossRef]
- Martic, I.; Jansen-Dürr, P.; Cavinato, M. Effects of Air Pollution on Cellular Senescence and Skin Aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Kümper, M.; Steinkamp, J.; Zigrino, P. Metalloproteinases in dermal homeostasis. Am. J. Physiol. Physiol. 2022, 323, C1290–C1303. [Google Scholar] [CrossRef]
- Lynch, K.; Pei, M. Age associated communication between cells and matrix: A potential impact on stem cell-based tissue regeneration strategies. Organogenesis 2014, 10, 289–298. [Google Scholar] [CrossRef]
- Sethe, S.; Scutt, A.; Stolzing, A. Aging of mesenchymal stem cells. Ageing Res. Rev. 2006, 5, 91–116. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Kaul, A.; Short, W.D.; Keswani, S.G.; Wang, X. Immunologic Roles of Hyaluronan in Dermal Wound Healing. Biomolecules 2021, 11, 1234. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Croce, M.; Dyne, K.; Boraldi, F.; Quaglino, D.; Cetta, G.; Tiozzo, R.; Ronchetti, I.P. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell 2001, 33, 326–331. [Google Scholar] [CrossRef]
- Mildmay-White, A. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef]
- Hemmrich, K.; Van de Sijpe, K.; Rhodes, N.P.; Hunt, J.A.; Di Bartolo, C.; Pallua, N.; Blondeel, P.; von Heimburg, D. Autologous In Vivo Adipose Tissue Engineering in Hyaluronan-Based Gels—A Pilot Study. J. Surg. Res. 2008, 144, 82–88. [Google Scholar] [CrossRef]
- Svolacchia, F.; Svolacchia, L. Adipose tissue micrograft in a scaffold of plasma-gel combined with platelet-derived growth factors in dermal wrinkle regeneration. Scr. Med. 2021, 52, 42–48. [Google Scholar] [CrossRef]
- Svolacchia, F.; Svolacchia, L. Adult Mesenchymal Stem Cells (MSCa) derived from adipose tissue (ADSCa) in a scaffold of free hyaluronic acid in the regeneration of periocular tissues. J. Appl. Cosmetol. 2019, 37. [Google Scholar]
- Rusanov, A.L.; Biryukova, Y.K.; Shoshina, O.O.; Luzgina, E.D.; Luzgina, N.G. Activation of TLR4 of Mesenchymal Stem Cells Enhances the Regenerative Properties of Their Secretomes. Bull. Exp. Biol. Med. 2021, 170, 544–549. [Google Scholar] [CrossRef]
- Luo, L.; Lucas, R.M.; Liu, L.; Stow, L.J. Signalling, sorting and scaffolding adaptors for Toll-like receptors. J. Cell Sci. 2020, 133, 239194. [Google Scholar] [CrossRef]
- Svolacchia, F.; Svolacchia, L. Use of microfiltered vs only disaggregated mesenchymal stem cells from adipose tissue in regenerative medicine. Scr. Med. 2020, 51, 152–157. [Google Scholar] [CrossRef]
- Bi, H.-S.; Zhang, C.; Nie, F.-F.; Pan, B.-L.; Xiao, E. Basic and Clinical Evidence of an Alternative Method to Produce Vivo Nanofat. Chin. Med. J. 2018, 131, 588–593. [Google Scholar] [CrossRef]
- Furno, D.L.; Tamburino, S.; Mannino, G.; Gilia, E.; Lombardo, G.A.G.; Tarico, M.S.; Vancheri, C.; Giuffrida, R.; Perrotta, R.E. Nanofat 2.0: Experimental Evidence for a Fat Grafting Rich in Mesenchymal Stem Cells. Physiol. Res. 2017, 66, 663–671. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; Francois, M.; Boivin, M.-N.; Bouchentouf, M.; Spaner, D.E.; Galipeau, J. Cytokine Modulation of TLR Expression and Activation in Mesenchymal Stromal Cells Leads to a Proinflammatory Phenotype. J. Immunol. 2009, 182, 7963–7973. [Google Scholar] [CrossRef]
- Tesar, B.M.; Goldstein, D.R. Toll-like receptors and their role in transplantation. Front. Biosci. 2007, 12, 4221–4238. [Google Scholar] [CrossRef][Green Version]
- DelaRosa, O.; Dalemans, W.; Lombardo, E. Toll-Like Receptors as Modulators of Mesenchymal Stem Cells. Front. Immunol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Jia, P.; Cheng, H.; Wang, C.; Chen, S.; Huang, H.; Han, Z.; Han, Z.-C.; Marycz, K.; et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res. Ther. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Svolacchia, F.; Svolacchia, L. A protocol of a new regenerative treatment of chrono-aging and photo-aging with progenitors cells from adipose Micrograft obtained from MilliGraft® Kit. Acta Sci. Med. Sci. 2019, 3, 30–35. [Google Scholar]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- PROCEDURE: Milligraft n.d. Available online: https://www.milligraft.com/en-procedure/ (accessed on 6 November 2022).
- Berardesca, E.; Distante, F.; Anthoine, P.; Rabbiosi, G.; Aubert, L. Clinical and instrumental evaluation of the activity of an anti-wrinkle cosmetic product on cutaneous relief and photoaged skin. J. Appl. Cosmetol. 1997, 15, 69–75. [Google Scholar]
- Sullivan, T.; Smith, J.; Kermode, J.; Mclver, E.; Courtemanche, D.J. Rating the Burn Scar. J. Burn Care Rehabil. 1990, 11, 256–260. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef]
- Cittadini, E.; Brucculeri, A.M.; Quartararo, F.; Vaglica, R.; Miceli, V.; Conaldi, P.G. Stem cell therapy in the treatment of organic and dysfunctional endometrial pathology. Minerva Obstet. Gynecol. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and Properties of Mesenchymal Stem Cells Derived from Microfragmented Adipose Tissue. Cell Transplant. 2015, 24, 1233–1252. [Google Scholar] [CrossRef]
- Nava, S.; Sordi, V.; Pascucci, L.; Tremolada, C.; Ciusani, E.; Zeira, O.; Cadei, M.; Soldati, G.; Pessina, A.; Parati, E.; et al. Long-Lasting Anti-Inflammatory Activity of Human Microfragmented Adipose Tissue. Stem Cells Int. 2019, 2019, 5901479. [Google Scholar] [CrossRef]
- Han, C.; Weng, X.S.; Cui, Y. Microfragmented adipose tissue and its initial application in articular disease. Chin. Med. J. 2019, 132, 2745–2748. [Google Scholar] [CrossRef]
- Sembronio, S.; Tel, A.; Tremolada, C.; Lazzarotto, A.; Isola, M.; Robiony, M. Temporomandibular Joint Arthrocentesis and Microfragmented Adipose Tissue Injection for the Treatment of Internal Derangement and Osteoarthritis: A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2021, 79, 1447–1456. [Google Scholar] [CrossRef]
- Laureti, S.; Gionchetti, P.; Cappelli, A.; Vittori, L.; Contedini, F.; Rizzello, F.; Golfieri, R.; Campieri, M.; Poggioli, G. Refractory Complex Crohn’s Perianal Fistulas: A Role for Autologous Microfragmented Adipose Tissue Injection. Inflamm. Bowel Dis. 2020, 26, 321–330. [Google Scholar] [CrossRef]
- Suh, A.; Pham, A.; Cress, M.J.; Pincelli, T.; TerKonda, S.P.; Bruce, A.J.; Zubair, A.C.; Wolfram, J.; Shapiro, S.A. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res. Rev. 2019, 54, 100933. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svolacchia, L.; Prisco, C.; Giuzio, F.; Svolacchia, F. Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report. Medicina 2022, 58, 1692. https://doi.org/10.3390/medicina58111692
Svolacchia L, Prisco C, Giuzio F, Svolacchia F. Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report. Medicina. 2022; 58(11):1692. https://doi.org/10.3390/medicina58111692
Chicago/Turabian StyleSvolacchia, Lorenzo, Claudia Prisco, Federica Giuzio, and Fabiano Svolacchia. 2022. "Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report" Medicina 58, no. 11: 1692. https://doi.org/10.3390/medicina58111692
APA StyleSvolacchia, L., Prisco, C., Giuzio, F., & Svolacchia, F. (2022). Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report. Medicina, 58(11), 1692. https://doi.org/10.3390/medicina58111692




