Atrial Fibrillation and Transvenous Lead Extraction—A Comprehensive Subgroup Analysis of the GermAn Laser Lead Extraction RegistrY (GALLERY)
Abstract
1. Introduction
2. Methods
2.1. Patients Population & Study Design
2.2. Definitions
2.3. Study Objectives
2.4. Lead Extraction Technique
2.5. Statistics
3. Results
3.1. Patient & Device Characteristics
3.2. Procedure and Outcome
3.3. Risk Factors for All-Cause Mortality
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Raatikainen, M.J.P.; Arnar, D.O.; Merkely, B.; Camm, A.J.; Hindricks, G. Access to and clinical use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2016 Report from the European Heart Rhythm Association. Europace 2016, 18, iii1–iii79. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Cai, C.; Vaibhav, V.; Sohail, M.R.; Hayes, D.L.; Hodge, D.O.; Tian, Y.; Asirvatham, R.; Cochuyt, J.J.; Huang, C.; et al. Trends of Cardiovascular Implantable Electronic Device Infection in 3 Decades: A Population-Based Study. JACC Clin. Electrophysiol. 2019, 5, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Eby, E.L.; Bengtson, L.G.S.; Johnson, M.P.; Burton, M.L.; Hinnenthal, J. Economic impact of cardiac implantable electronic device infections: Cost analysis at one year in a large U.S. health insurer. J. Med. Econ. 2020, 23, 698–705. [Google Scholar] [CrossRef]
- Olsen, T.; Jørgensen, O.D.; Nielsen, J.C.; Thøgersen, A.M.; Philbert, B.T.; Johansen, J.B. Incidence of device-related infection in 97 750 patients: Clinical data from the complete Danish device-cohort (1982–2018). Eur. Heart J. 2019, 40, 1862–1869. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Maisel, W.H.; Stevenson, L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003, 91, 2–8. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.; Clancy, J.; Deharo, J.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Burri, H.; Deharo, J.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Le, K.Y.; Sohail, M.R.; Friedman, P.A.; Uslan, D.Z.; Cha, S.S.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M.; Mayo Cardiovascular Infections Study Group; et al. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm 2011, 8, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.-U.; Burger, H.; Kaiser, L.; Osswald, B.; Bärsch, V.; Nägele, H.; Knaut, M.; Reichenspurner, H.; Gessler, N.; Willems, S.; et al. Transvenous lead extraction in patients with systemic cardiac device-related infection–Procedural outcome and risk prediction: A GALLERY subgroup-analysis. Heart Rhythm, 2022, in press. [CrossRef]
- Pecha, S.; Burger, H.; Chung, D.; Möller, V.; Madej, T.; Maali, A.; Osswald, B.; De Simone, R.; Monsefi, N.; Ziaukas, V.; et al. The GermAn Laser Lead Extraction RegistrY: GALLERY. Europace 2022, 24, 1627–1635. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.; Kutarski, A.; Rinaldi, C.A.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: A European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef]
- Starck, C.T.; Gonzalez, E.; Al-Razzo, O.; Mazzone, P.; Delnoy, P.P.; Breitenstein, A.; Steffel, J.; Eulert-Grehn, J.; Lanmuller, P.; Melillo, F.; et al. Results of the Patient-Related Outcomes of Mechanical lead Extraction Techniques (PROMET) study: A multicentre retrospective study on advanced mechanical lead extraction techniques. Europace 2020, 22, 1103–1110. [Google Scholar] [CrossRef]
- Mazzone, P.; Migliore, F.; Bertaglia, E.; Facchin, D.; Daleffe, E.; Calzolari, V.; Crosato, M.; Melillo, F.; Peruzza, F.; Marzi, A.; et al. Safety and efficacy of the new bidirectional rotational Evolution(R) mechanical lead extraction sheath: Results from a multicentre Italian registry. Europace 2018, 20, 829–834. [Google Scholar] [CrossRef]
- Witte, O.A.; Adiyaman, A.; Smit, J.J.J.; Ramdat Misier, A.R.; Elvan, A.; Ghani, A.; Delnoy, P.P. Success and complication rates of lead extraction with the first- vs. the second-generation Evolution mechanical sheath. Europace 2017, 19, 1717–1722. [Google Scholar] [CrossRef]
- Oto, A.; Aytemir, K.; Canpolat, U.; Yorgun, H.; Şahiner, L.; Kaya, E.B.; Kabakçi, G.; Tokgözoğlu, L. Evolution in transvenous extraction of pacemaker and implantable cardioverter defibrillator leads using a mechanical dilator sheath. Pacing. Clin. Electrophysiol. 2012, 35, 834–840. [Google Scholar] [CrossRef]
- Wazni, O.; Epstein, L.M.; Carrillo, R.G.; Love, C.; Adler, S.W.; Riggio, D.W.; Karim, S.S.; Bashir, J.; Greenspon, A.J.; DiMarco, J.P.; et al. Lead extraction in the contemporary setting: The LExICon study: An observational retrospective study of consecutive laser lead extractions. J. Am. Coll. Cardiol. 2010, 55, 579–586. [Google Scholar] [CrossRef]
- Kennergren, C.; Bucknall, C.A.; Butter, C.; Charles, R.; Fuhrer, J.; Grosfeld, M.; Tavernier, R.; Morgado, T.B.; Mortensen, P.; Paul, V.; et al. Laser-assisted lead extraction: The European experience. Europace 2007, 9, 651–656. [Google Scholar] [CrossRef]
- Tanawuttiwat, T.; Gallego, D.; Carrillo, R.G. Lead extraction experience with high frequency excimer laser. Pacing. Clin. Electrophysiol. 2014, 37, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Pecha, S.; Linder, M.; Gosau, N.; Castro, L.; Vogler, J.; Willems, S.; Reichenspurner, H.; Hakmi, S. Lead extraction with high frequency laser sheaths: A single-centre experience. Eur. J. Cardiothorac. Surg. 2017, 51, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.P.; Cronin, E.M.; Duarte, V.E.; Yu, C.; Tarakji, K.G.; Martin, D.O.; Callahan, T.; Cantillon, D.J.; Niebauer, M.J.; Saliba, W.I.; et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014, 11, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.L.; Wilkoff, B.L.; Love, C.J.; Sellers, T.D.; Reiser, C. Clinical study of the laser sheath for lead extraction: The total experience in the United States. Pacing. Clin. Electrophysiol. 2002, 25, 804–808. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Byrd, C.L.; Love, C.J.; Hayes, D.L.; Sellers, T.D.; Schaerf, R.; Pasornet, V.; Epstein, L.M.; Sorrentino, R.A.; Reiser, C.; et al. Pacemaker Lead Extraction with the Laser Sheath: Results of the Pacing Lead Extraction With the Excimer Sheath (PLEXES) Trial. J. Am. Coll. Cardiol. 1999, 33, 1671–1676. [Google Scholar] [CrossRef]
- Essebag, V.; Verma, A.; Healey, J.S.; Krahn, A.D.; Kalfon, E.; Coutu, B.; Ayala-Paredes, F.; Tang, A.S.; Sapp, J.; Sturmer, M.; et al. Clinically Significant Pocket Hematoma Increases Long-Term Risk of Device Infection: Bruise control infection Study. J. Am. Coll. Cardiol. 2016, 67, 1300–1308. [Google Scholar] [CrossRef]
- LaMori, J.C.; Mody, S.H.; Gross, H.J.; daCosta DiBonaventura, M.; Patel, A.A.; Schein, J.R.; Nelson, W.W. Burden of comorbidities among patients with atrial fibrillation. Ther. Adv. Cardiovasc. Dis. 2013, 7, 53–62. [Google Scholar] [CrossRef]
- Friberg, L.; Hammar, N.; Pettersson, H.; Rosenqvist, M. Increased mortality in paroxysmal atrial fibrillation: Report from the Stockholm Cohort-Study of Atrial Fibrillation (SCAF). Eur. Heart J. 2007, 28, 2346–2353. [Google Scholar] [CrossRef]
- Wyse, D.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management, I. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar]
| Atrial Fibrillation (n = 510) | |
|---|---|
| Mean age, years ± SD | 74.0 ± 10.3 |
| Female sex, n (%) | 113 (22.2) |
| Mean BMI, kg/m2 ± SD | 27.4 ± 4.8 |
| LVEF ≤ 30%, n (%) | 134 (26.3) |
| Arterial hypertension, n (%) | 394 (77.3) |
| Coronary artery disease, n (%) | 267 (52.4) |
| Diabetes mellitus, n (%) | 196 (38.4) |
| Chronic kidney disease, n (%) | 205 (40.2) |
| Previous heart surgery, n (%) | 142 (27.8) |
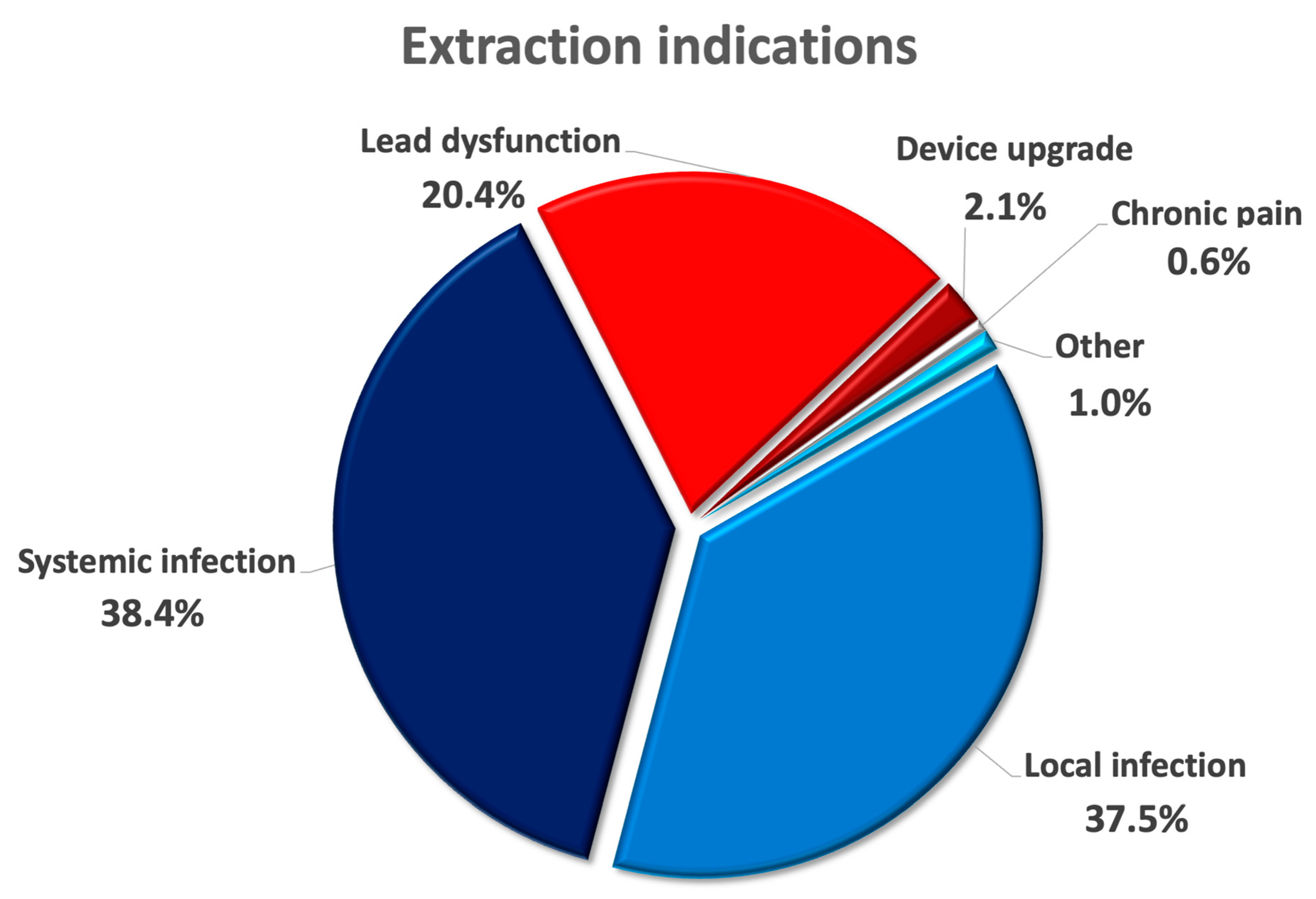
| Extraction indication | |
| Device infection, n (%) | 387 (75.9) |
| 197 (37.5) |
| 196 (38.4) |
| Lead dysfunction, n (%) | 104 (20.4) |
| System upgrade, n (%) | 11 (2.1) |
| Chronic pain, n (%) | 3 (0.6) |
| Other, n (%) | 5 (1.0) |
| Extracted devices | |
| Pacemaker, n (%) | 234 (45.9) |
| ICD, n (%) | 163 (31.9) |
| CRT, n (%) | 113 (22.2) |
| Leads | |
| Total number of leads, n | 1181 |
| Leads per patient, n ± SD | 2.3 ± 0.96 |
| Median age of the oldest leads, months [IQR] | 106.5 [66; 154] |
| Patients with abandoned leads, n (%) | 163 (32.0) |
| Patients with right-sided leads, n (%) | 174 (34.1) |
| Atrial Fibrillation (n = 510) | |
|---|---|
| Median hospital stay, days [IQR] | 10.0 [6; 18] |
| Median postoperative stay, days [IQR] | 7.0 [4; 14] |
| Median procedural time, minutes [IQR] | 77.5 [51.75; 120] |
| Overall complications, n (%) | 18 (3.6) |
| 9 (1.8) |
| 9 (1.8) |
| Complete procedural success, n (%) | 471 (92.4) |
| Clinical procedural success, n (%) | 496 (97.2) |
| Procedure-related mortality, n (%) | 5 (1.0) |
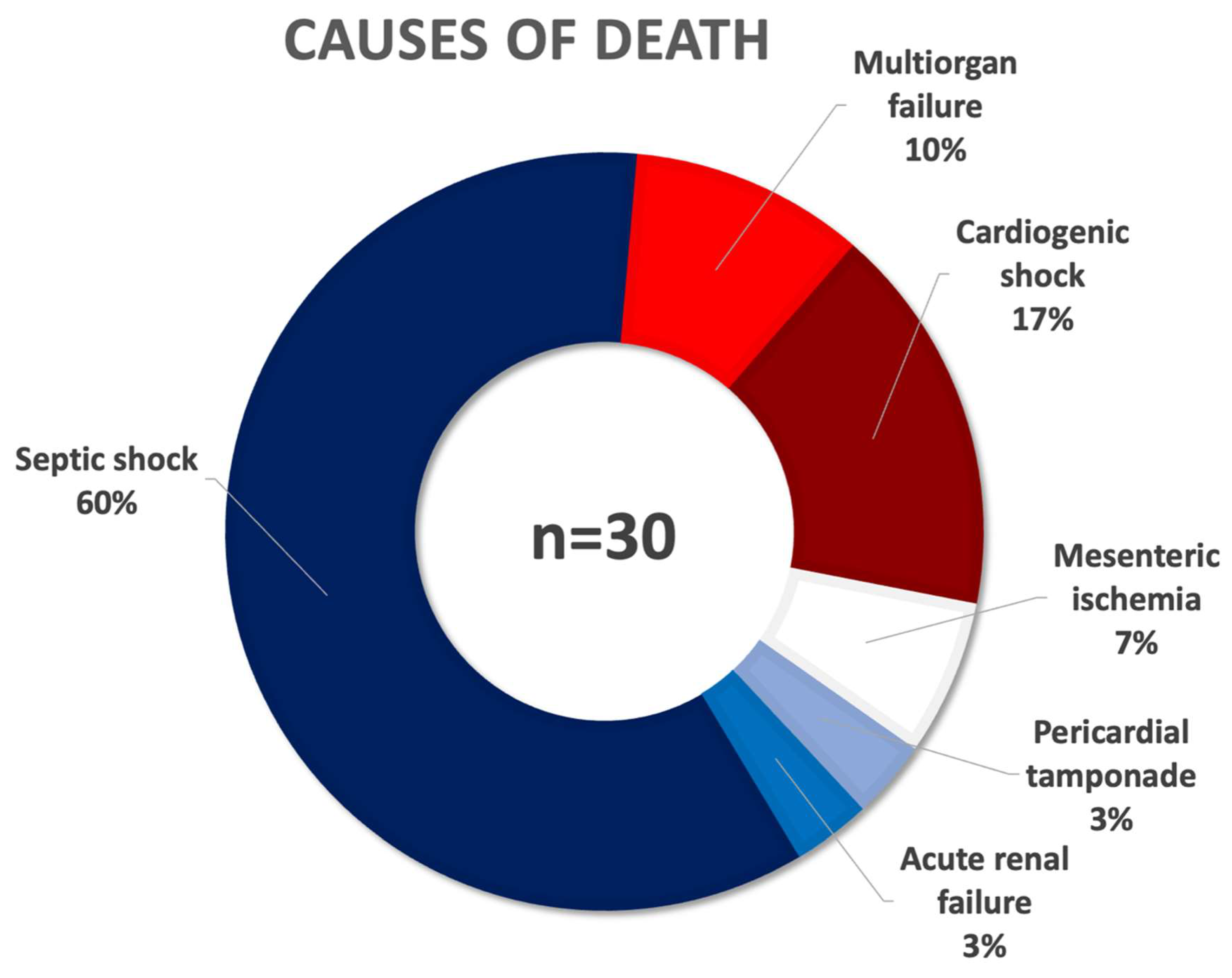
| All-cause mortality, n (%) | 30 (5.9) |
| 18 (3.6) |
| 3 (0.6) |
| 5 (1.0) |
| 1 (0.2) |
| 2 (0.4) |
| 1 (0.2) |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| BMI < 21 kg/m2 | 7.14 | 1.91–26.70 | 0.004 |
| BMI ≥ 35 kg/m2 | 0.41 | 0.05–3.47 | 0.410 |
| Arterial hypertension | 0.84 | 0.29–2.41 | 0.740 |
| Chronic kidney disease | 2.78 | 1.08–7.16 | 0.034 |
| Coronary artery disease | 1.62 | 0.63–4.14 | 0.316 |
| Diabetes mellitus | 0.94 | 0.38–2.32 | 0.893 |
| Right-sided leads | 1.06 | 0.41–2.77 | 0.900 |
| LVEF < 30% | 0.95 | 0.36–2.53 | 0.923 |
| Previous cardiac surgery | 1.11 | 0.42–2.95 | 0.830 |
| Abandoned leads | 1.32 | 0.44–3.92 | 0.621 |
| ≥4 leads in situ | 1.06 | 0.27–4.17 | 0.936 |
| Lead Age ≥ 10 years | 0.72 | 0.27–1.92 | 0.507 |
| Systemic infection | 50.66 | 6.70–382.9 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, D.-U.; Pecha, S.; Burger, H.; Anwar, O.; Eickholt, C.; Nägele, H.; Reichenspurner, H.; Gessler, N.; Willems, S.; Butter, C.; et al. Atrial Fibrillation and Transvenous Lead Extraction—A Comprehensive Subgroup Analysis of the GermAn Laser Lead Extraction RegistrY (GALLERY). Medicina 2022, 58, 1685. https://doi.org/10.3390/medicina58111685
Chung D-U, Pecha S, Burger H, Anwar O, Eickholt C, Nägele H, Reichenspurner H, Gessler N, Willems S, Butter C, et al. Atrial Fibrillation and Transvenous Lead Extraction—A Comprehensive Subgroup Analysis of the GermAn Laser Lead Extraction RegistrY (GALLERY). Medicina. 2022; 58(11):1685. https://doi.org/10.3390/medicina58111685
Chicago/Turabian StyleChung, Da-Un, Simon Pecha, Heiko Burger, Omar Anwar, Christian Eickholt, Herbert Nägele, Hermann Reichenspurner, Nele Gessler, Stephan Willems, Christian Butter, and et al. 2022. "Atrial Fibrillation and Transvenous Lead Extraction—A Comprehensive Subgroup Analysis of the GermAn Laser Lead Extraction RegistrY (GALLERY)" Medicina 58, no. 11: 1685. https://doi.org/10.3390/medicina58111685
APA StyleChung, D.-U., Pecha, S., Burger, H., Anwar, O., Eickholt, C., Nägele, H., Reichenspurner, H., Gessler, N., Willems, S., Butter, C., & Hakmi, S., on behalf of the GALLERY Investigators. (2022). Atrial Fibrillation and Transvenous Lead Extraction—A Comprehensive Subgroup Analysis of the GermAn Laser Lead Extraction RegistrY (GALLERY). Medicina, 58(11), 1685. https://doi.org/10.3390/medicina58111685






