EGFR Tyrosine Kinase Inhibitor Efficacy in Older Adult Patients with Advanced EGFR-Mutated Non-Small-Cell Lung Cancer: A Meta-Analysis and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Retrieval Strategies
2.2. Inclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kanazu, M.; Shimokawa, M.; Saito, R.; Mori, M.; Tamura, A.; Okano, Y.; Fujita, Y.; Endo, T.; Motegi, M.; Takata, S.; et al. Predicting systemic therapy toxicity in older adult patients with advanced non-small cell lung cancer: A prospective multicenter study of National Hospital Organization in Japan. J. Geriatr. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Stat Fact Sheets: Lung and Bronchus Cancer. Available online: http://seer.cancer.gov/statfacts/html/lungb.html (accessed on 2 September 2022).
- Orimo, H.; Ito, H.; Suzuki, T.; Araki, A.; Hosoi, T.; Sawabe, M. Reviewing the definition of “elderly”. Geriatr. Gerontol. Int. 2006, 6, 149–158. [Google Scholar]
- Blanco, R.; Maestu, I.; de la Torre, M.; Cassinello, A.; Nuñez, I. A review of the management of elderly patients with non-small cell lung cancer. Ann. Oncol. 2015, 26, 451–463. [Google Scholar] [CrossRef]
- Passiglia, F.; Bironzo, P.; Bertaglia, V.; Listì, A.; Garbo, E.; Scagliotti, G.V. Optimizing the clinical management of EGFR-mutant advanced non-small cell lung cancer: A literature review. Transl. Lung Cancer Res. 2022, 11, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Pallis, A.G.; Gridelli, C.; Wedding, U.; Faivre-Finn, C.; Veronesi, G.; Jaklitsch, M.; Luciani, A.; O’Brien, M. Management of elderly patients with NSCLC; updated expert’s opinion paper: EORTC Elderly Task Force, Lung Cancer Group and International Society for Geriatric Oncology. Ann. Oncol. 2014, 25, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Sequist, L.V.; Tan, E.H.; Geater, S.L.; Orlov, S.; Zhang, L.; Lee, K.H.; Tsai, C.M.; Kato, T.; Barrios, C.H.; et al. Afatinib as First-line Treatment of Older Patients with EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Subgroup Analyses of the LUX-Lung 3, LUX-Lung 6, and LUX-Lung 7 Trials. Clin. Lung Cancer 2018, 19, e465–e479. [Google Scholar] [CrossRef]
- Roviello, G.; Zanotti, L.; Cappelletti, M.R.; Gobbi, A.; Dester, M.; Paganini, G.; Pacifico, C.; Generali, D.; Roudi, R. Are EGFR tyrosine kinase inhibitors effective in elderly patients with EGFR-mutated non-small cell lung cancer? Clin. Exp. Med. 2018, 18, 15–20. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and me-ta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Hirsh, V.; Boyer, M.; Yang, J.-H.; Mok, T.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-Lung 7 trial. Ann. Oncol. 2017, 28, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J.; et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib with Gefitinib in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Boland, A.; Bates, V.; Vecchio, F.; Dundar, Y.; Chaplin, M.; Green, J.A. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst. Rev. 2021, 3, CD010383. [Google Scholar]
- Yang, J.J.; Zhou, Q.; Yan, H.H.; Zhang, X.C.; Chen, H.J.; Tu, H.Y.; Wang, Z.; Xu, C.R.; Su, J.; Wang, B.C.; et al. A phase III randomised controlled trial of erlotinib vs. gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br. J. Cancer 2017, 116, 568–574. [Google Scholar] [CrossRef]
- Lin, J.Z.; Ma, S.K.; Wu, S.X.; Yu, S.H.; Li, X.Y. A network meta-analysis of nonsmall-cell lung cancer patients with an activating EGFR mutation: Should osimertinib be the first-line treatment? Medicine 2018, 97, e11569. [Google Scholar] [CrossRef]
- Holleman, M.S.; van Tinteren, H.; Groen, H.J.; Al, M.J.; Uyl-de Groot, C.A. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: A network meta-analysis. Oncol. Targets Ther. 2019, 12, 1413–1421. [Google Scholar] [CrossRef]
- Zhao, Y.I.; Liu, J.; Cai, X.; Pan, Z.; Liu, J.; Yin, W.; Chen, H.; Xie, Z.; Liang, H.; Wang, W.; et al. Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: Systematic review and network meta-analysis. BMJ 2019, 367, L5460. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Shah, D.R. Safety and Tolerability of Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors in Oncology. Drug Saf. 2019, 42, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cheng, B.; Chen, Z.; Li, J.; Liang, H.; Chen, Y.; Zhu, F.; Li, C.; Xu, K.; Xiong, S.; et al. Toxicity profile of epidermal growth factor receptor tyrosine kinase inhibitors for patients with lung cancer: A systematic review and network meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103305. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines of Non-Small Cell Lung Cancer Version 4. 2022. Available online: https://www.nccn.org/ (accessed on 2 September 2022).

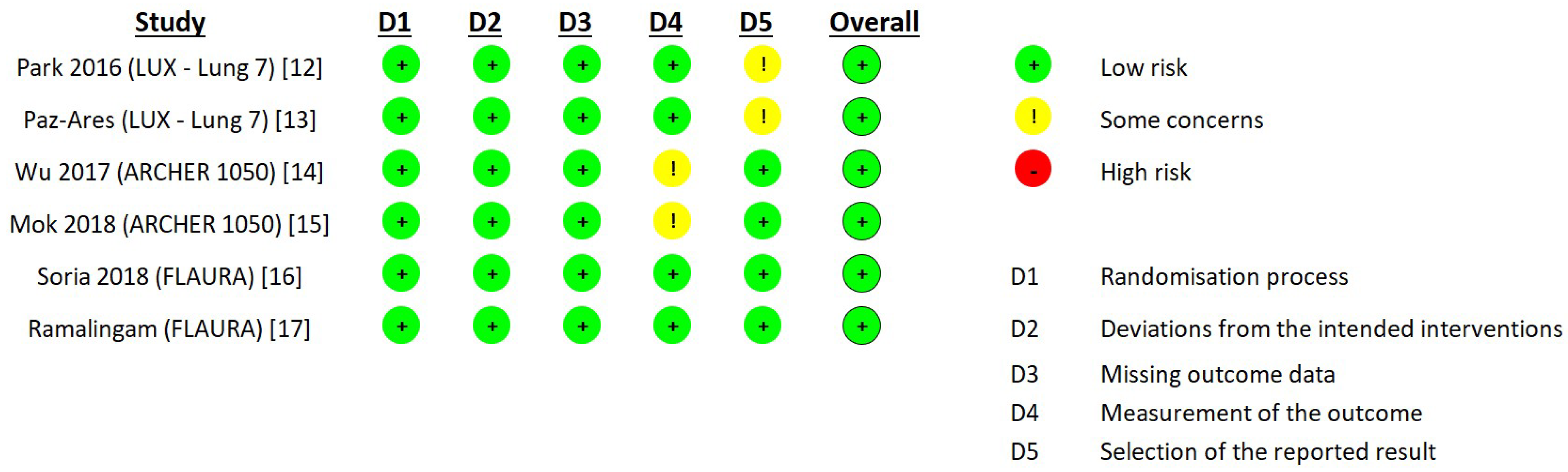

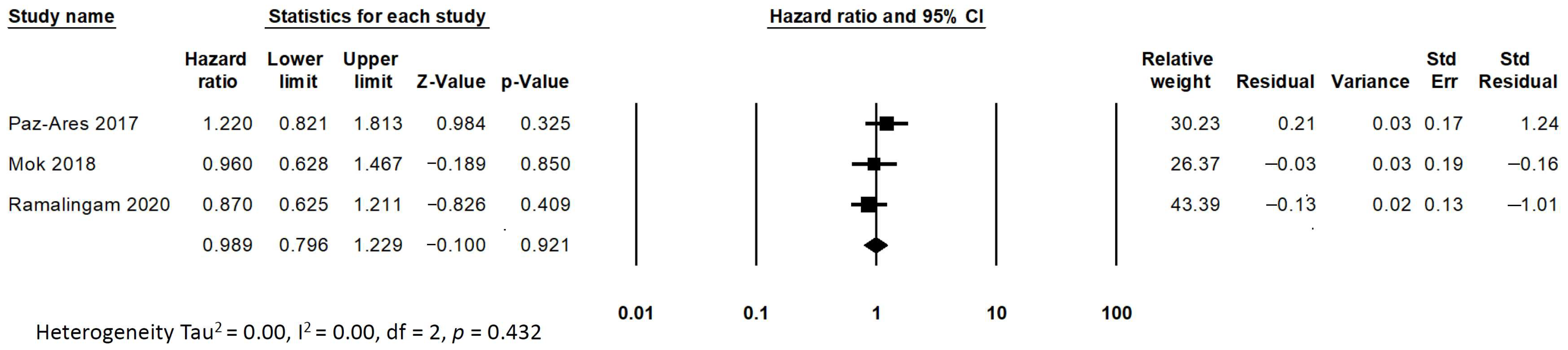
| Study Authors Year | Phase | Treatment Experimental/Control (EGFR TKIs) Participants | Old Adult Participants Old Adults (>65 Years Old)/Total | Primary Endpoint PFS and OS 95% CI |
|---|---|---|---|---|
| LUX-Lung 7 Park K. et al. 2016 [12] Paz-Ares L. et al. 2017 [13] | IIB | Afatinib/Gefitinib 160/159 | 142/319 (44%) | PFS (Total/Old adults) HR 0.73 (0.57–0.95)/HR 0.85 (0.59–1.22) OS (Total/Old adults) HR 0.86 (0.66–1.12)/HR 1.22 (0.82–1.81) |
| ARCHER 1050 Wu YL. et al. 2017 [14] Mok TS. et al. 2018 [15] | III | Dacomitinib/Gefitinib 227/225 | 179/452 (39%) | PFS (Total/Old adults) HR 0.59 (0.47–0.74)/HR 0.69 (0.48–0.99) OS (Total/Old adults) HR 0.76 (0.58–0.99)/HR 0.96 (0.63–1.47) |
| FLAURA Soria JC. et al. 2018 [16] Ramalingam SS. et al. 2020 [17] | III | Osimertinib/Erlotinib & Gefitinib 279/277 | 258/556 (46%) | PFS (Total/Old adults) HR 0.46 (0.37–0.57) / HR 0.49 (0.35–0.67) OS (Total/Old adults) HR 0.80 (0.64–1.00)/HR 0.87 (0.63–1.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Chou, D.-W.; Chung, K.-M.; Chang, H.-Y. EGFR Tyrosine Kinase Inhibitor Efficacy in Older Adult Patients with Advanced EGFR-Mutated Non-Small-Cell Lung Cancer: A Meta-Analysis and Systematic Review. Medicina 2022, 58, 1645. https://doi.org/10.3390/medicina58111645
Chen C-H, Chou D-W, Chung K-M, Chang H-Y. EGFR Tyrosine Kinase Inhibitor Efficacy in Older Adult Patients with Advanced EGFR-Mutated Non-Small-Cell Lung Cancer: A Meta-Analysis and Systematic Review. Medicina. 2022; 58(11):1645. https://doi.org/10.3390/medicina58111645
Chicago/Turabian StyleChen, Chang-Hung, Deng-Wei Chou, Kuo-Mou Chung, and Han-Yu Chang. 2022. "EGFR Tyrosine Kinase Inhibitor Efficacy in Older Adult Patients with Advanced EGFR-Mutated Non-Small-Cell Lung Cancer: A Meta-Analysis and Systematic Review" Medicina 58, no. 11: 1645. https://doi.org/10.3390/medicina58111645
APA StyleChen, C.-H., Chou, D.-W., Chung, K.-M., & Chang, H.-Y. (2022). EGFR Tyrosine Kinase Inhibitor Efficacy in Older Adult Patients with Advanced EGFR-Mutated Non-Small-Cell Lung Cancer: A Meta-Analysis and Systematic Review. Medicina, 58(11), 1645. https://doi.org/10.3390/medicina58111645






