Protective Role of IL7A-197 A/G Heterozygosity in the Development and Severity of Colorectal Cancer in the Bulgarian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. DNA Extraction
2.3. Genotyping
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of CRC Cases and Controls
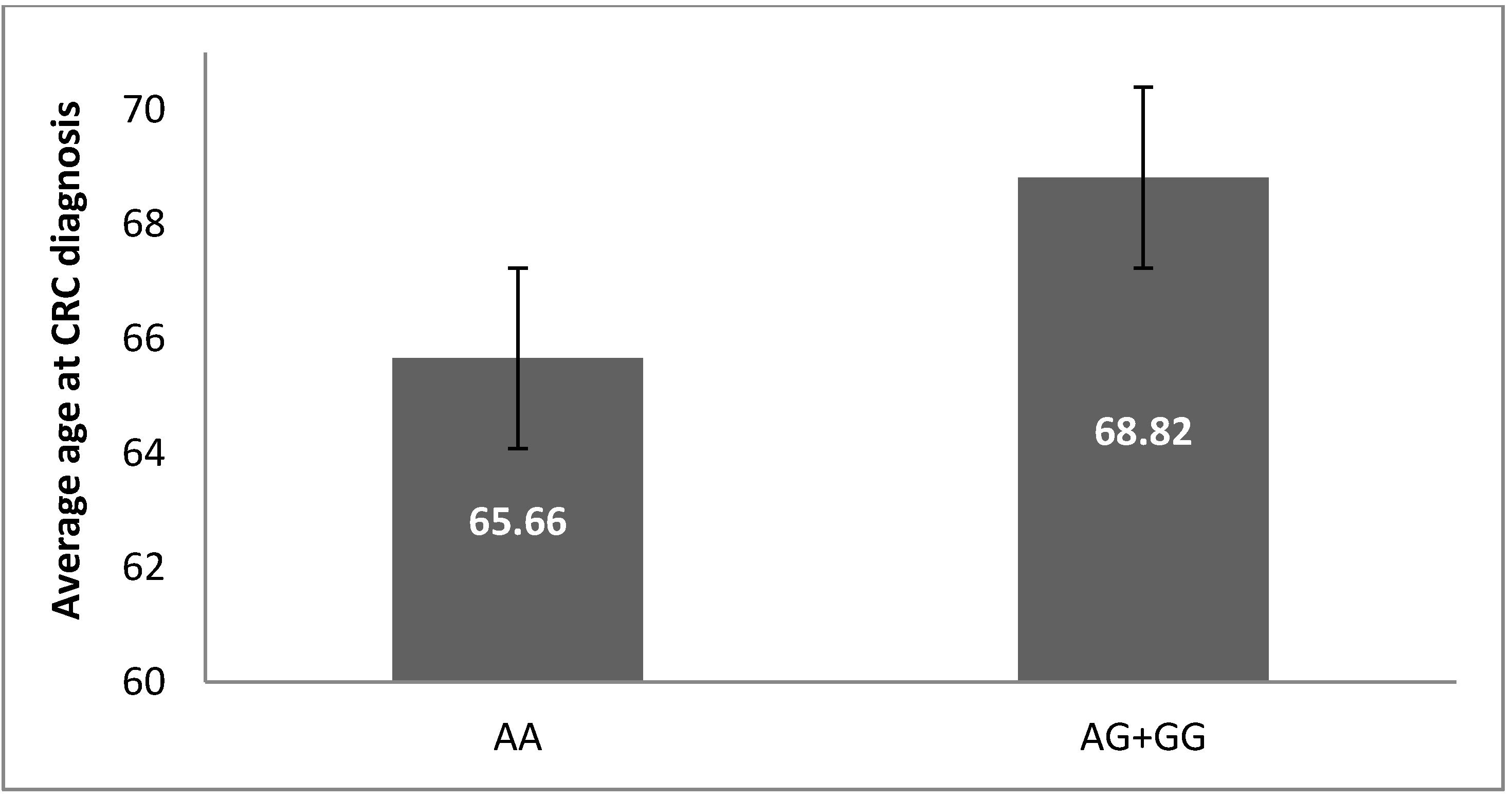
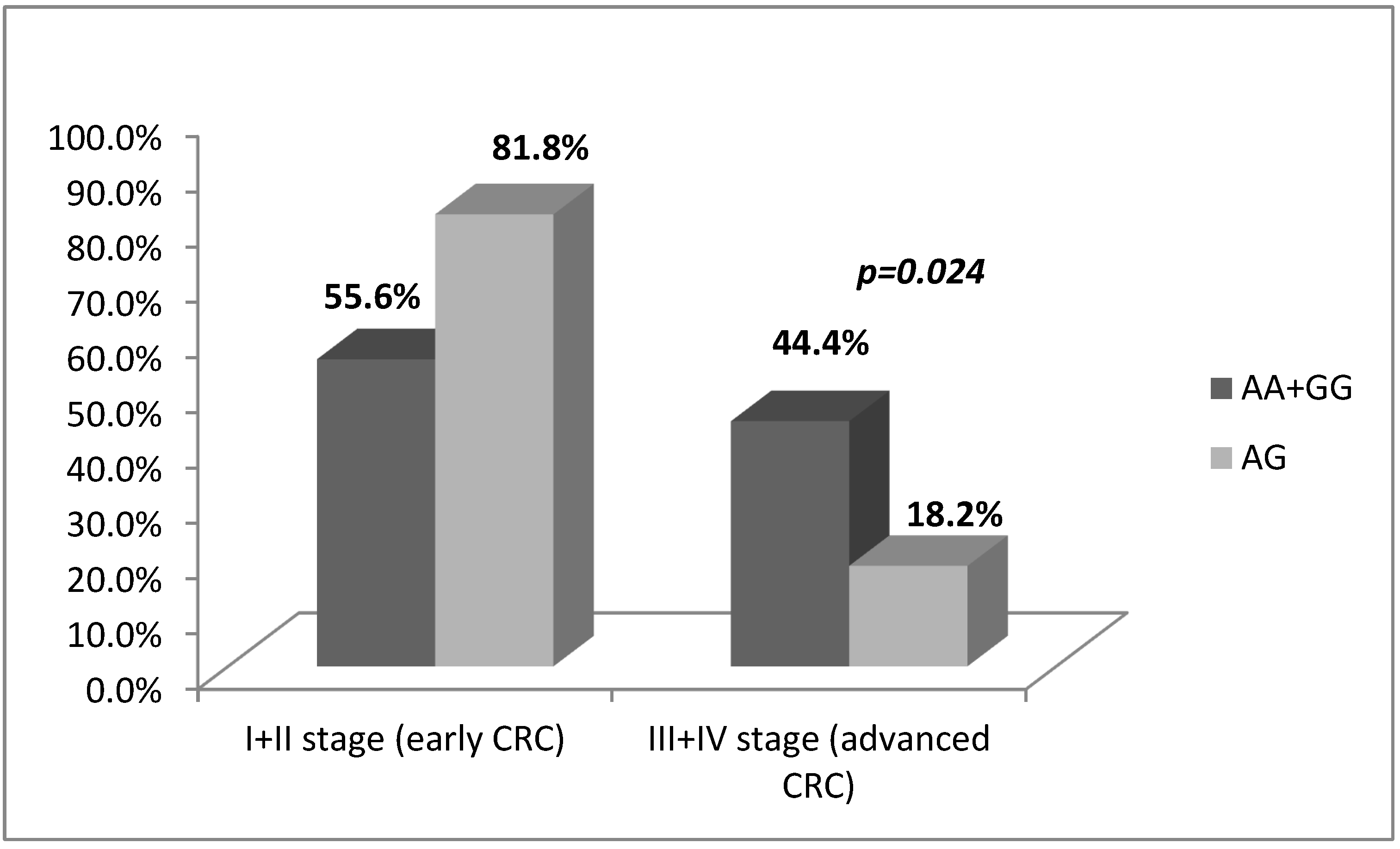
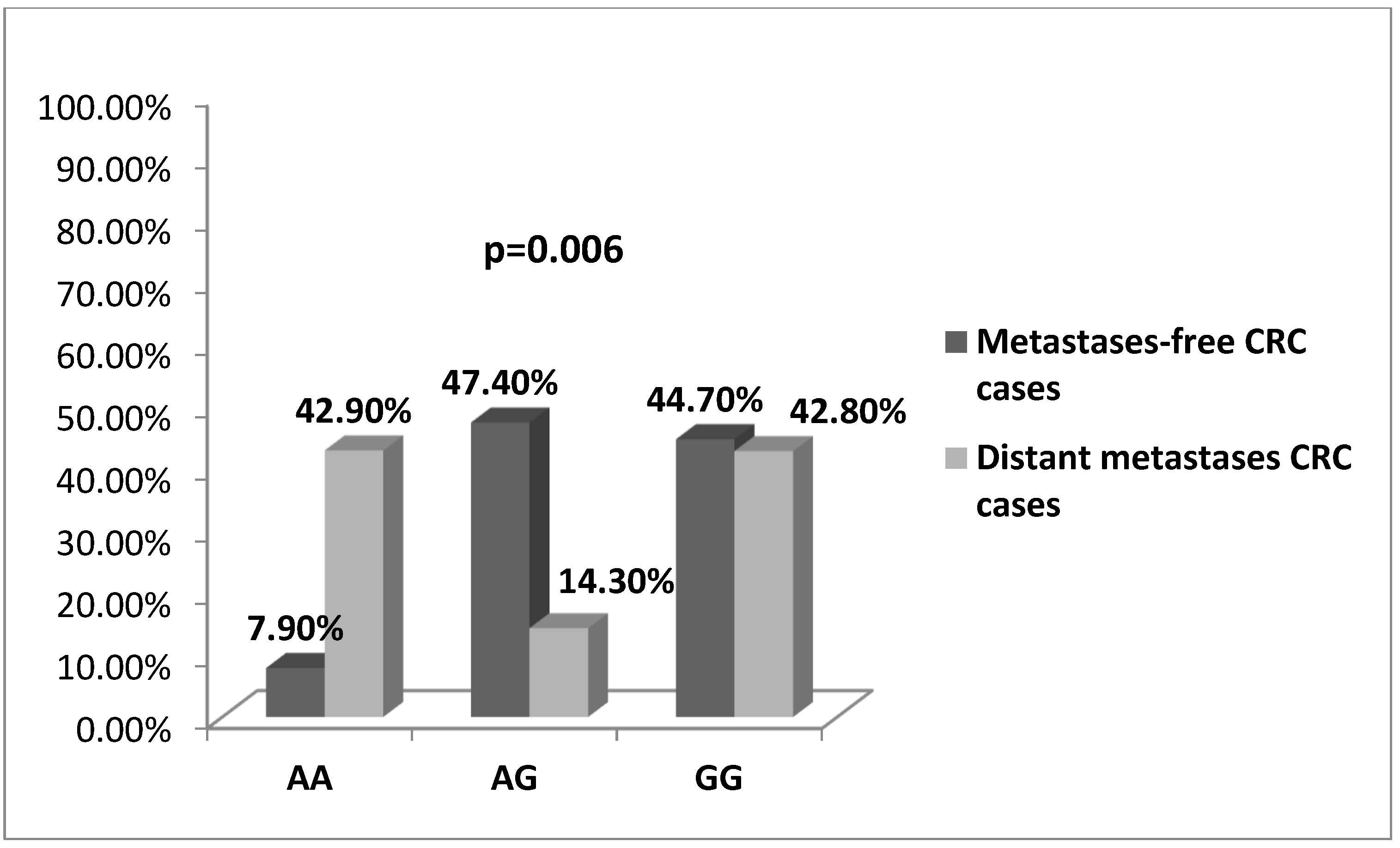
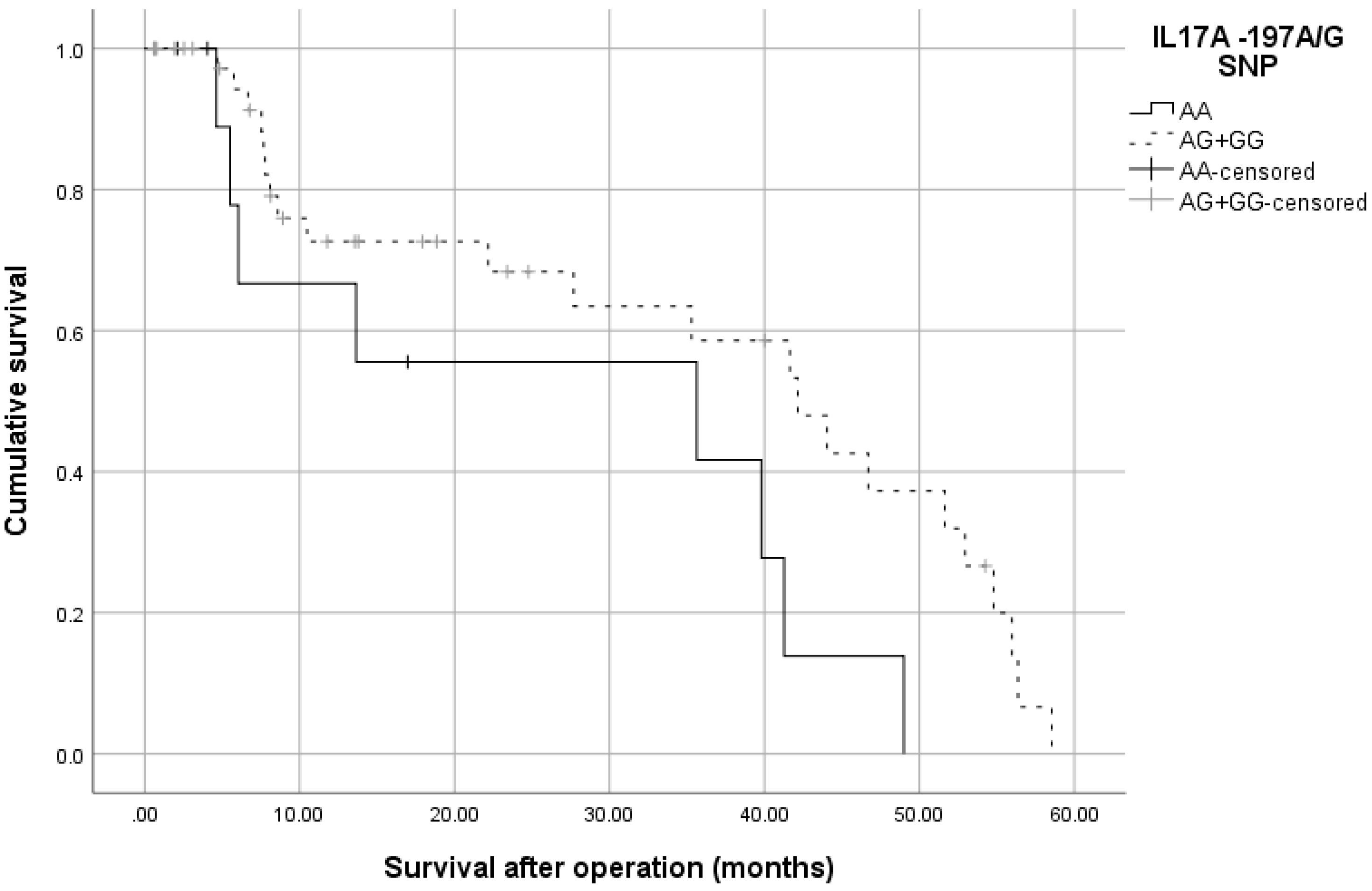
3.2. -197 G/A SNP (rs2275913) in IL17A Gene Is Associated with CRC Risk and Severity of the Disease
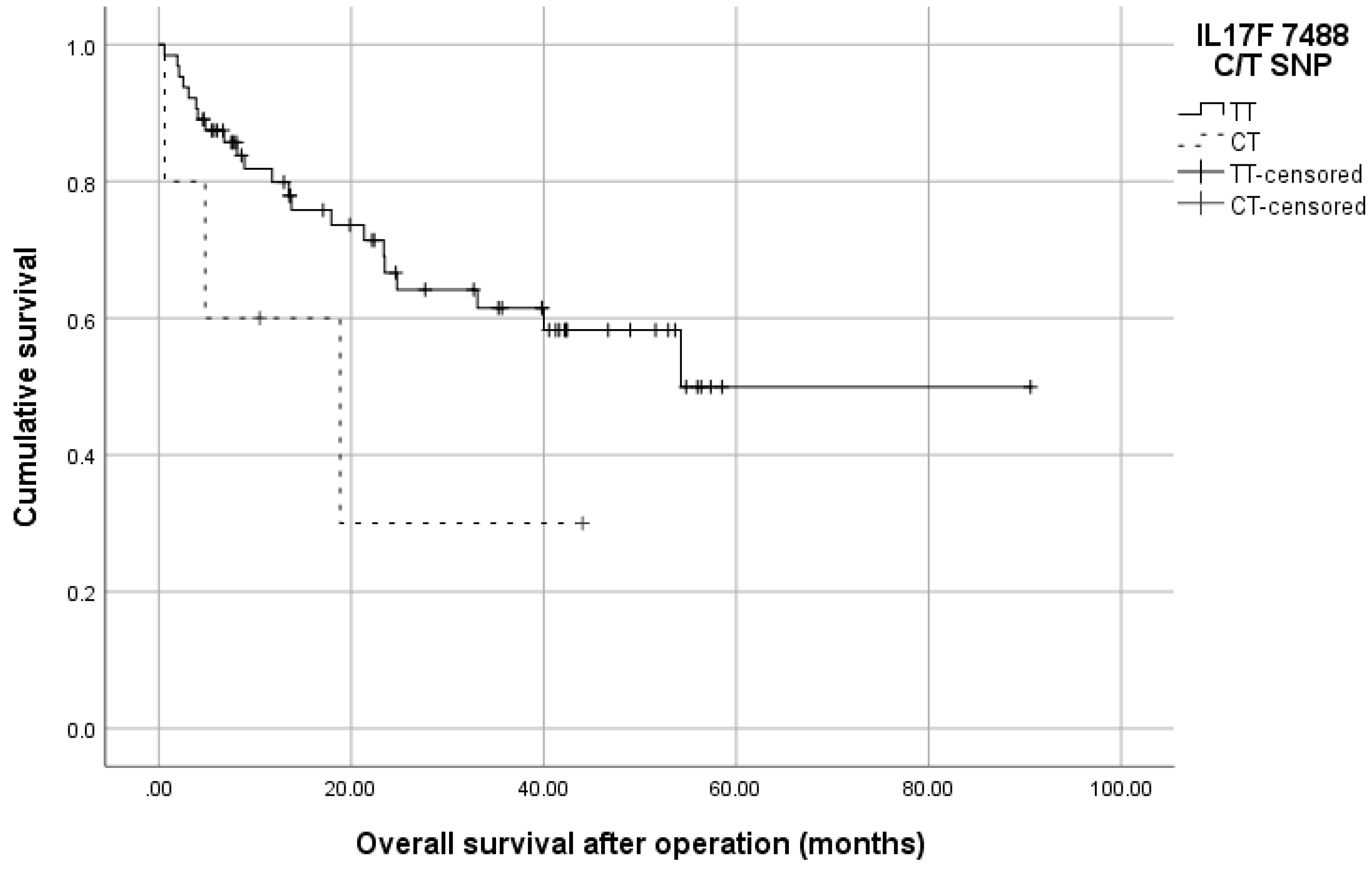
3.3. Distribution of 7488 T/C SNP (rs763780) in IL17F Gene
3.4. Haplotypes Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. Chapter 11, Multi-step Tumorigenesis. In The Biology of Cancer, 2nd ed.; Garland Science, Taylor & Francis Group: Oxford, UK, 2014; pp. 439–509. [Google Scholar]
- West, N.R.; McCuaig, S.; Franchini, F.; Powrie, F. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 2015, 15, 615–629. [Google Scholar] [CrossRef]
- Chang, S.H.; Dong, C. IL-17F: Regulation, signaling and function in inflammation. Cytokine 2009, 46, 7–11. [Google Scholar] [CrossRef]
- Hymowitz, S.G.; Filvaroff, E.H.; Yin, J.P.; Lee, J.; Cai, L.; Risser, P.; Maruoka, M.; Mao, W.; Foster, J.; Kelley, R.F.; et al. IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001, 20, 5332–5341. [Google Scholar] [CrossRef] [PubMed]
- Moseley, T.A.; Haudenschild, D.R.; Rose, L.; Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pagès, F.; et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Wilke, C.M.; Bishop, K.; Fox, D.; Zou, W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011, 32, 603–611. [Google Scholar] [CrossRef]
- Kryczek, I.; Banerjee, M.; Cheng, P.; Vatan, L.; Szeliga, W.; Wei, S.; Huang, E.; Finlayson, E.; Simeone, D.; Welling, T.H.; et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009, 114, 1141–1149. [Google Scholar] [CrossRef]
- Dubin, P.J.; Kolls, J.K. Th17 cytokines and mucosal immunity. Immunol. Rev. 2008, 226, 160–171. [Google Scholar] [CrossRef]
- Greten, T.F.; Zhao, F.; Gamrekelashvili, J.; Korangy, F. Human Th17 cells in patients with cancer: Friends or foe? Oncoimmunology 2012, 1, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef] [PubMed]
- De Simone, V.; Pallone, F.; Monteleone, G.; Stolfi, C. Role of T. Oncoimmunology 2013, 2, e26617. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yang, X.O.; Yan, H.; Liu, W.; Niu, X.; Shi, Y.; Fang, W.; Xiong, B.; Wan, Y.; Dong, C. A protective role by interleukin-17F in colon tumorigenesis. PLoS ONE 2012, 7, e34959. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A.; Goll, R.; Florholmen, J. IL-17A in the tumor microenvironment of the human colorectal adenoma-carcinoma sequence. Scand. J. Gastroenterol. 2012, 47, 1304–1312. [Google Scholar] [CrossRef]
- Nemati, K.; Golmoghaddam, H.; Hosseini, S.V.; Ghaderi, A.; Doroudchi, M. Interleukin-17FT7488 allele is associated with a decreased risk of colorectal cancer and tumor progression. Gene 2015, 561, 88–94. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, Z.; Chen, B.; Yu, J.; Xue, L.; Hao, Y.; Chen, M.; Sung, J.J.; Hu, P. Association between polymorphisms in interleukin-17A and interleukin-17F genes and risks of gastric cancer. Int. J. Cancer 2010, 127, 86–92. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Zhang, Y.; Wang, Y.; Huang, S.; Wang, Z.; Tian, B.; Yang, Y.; Jiang, W.; Pang, D. Association analysis of IL-17A and IL-17F polymorphisms in Chinese Han women with breast cancer. PLoS ONE 2012, 7, e34400. [Google Scholar] [CrossRef]
- Arisawa, T.; Tahara, T.; Shibata, T.; Nagasaka, M.; Nakamura, M.; Kamiya, Y.; Fujita, H.; Yoshioka, D.; Arima, Y.; Okubo, M.; et al. The influence of polymorphisms of interleukin-17A and interleukin-17F genes on the susceptibility to ulcerative colitis. J. Clin. Immunol. 2008, 28, 44–49. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, Z.; Hou, J.; Chen, M.; Gao, X.; Hu, P. Association of interleukin-17F 7488 single nucleotide polymorphism and inflammatory bowel disease in the Chinese population. Scand. J. Gastroenterol. 2009, 44, 720–726. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chung, J.H.; Kim, S.K.; Rhee, S.Y.; Chon, S.; Oh, S.J.; Hong, I.K.; Eun, Y.G. Association between interleukin 17/interleukin 17 receptor gene polymorphisms and papillary thyroid cancer in Korean population. Cytokine 2015, 71, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Al Obeed, O.A.; Vaali-Mohamed, M.A.; Alkhayal, K.A.; Bin Traiki, T.A.; Zubaidi, A.M.; Arafah, M.; Harris, R.A.; Khan, Z.; Abdulla, M.H. IL-17 and colorectal cancer risk in the Middle East: Gene polymorphisms and expression. Cancer Manag. Res. 2018, 10, 2653–2661. [Google Scholar] [CrossRef]
- Kolls, J.K.; Lindén, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.A.; Barbirou, M.; Stayoussef, M.; Dallel, M.; Mokrani, A.; Makni, L.; Mezlini, A.; Bouhaouala, B.; Yacoubi-Loueslati, B.; Almawi, W.Y. Association of interleukin-17A polymorphisms with the risk of colorectal cancer: A case-control study. Cytokine 2018, 110, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Numasaki, M.; Fukushi, J.; Ono, M.; Narula, S.K.; Zavodny, P.J.; Kudo, T.; Robbins, P.D.; Tahara, H.; Lotze, M.T. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003, 101, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, J.; Liu, J.; Xie, W.; Diao, X.; Xu, J.; Zhu, B.; Chen, Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 2010, 69, 348–354. [Google Scholar] [CrossRef]
- Liao, R.; Sun, J.; Wu, H.; Yi, Y.; Wang, J.X.; He, H.W.; Cai, X.Y.; Zhou, J.; Cheng, Y.F.; Fan, J.; et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2013, 32, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lai, Y.H.; Chen, H.Y.; Guo, H.R.; Su, I.J.; Chen, H.H. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013, 63, 225–233. [Google Scholar] [CrossRef]
- Xie, Y.; Sheng, W.; Xiang, J.; Ye, Z.; Yang, J. Interleukin-17F suppresses hepatocarcinoma cell growth via inhibition of tumor angiogenesis. Cancer Investig. 2010, 28, 598–607. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Nakata, K.; Onizuka, M.; Kawase, T.; Akiyama, H.; Miyamura, K.; Morishima, Y.; Fukuda, T.; Kodera, Y.; et al. A genetic variant in the IL-17 promoter is functionally associated with acute graft-versus-host disease after unrelated bone marrow transplantation. PLoS ONE 2011, 6, e26229. [Google Scholar] [CrossRef]
- Samiei, G.; Yip, W.K.; Leong, P.P.; Jabar, M.F.; Dusa, N.M.; Mohtarrudin, N.; Seow, H.F. Association between polymorphisms of interleukin-17A G197A and interleukin-17F A7488G and risk of colorectal cancer. J. Cancer Res. Ther. 2018, 14, S299–S305. [Google Scholar] [CrossRef] [PubMed]
- Omrane, I.; Marrakchi, R.; Baroudi, O.; Mezlini, A.; Ayari, H.; Medimegh, I.; Stambouli, N.; Kourda, N.; Bouzaienne, H.; Uhrhammer, N.; et al. Significant association between interleukin-17A polymorphism and colorectal cancer. Tumour Biol. 2014, 35, 6627–6632. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Takahashi, D.; Hizawa, N.; Suzuki, S.; Matsukura, S.; Kokubu, F.; Maeda, Y.; Fukui, Y.; Konno, S.; Huang, S.K.; et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J. Allergy Clin. Immunol. 2006, 117, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, T.; Gębura, K.; Wysoczańska, B.; Jaźwiec, B.; Dobrzyńska, O.; Mazur, G.; Kuliczkowski, K.; Bogunia-Kubik, K. IL-17F gene polymorphism is associated with susceptibility to acute myeloid leukemia. J. Cancer Res. Clin. Oncol. 2014, 140, 1551–1555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, H.Y.; Wang, R.; Ma, W. IL-17A G197A and IL-17F T7488C polymorphisms and cancer risk in Asian populations: A meta-analysis. J. BUON 2014, 19, 562–566. [Google Scholar]

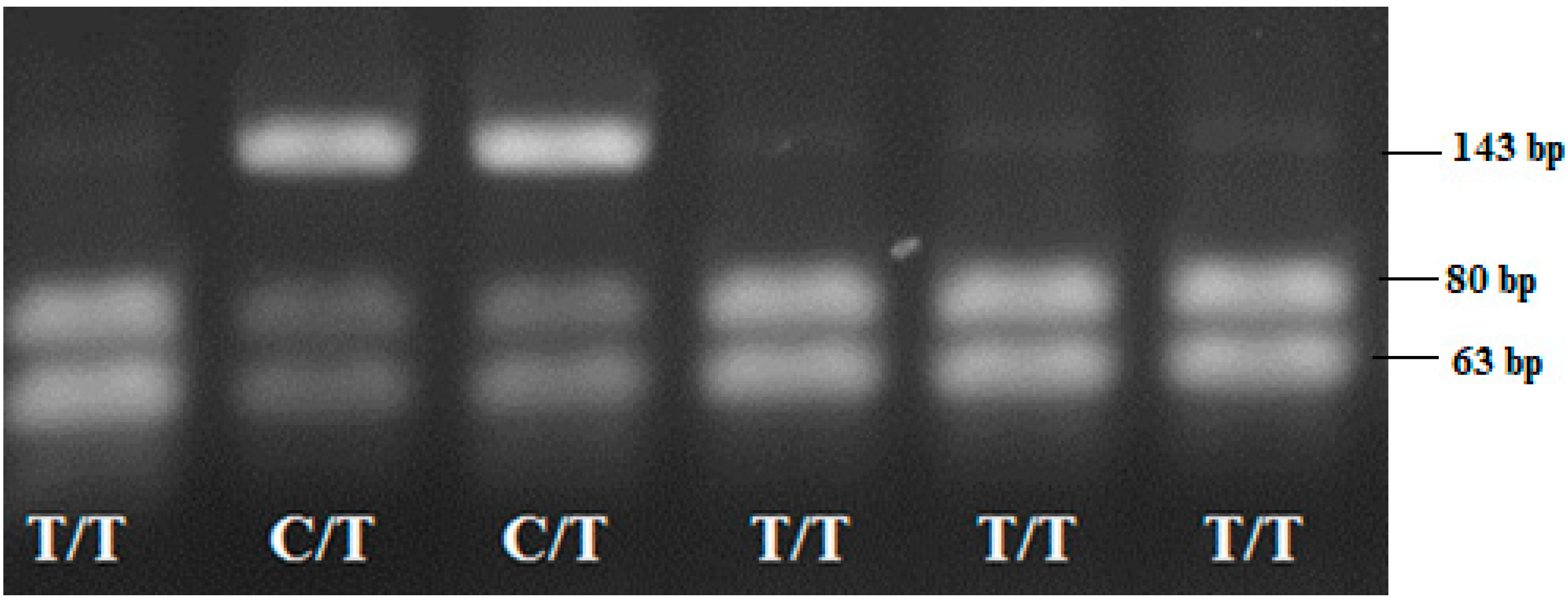
| SNP | PCR Product Size (bp) | Restriction Enzyme | Restriction Product | |
|---|---|---|---|---|
| Genotype | Restriction Products Size (bp) | |||
| IL17A-197 G/A | 102 | XagI (EcoNI) | AA | 102 |
| AG | 102, 68, 34 | |||
| GG | 68, 34 | |||
| IL17F 7488 T/C | 143 | HinlII (NlaIII) | CC | 143 |
| CT | 143, 80, 63 | |||
| TT | 80, 63 | |||
| Parameter | Number (%) |
|---|---|
| Patients | n = 136 |
| Gender | n = 136 |
| Male | 67 (49.3) |
| Female | 69 (50.7) |
| Age at diagnosis (years) | n = 136 |
| Range | 44–88 |
| Mean (±SD) | 67.14 (±8.41) |
| Localization of primary tumor | n = 136 |
| Colon | 50 (36.8) |
| Rectum | 65 (47.8) |
| Sigma | 21 (15.4) |
| Local tumor invasion groups | n = 136 |
| T 1-2 (early CRC) | 37 (27.2) |
| T 3-4 (advanced CRC) | 99 (72.8) |
| Grade of differentiation | n = 136 |
| low | 26 (19.1) |
| moderate | 102 (75.0) |
| high | 8 (5.9) |
| N staging | n = 136 |
| N1-2 | 17 (12.5) |
| N0 | 119 (87.5) |
| M staging | n = 73 |
| M1 | 18 (24.7) |
| M0 | 55 (75.3) |
| Survival status (total) | n = 73 |
| alive | 28 (38.4) |
| deceased | 45 (61.6) |
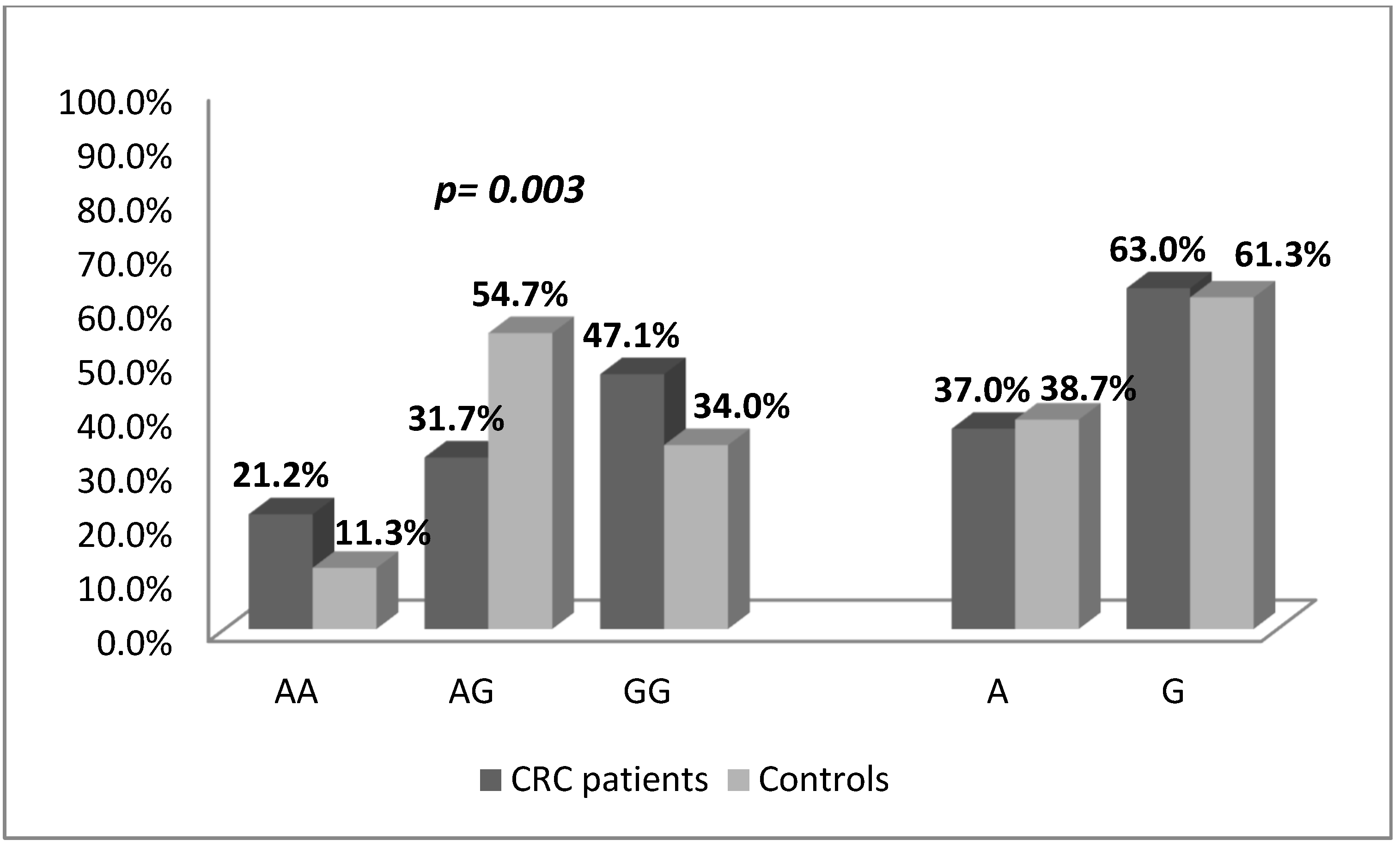
| IL17A-197G/A SNP | CRC patients | Controls | OR (95% CI), p-Value | ||
|---|---|---|---|---|---|
| Number | Frequency | Number | Frequency | ||
| n = 104 | n = 106 | ||||
| Genotype | |||||
| AA | 22 | 0.21 | 12 | 0.11 | 1.347 (0.591–3.072), p = 0.539 |
| AG | 33 | 0.32 | 58 | 0.55 | 0.418 (0.228–0.767), p = 0.006 |
| GG | 49 | 0.47 | 36 | 0.34 | Ref. (1.0) |
| AA + AG vs GG (dominant model) | 55 | 0.53 | 70 | 0.66 | 0.577 (0.331–1.007), p = 0.057 |
| AG + GG vs AA (recessive model) | 82 | 0.78 | 94 | 0.88 | 0.476 (0.222–1.021), p = 0.062 |
| Allele | |||||
| A | 77 | 0.37 | 82 | 0.39 | 0.932 (0.628–1.383), p = 0.763 |
| G | 131 | 0.63 | 130 | 0.61 | Ref. (1.0) |
| Genotype IL17A-197G/A | Controls n = 106 | Colon n = 39 | OR (95% CI) | p | Rectum n = 51 | OR (95% CI) | p | Sigma n = 14 | OR (95% CI) | p |
|---|---|---|---|---|---|---|---|---|---|---|
| GG | 36 | 19 | 1.000 (reference) | - | 25 | 1.000 (reference) | - | 5 | 1.000 (reference) | - |
| GA | 58 | 13 | 0.425 (0.187–0.963) | 0.042 | 17 | 0.422 (0.201–0.888) | 0.026 | 3 | 0.372 (0.084–1.653) | 0.262 |
| AA | 12 | 7 | 1.105 (0.373–3.272) | 1.000 | 9 | 1.080 (0.396–2.946) | 1.000 | 6 | 3.600 (0.929–13.953) | 0.074 |
| Dominant (GG vs. GA + AA) | 70 | 20 | 0.541 (0.257–1.141) | 0.124 | 26 | 0.535 (0.271–1.056) | 0.082 | 9 | 0.926 (0.289–2.967) | 1.000 |
| Recessive (AA vs. GA + GG) | 94 | 32 | 0.584 (0.212–1.610) | 0.404 | 42 | 0.596 (0.233–1.522) | 0.319 | 8 | 0.170 (0.050–0.575) | 0.007 |
| IL-17F 7488T/C | CRC Patients | Controls | OR (95% CI), p-Value | ||
|---|---|---|---|---|---|
| Number | Frequency | Number | Frequency | ||
| n = 113 | n = 116 | ||||
| Genotype | |||||
| TT | 107 | 0.95 | 110 | 0.95 | 1.0 (referent) |
| TC | 6 | 0.05 | 6 | 0.05 | 1.028 (0.283–3.738), p = 0.963 |
| CC | 0 | 0 | 0 | 0 | Na/N |
| Allele | |||||
| T | 220 | 0.97 | 214 | 0.97 | 1.0 (referent) |
| C | 6 | 0.06 | 6 | 0.03 | 0.973 (0.273–3.464), p = 0.962 |
| CRC Stage | p (χ2-Test) | N Stage | p (χ2-Test) | M Stage | p (χ2-Test) | ||||
|---|---|---|---|---|---|---|---|---|---|
| IL17F 7488 T/C | T 1-2 | T 3-4 | 0.353 | N1-2 | N0 | 0.278 | M1 | M0 | 0.589 |
| TT | 96.7% | 92.6% | 95.1% | 88.2% | 94.3% | 88.2% | |||
| CT | 3.3% | 7.4% | 4.9% | 11.8% | 5.7% | 11.8% | |||
| IL-17A/IL-17F | CRC Patients | Controls | OR (95% CI), p-Value | ||
|---|---|---|---|---|---|
| n | Frequency | n | Frequency | ||
| n = 81 | n = 95 | ||||
| Haplotypes | |||||
| G/T | 48 | 0.593 | 57 | 0.597 | 1.0 (referent) |
| A/T | 31 | 0.380 | 35 | 0.363 | 1.052 (0.541–2.043), p = 0.876 |
| A/C | 1 | 0.008 | 1 | 0.016 | 1.188 (0.031–44.855), p = 0.904 |
| G/C | 1 | 0.019 | 2 | 0.024 | 0.594 (0.021–8.737), p = 0.671 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, E.; Vlaykova, T.; Ananiev, J.; Gulubova, M. Protective Role of IL7A-197 A/G Heterozygosity in the Development and Severity of Colorectal Cancer in the Bulgarian Population. Medicina 2022, 58, 1632. https://doi.org/10.3390/medicina58111632
Aleksandrova E, Vlaykova T, Ananiev J, Gulubova M. Protective Role of IL7A-197 A/G Heterozygosity in the Development and Severity of Colorectal Cancer in the Bulgarian Population. Medicina. 2022; 58(11):1632. https://doi.org/10.3390/medicina58111632
Chicago/Turabian StyleAleksandrova, Elina, Tatyana Vlaykova, Julian Ananiev, and Maya Gulubova. 2022. "Protective Role of IL7A-197 A/G Heterozygosity in the Development and Severity of Colorectal Cancer in the Bulgarian Population" Medicina 58, no. 11: 1632. https://doi.org/10.3390/medicina58111632
APA StyleAleksandrova, E., Vlaykova, T., Ananiev, J., & Gulubova, M. (2022). Protective Role of IL7A-197 A/G Heterozygosity in the Development and Severity of Colorectal Cancer in the Bulgarian Population. Medicina, 58(11), 1632. https://doi.org/10.3390/medicina58111632







