Three Weeks of Pulmonary Rehabilitation Do Not Influence Oscillometry Parameters in Postoperative Lung Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physiological Measurements
2.3. Pulmonary Rehabilitation
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2013; American Cancer Society: Atlanta, GA, USA, 2013; Available online: https://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf (accessed on 11 November 2013).
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of Stage I and II Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed.; American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143 (Suppl. 5), e278S–e313S. [Google Scholar] [CrossRef]
- Villeneuve, P.J. Interventions to avoid pulmonary complications after lung cancer resection. J. Thorac. Dis. 2018, 10 (Suppl. 32), S3781–S3788. [Google Scholar] [CrossRef] [PubMed]
- Novoa, N.; Ballesteros, E.; Aranda, J.L.; Jiménez, M.F.; Varela, G. Chest physiotherapy revisited: Evaluation of its influence on the pulmonary morbidity after pulmonary resection. Eur. J. Cardio-Thorac. Surg. 2011, 40, 130–134. [Google Scholar] [CrossRef]
- Mujovic, N.; Mujovic, N.; Subotic, D.; Ercegovac, M.; Milovanovic, A.; Nikcevic, L.; Zugic, V.; Nikolic, D. Influence of Pulmonary Rehabilitation on Lung Function Changes After the Lung Resection for Primary Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. Aging Dis. 2015, 6, 466–477. [Google Scholar] [CrossRef]
- Weiner, P.; Man, A.; Weiner, M.; Rabner, M.; Waizman, J.; Magadle, R.; Zamir, D.; Greiff, Y. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. J. Thorac. Cardiovasc. Surg. 1997, 113, 552–557. [Google Scholar] [CrossRef]
- Sano, Y. Perioperative Pulmonary Rehabilitation for Lung Cancer Surgeries in Patients with Poor Pulmonary Function. Kyobu Geka 2016, 69, 41–46. [Google Scholar]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Bolton, C.E.; Bevan-Smith, E.F.; Blakey, J.D.; Crowe, P.; Elkin, S.L.; Garrod, R.; Greening, N.J.; Heslop, K.; Hull, J.H.; Man, W.D.; et al. British Thoracic Society Pulmonary Rehabilitation Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013, 68 (Suppl. 2), ii1–ii30. [Google Scholar] [CrossRef] [PubMed]
- Tomalak, W.; Radliński, J.; Latawiec, W. Quality of spirometric measurements in children younger than 10 years of age in the light of the recommendations, Pneumonol. Alergol. Pol. 2008, 6, 421–425. [Google Scholar]
- Czajkowska-Malinowska, M.; Tomalak, W.; Radliński, J. Quality of spirometry in elderly. Pneumonol. Alergol. Pol. 2013, 81, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Tomalak, W.; Radliński, J.; Czajkowska-Malinowska, M. Impulse oscillometry in assessing small airways properties. Electr. Rev. 2014, 90, 157–159. [Google Scholar]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farre, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef]
- Kaczka, D.W.; Dellacá, R.L. Oscillation mechanics of the respiratory system: Applications to lung disease. Crit. Rev. Biomed. Eng. 2011, 39, 337–359. [Google Scholar] [CrossRef]
- Mori, K.; Shirai, T.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Fujii, M.; Suda, T.; Chida, K. Colored 3-dimenssional analyses of respiratory resistance and reactance in COPD and asthma. COPD 2011, 8, 456–463. [Google Scholar] [CrossRef]
- Kanda, S.; Fujimoto, K.; Komatsu, Y.; Yasuo, M.; Hanaoka, M.; Kubo, K. Evaluation of Respiratory Impedance in Asthma and COPD by an Impulse Oscillation System. Intern. Med. 2010, 49, 23–30. [Google Scholar] [CrossRef]
- Dandurand, R.; Dandurand, M.; Estepar, R.; Bourbeau, J.; Eidelman, D. Oscillometry in community practice ild is characterized by abnormal reactance but normal resistance. Quart. J. Med. 2016, 109, S50. [Google Scholar]
- Fujii, M.; Shirai, T.; Mori, K.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Suda, T. Inspiratory resonant frequency of forced oscillation technique as a predictor of the composite physiologic index in interstitial lung disease. Respir. Physiol. Neurobiol. 2015, 207, 22–27. [Google Scholar] [CrossRef]
- Mori, K.; Shirai, T.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Fujii, M.; Hozumi, H.; Suda, T.; et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respir. Physiol. Neurobiol. 2013, 185, 235–240. [Google Scholar] [CrossRef]
- Kaku, R.; Yoden, M.; Shiratori, T.; Hayashi, K.; Okamoto, K.; Oshio, Y.; Nakano, Y.; Hanaoka, J. Perioperative changes in respiratory impedance in lobectomy and their clinical impact. J. Thorac. Dis. 2021, 13, 1347–1357. [Google Scholar] [CrossRef]
- Kostorz-Nosal, S.; Jastrzębski, D.; Ziora, D. Forced oscillation measurements in patients after lobectomy—A comparative analysis with IPF and COPD patients. Clin. Respir. J. 2021, 15, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2021 Report). Available online: https://goldcopd.org/ (accessed on 22 November 2020).
- Roman-Rodriguez, M.; Kaplan, A. GOLD 2021 Strategy Report: Implications for Asthma-COPD Overlap. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 1709–1715. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–656. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Sallinen, J.; Stenholm, S.; Rantanen, T.; Heliövaara, M.; Sainio, P.; Koskinen, S. Hand-Grip Strength Cut Points to Screen Older Persons at Risk for Mobility Limitation. J. Am. Geriatr. Soc. 2010, 58, 1721–1726. [Google Scholar] [CrossRef]
- Kostorz-Nosal, S.; Jastrzębski, D.; Kubicki, P.; Galle, D.; Gałeczka-Turkiewicz, A.; Toczylowska, B.; Ziora, D. Forced Oscillation Measurements in Patients with Idiopathic Interstitial Pneumonia Subjected to Pulmonary Rehabilitation. J. Clin. Med. 2022, 11, 3657. [Google Scholar] [CrossRef]
- Gloeckl, R.; Marinov, B.; Pitta, F. Practical recommendations for exercise training in patients with COPD. Eur. Respir. Rev. 2013, 22, 178–186. [Google Scholar] [CrossRef]
- Kimihiro, N.; Gel, Y.R.; Brunner, E.; Konietschke, F. NparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaré, R.; Dekhuijzen, P.N.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef] [PubMed]
- Illini, O.; Valipour, A.; Gattinger, D.; Petrovic, M.; Fabikan, H.; Hochmair, M.J.; Zwick, R.H. Effectiveness of Outpatient Pulmonary Rehabilitation in Patients with Surgically Resected Lung Cancer: A Retrospective Real-World Analysis. Cancers 2022, 14, 3479. [Google Scholar] [CrossRef]
- Franssen, F.M.; Broekhuizen, R.; Janssen, P.P.; Wouters, E.F.; Schols, A.M. Effects of Whole-Body Exercise Training on Body Composition and Functional Capacity in Normal-Weight Patients with COPD. Chest 2004, 125, 2021–2028. [Google Scholar] [CrossRef]
- Spruit, M.; Gosselink, R.; Troosters, T.; De Paepe, K.; Decramer, M. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur. Respir. J. 2002, 19, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Danoff, S.K.; Schonhoft, E.H. Role of support measures and palliative care. Curr. Opin. Pulm. Med. 2013, 19, 480–484. [Google Scholar] [CrossRef]
- Bobbio, A.; Chetta, A.; Ampollini, L.; Primomo, G.L.; Internullo, E.; Carbognani, P.; Rusca, M.; Olivieri, D. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2008, 33, 95–98. [Google Scholar] [CrossRef]
- Jones, L.W.; Peddle, C.J.; Eves, N.D.; Haykowsky, M.J.; Courneya, K.S.; Mackey, J.R.; Joy, A.A.; Kumar, V.; Winton, T.W.; Reiman, T. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer 2007, 110, 590–598. [Google Scholar] [CrossRef]
- Man, W.D.-C.; Kemp, P.; Moxham, J.; Polkey, M.I. Skeletal muscle dysfunction in COPD: Clinical and laboratory observations. Clin. Sci. 2009, 117, 251–264. [Google Scholar] [CrossRef]
- Geddes, E.L.; O’Brien, K.; Reid, W.D.; Brooks, D.; Crowe, J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: An update of a systematic review. Respir. Med. 2008, 102, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.; De Vos, J.; van den Heuvel, S.P.; Segers, J.; Decramer, M.; Kwakkel, G. Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur. Respir. J. 2011, 37, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Brocki, B.C.; Andreasen, J.J.; Langer, D.; Souza, D.S.R.; Westerdahl, E. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: A randomized controlled trial. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1483–1491. [Google Scholar] [CrossRef]
- Hillegass, E. Breathing retraining for individuals with chronic obstructive pulmonary disease—A role for clinicians. Chronic Respir. Dis. 2009, 6, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.P.; Varela, G.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; Clini, E.M.; et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef]
- Makhabah, D.; Martino, F.; Ambrosino, N. Peri-operative physiotherapy. Multidisc. Respir. Med. 2013, 8, 4. [Google Scholar] [CrossRef]
- Rodriguez-Larrad, A.; Aguirrebeña, I.L.; Inchaurregui, L.C.A.; Seco, J. Perioperative physiotherapy in patients undergoing lung cancer resection. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 269–281. [Google Scholar] [CrossRef]
- Liu, W.; Pan, Y.-L.; Gao, C.-X.; Shang, Z.; Ning, L.-J.; Liu, X. Breathing exercises improve post-operative pulmonary function and quality of life in patients with lung cancer: A meta-analysis. Exp. Ther. Med. 2013, 5, 1194–1200. [Google Scholar] [CrossRef]
- Divisi, D.; Di Francesco, C.; Di Leonardo, G.; Crisci, R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur. J. Cardio-Thorac. Surg. 2013, 43, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, Y.; Martin, S.; Lasserson, T.J.; Goldstein, R.S. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Cochrane Syst. Rev. Eur. Med. 2007, 43, 475–485. [Google Scholar] [CrossRef]
- Ries, A.L.; Bauldoff, G.S.; Carlin, B.W.; Casaburi, R.; Emery, C.F.; Mahler, D.A.; Make, B.; Rochester, C.L.; Zuwallack, R.; Herrerias, C. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007, 131 (Suppl. 5), 4S–42S. [Google Scholar] [CrossRef]
- Eliason, G.; Abdel-Halim, S.; Arvidsson, B.; Kadi, F.; Piehl-Aulin, K. Physical performance and muscular characteristics in different stages of COPD. Scand. J. Med. Sci. Sports 2009, 19, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Puente-Maestu, L.; Garcia de Pedro, J.; Martinez-Abad, Y.; Ruíz de Oña, J.M.; Llorente, D.; Cubillo, J.M. Dyspnea, ventilatory pattern, and changes in dynamic hyperinflation related to the intensity of constant work rate exercise in OPD. Chest 2005, 128, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.C.; Thamrin, C.; Chan, A.S.; Bertolin, A.; Chapman, D.G.; King, G.G. Relationships Between Forced Oscillatory Impedance and 6-min Walk Distance After Pulmonary Rehabilitation in COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2020, 15, 157–166. [Google Scholar] [CrossRef]
- Brashier, B.; Salvi, S. Measuring lung function using sound waves: Role of the forced oscillation technique and impulse oscillometry system. Breathe 2015, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Rock, P.; Rich, P.B. Postoperative pulmonary complications. Curr. Opin. Anaesthesiol. 2003, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Serejo, L.G.; da Silva-Junior, F.P.; Bastos, J.P.; de Bruin, G.S.; Mota, R.M.; de Bruin, P.F. Risk factors for pulmonary complications after emergency abdominal surgery. Respir. Med. 2007, 101, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, V.A.; Cornell, J.E.; Smetana, G.W. Strategies To Reduce Postoperative Pulmonary Complications after Noncardiothoracic Surgery: Systematic Review for the American College of Physicians. Ann. Intern. Med. 2006, 144, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Snow, V.; Fitterman, N.; Hornbake, R.; Lawrence, V.A.; Smetana, G.W.; Weiss, K.; Owens, D.K.; Aronson, M.; Barry, P. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: A guideline from the American college of physicians. Ann. Intern. Med. 2006, 144, 575–580. [Google Scholar] [CrossRef] [PubMed]
- FOT GUIDE. Resmon Pro Full Device; Restech: Milan, Italy, 2018; p. 24. [Google Scholar]
- Matsumoto, R.; Takamori, S.; Yokoyama, S.; Hashiguchi, T.; Murakami, D.; Yoshiyama, K.; Nishi, T.; Kashihara, M.; Mitsuoka, M.; Hayashida, R.; et al. Lung function in the late postoperative phase and influencing factors in patients undergoing pulmonary lobectomy. J. Thorac. Dis. 2018, 10, 2916–2923. [Google Scholar] [CrossRef]
- Cukic, V. Reduction of Pulmonary Function After Surgical Lung Resections of Different Volume. Med. Arch. 2014, 68, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qin, Y.; Ma, D.; Liu, H. The impact of segmentectomy versus lobectomy on pulmonary function in patients with non-small-cell lung cancer: A meta-analysis. J. Cardiothorac. Surg. 2022, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Drakou, E.; Meletios, A.K.; Papadimitriou, L.; Lacovidou, N.; Vrachnis, N.; Nicolouzos, S.; Loukas, C.; Lioulias, A. Changes in Simple Spirometric Parameters After Lobectomy for Bronchial Carcinoma. J. Cardiovasc. Thorac. Res. 2015, 7, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Nomori, H.; Shiraishi, A.; Cong, Y.; Sugimura, H.; Mishima, S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur. J. Cardio-Thorac. Surg. 2018, 53, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Nomori, H.; Mori, T.; Ohba, Y.; Shiraishi, K.; Tashiro, K.; Shiraishi, S. Postoperative change in pulmonary function of the ipsilateral preserved lung after segmentectomy versus lobectomy. Eur. J. Cardio-Thorac. Surg. 2010, 37, 36–39. [Google Scholar] [CrossRef]
- Santus, P.; Franceschi, E.; Radovanovic, D. Sublobar resection: Functional evaluation and pathophysiological considerations. J. Thorac. Dis. 2020, 12, 3363–3368. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kikuchi, S.; Goto, Y.; Ichimura, H.; Endo, K.; Sato, Y. Long-term pulmonary function after surgery for lung cancer. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 727–732. [Google Scholar] [CrossRef]
- Nakano, S.; Nakahira, J.; Sawai, T.; Kuzukawa, Y.; Ishio, J.; Minami, T. Perioperative evaluation of respiratory impedance using the forced oscillation technique: A prospective observational study. BMC Anesthesiol. 2016, 16, 32. [Google Scholar] [CrossRef]
- Subotic, D.; Mandaric, D.; Radosavljevic, G.; Stojsic, J.; Gajic, M. Lung function changes and complications after lobectomy for lung cancer in septuagenarians. Ann. Thorac. Med. 2009, 4, 54–59. [Google Scholar] [CrossRef]
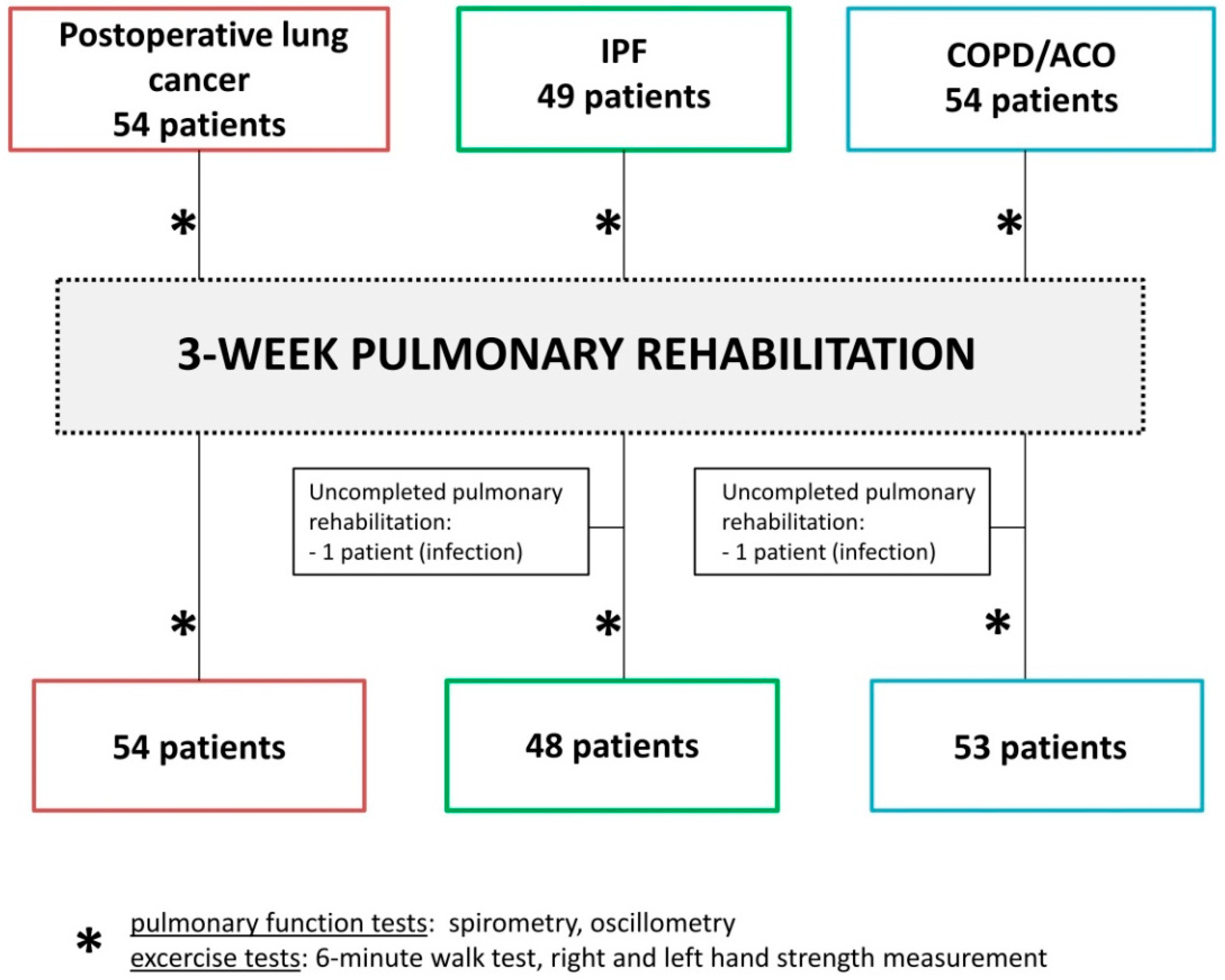
| Postoperative Lung Cancer (54) | IPF (48) | COPD/ACO (53) | p | |
|---|---|---|---|---|
| Age (years) | 67.4 ± 8.8 | 66.3 ± 6.1 | 65.9 ± 9.9 | 0.69 |
| Sex (M/F) | 23/31 | 34/14 | 32/21 | 0.014 |
| BMI (kg/m2) | 28.6 ± 4.5 | 27.2 ± 4.2 | 28.1 ± 6.0 | 0.308 |
| Characteristics of the patient group | Histopathological diagnosis
percentage of the lung tissue removal)
Adjuvant therapy: 23 pts | Antifibrotic treatment (77%): Nintedanib: 9 pts Pirfenidone: 28 pts GAP Index: Stage I: 31 pts (65%) Stage II: 15 pts (31%) Stage III: 2 pts (4%) | COPD: 41 pts (77%) ACO: 12 pts (23%) | - |
| Comorbidities | 0.908 | |||
| Cardiovascular diseases | 42 (78%) | 38 (79%) | 39 (74%) | |
| Diabetes mellitus | 13 (24%) | 8 (17%) | 14 (26%) | |
| Musculoskeletal disorders | 22 (41%) | 19 (40%) | 31 (58%) | |
| Gastrointestinal disorders | 13 (24%) | 13 (27%) | 11 (21%) | |
| Urogenital disorders | 20 (37%) | 16 (33%) | 16 (30%) | |
| Psychiatric disorders | 3 (6%) | 3 (6%) | 2 (4%) | |
| Smoking history | 0.119 | |||
| Current | 4 (7%) | - | 6 (11%) | |
| Previous | 36 (67%) | 30 (63%) | 35 (66%) | |
| Never | 14 (26%) | 18 (38%) | 12 (23%) |
| Parameter | Postoperative Lung Cancer (54) | IPF (48) | COPD/ACO (53) | npar LD Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After PR | Baseline | After PR | Baseline | After PR | Group | Time | Group: Time | |
| FEV1 (%pred.) | 76.31 ± 21.5 | 79.03 ± 19.8 | 82.98 ± 16.9 | 84.06 ± 17.9 | 57.55 ± 23.5 | 57.5 ± 22.7 | <0.001 1,3 | 0.409 | 0.313 |
| FVC (%pred.) | 92.33 ± 21.0 | 94.2 ± 21.7 | 80.78 ± 18.1 | 81.69 ± 18.9 | 80.62 ± 21.7 | 80.59 ± 21.2 | 0.003 1,2 | 0.218 | 0.392 |
| FEV1/FVC (%) | 67.84 ± 8.8 | 67.81 ± 9.9 | 81.73 ± 6.9 | 81.92 ± 6.5 | 55.76 ± 14.4 | 56.12 ± 13.8 | <0.001 4 | 0.643 | 0.781 |
| SpO2 (%) | 95.88 ± 1.3 | 96.38 ± 1.7 | 94.77 ± 1.9 | 95.0 ± 2.4 | 94.68 ± 1.8 | 94.79 ± 1.5 | <0.001 1,2 | 0.123 | 0.592 |
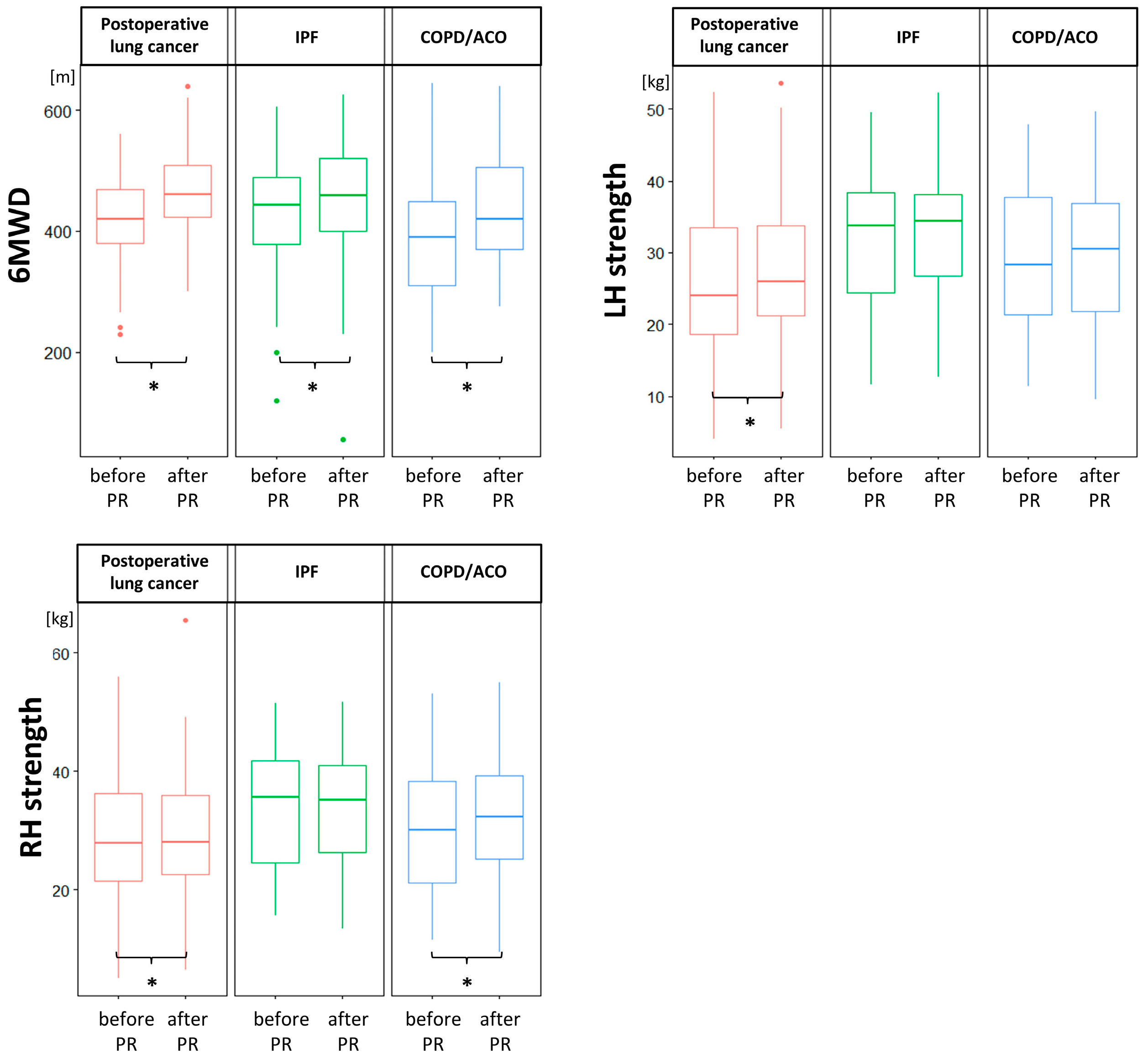
| 6MWD (m) | 415.7 ± 77.1 | 469.3 ± 71.5 | 426.6 ± 106.3 | 456.6 ± 105.5 | 382.3 ± 105.5 | 431.8 ± 91.7 | 0.032 1,3 | <0.001 *** | 0.072 |
| SpO2 after 6MWT (%) | 92.53 ± 3.1 | 92.26 ± 3.4 | 84.83 ± 8.9 | 86.82 ± 7.5 | 89.76 ± 6.2 | 90.39 ± 4.8 | <0.001 4 | 0.491 | 0.263 |
| RH strength (kg) | 25.76 ± 10.6 | 27.19 ± 10.3 | 32.27 ± 9.8 | 32.54 ± 8.9 | 28.63 ± 10.4 | 29.57 ± 10.0 | 0.039 2 | 0.002 *,** | 0.007 **** |
| LH strength (kg) | 28.37 ± 10.7 | 29.52 ± 11.2 | 34.19 ± 9.9 | 33.85 ± 9.3 | 30.33 ± 11.4 | 32.34 ± 10.5 | 0.007 2 | 0.012 * | 0.74 |
| Parameter | Postoperative Lung Cancer (54) | IPF (48) | COPD/ACO (53) | npar LD Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After PR | Baseline | After PR | Baseline | After PR | Group | Time | Group:Time | |
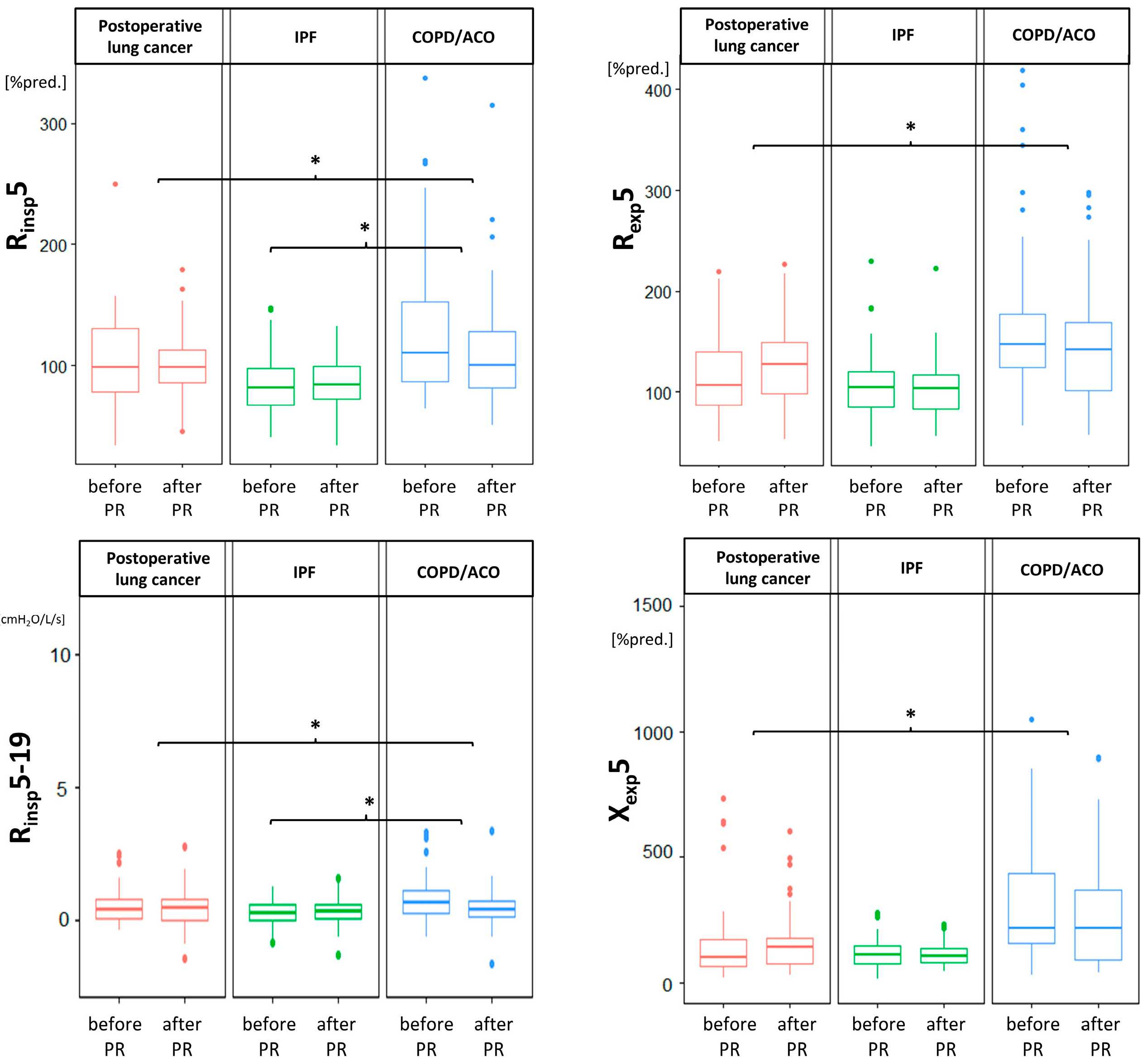
| Rinsp5 (%pred.) | 104.97 ± 37.9 | 101.3 ± 28.7 | 85.79 ± 26.5 | 85.66 ± 22.6 | 129.08 ± 60.8 | 109.36 ± 46.9 | <0.001 2,3 | 0.09 | 0.029 1,3 |
| Rexp5 (%pred.) | 119.69 ± 44.5 | 127.99 ± 39.9 | 105.08 ± 34.8 | 103.38 ± 31.4 | 167.95 ± 80.1 | 148.89 ± 59.6 | <0.001 4 | 0.85 | 0.005 1 |
| Rinsp11 (%pred.) | 108.65 ± 34.1 | 108.49 ± 24.8 | 92.91 ± 22.3 | 91.04 ± 17.6 | 129.07 ± 54.9 | 116.01 ± 43.0 | <0.001 2,3 | 0.408 | 0.11 |
| Rexp11 (%pred.) | 122.34 ± 36.3 | 130.93 ± 34.7 | 113.76 ± 31.6 | 115.36 ± 28.2 | 149.97 ± 61.5 | 139.43 ± 47.3 | <0.001 4 | 0.224 | 0.068 |
| Rinsp19 (%pred.) | 95.99 ± 28.8 | 95.88 ± 21.0 | 81.94 ± 18.0 | 79.92 ± 14.6 | 109.48 ± 44.8 | 99.83 ± 31.5 | <0.001 2,3 | 0.393 | 0.206 |
| Rexp19 (%pred.) | 100.98 ± 28.5 | 108.68 ± 28.9 | 95.5 ± 24.5 | 96.43 ± 22.0 | 118.46 ± 47.0 | 111.52 ± 33.4 | 0.005 2,3 | 0.156 | 0.133 |
| Rinsp5-19 (cmH2O/L/s) | 0.51 ± 0.6 | 0.36 ± 0.7 | 0.25 ± 0.5 | 0.28 ± 0.6 | 0.77 ± 0.8 | 0.82 ± 3.2 | 0.021 1,3 | 0.145 | 0.017 1,3 |
| Rexp5-19 (cmH2O/L/s) | 0.89 ± 1.0 | 0.8 ± 0.9 | 0.47 ± 0.6 | 0.3 ± 0.5 | 1.69 ± 1.1 | 1.2 ± 1.0 | <0.001 4 | 0.092 | 0.074 |
| Xinsp5 (%pred.) | 82.89 ± 41.8 | 87.37 ± 40.4 | 103.29 ± 54.2 | 110.33 ± 45.9 | 108.85 ± 58.5 | 106.89 ± 87.1 | 0.039 1,2 | 0.414 | 0.089 |
| Xexp5 (%pred.) | 147.54 ± 154.4 | 161.86 ± 122.8 | 116.4 ± 55.6 | 113.29 ± 49.0 | 318.26 ± 318.8 | 293.25 ± 294.8 | <0.001 1,3 | 0.656 | 0.048 1 |
| Xinsp11 (cmH2O/L/s) | −0.37 ± 0.5 | −0.45 ± 0.6 | −0.37 ± 0.4 | −0.41 ± 0.5 | −0.68 ± 0.7 | −0.59 ± 0.6 | 0.029 1,3 | 0.415 | 0.205 |
| Xexp11 (cmH2O/L/s) | −1.19 ± 1.5 | −1.4 ± 1.3 | −0.65 ± 0.5 | −0.76 ± 0.7 | −2.28 ± 1.5 | −2.13 ± 1.5 | <0.001 4 | 0.117 | 0.025 * |
| Xinsp19 (cmH2O/L/s) | 0.3 ± 0.5 | 0.27 ± 0.6 | −0.26 ± 4.5 | 0.33 ± 0.5 | 0.02 ± 0.7 | 0.04 ± 0.6 | 0.002 1,3 | 0.617 | 0.999 |
| Xexp19 (cmH2O/L/s) | −0.38 ± 1.0 | −0.55 ± 0.9 | 0.02 ± 0.5 | −0.03 ± 0.5 | −1.07 ± 0.9 | −1.01 ± 0.9 | <0.001 4 | 0.151 | 0.057 |
| Fres (Hz) | 15.84 ± 5.0 | 16.31 ± 5.6 | 15.2 ± 3.2 | 15.24 ± 3.6 | 19.08 ± 6.9 | 18.78 ± 6.3 | 0.003 1,3 | 0.53 | 0.838 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostorz-Nosal, S.; Jastrzębski, D.; Żebrowska, A.; Bartoszewicz, A.; Ziora, D. Three Weeks of Pulmonary Rehabilitation Do Not Influence Oscillometry Parameters in Postoperative Lung Cancer Patients. Medicina 2022, 58, 1551. https://doi.org/10.3390/medicina58111551
Kostorz-Nosal S, Jastrzębski D, Żebrowska A, Bartoszewicz A, Ziora D. Three Weeks of Pulmonary Rehabilitation Do Not Influence Oscillometry Parameters in Postoperative Lung Cancer Patients. Medicina. 2022; 58(11):1551. https://doi.org/10.3390/medicina58111551
Chicago/Turabian StyleKostorz-Nosal, Sabina, Dariusz Jastrzębski, Aleksandra Żebrowska, Agnieszka Bartoszewicz, and Dariusz Ziora. 2022. "Three Weeks of Pulmonary Rehabilitation Do Not Influence Oscillometry Parameters in Postoperative Lung Cancer Patients" Medicina 58, no. 11: 1551. https://doi.org/10.3390/medicina58111551
APA StyleKostorz-Nosal, S., Jastrzębski, D., Żebrowska, A., Bartoszewicz, A., & Ziora, D. (2022). Three Weeks of Pulmonary Rehabilitation Do Not Influence Oscillometry Parameters in Postoperative Lung Cancer Patients. Medicina, 58(11), 1551. https://doi.org/10.3390/medicina58111551








