Patterns of Locus of Control in People Suffering from Heart Failure: An Approach by Clustering Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Instruments
2.4. Assumptions Related to MHLC Subscales [35]
2.5. Statistical Analysis
2.6. Data Clustering
- K-means based on the Euclidean distance between observations.
- Gaussian Mixture Model (GMM) assumes that a finite number of particular features follow a normal distribution.
- Agglomerative clustering using Ward’s linkage based on a classical sum-of-squares criterion produces clusters that minimize within-group dispersion at each binary fusion.
- 1.
- Selection of features for the grouping procedure.
- 2.
- Visualization of the distribution in 3D space to identify potential clusters using an expert method.
- 3.
- Data scaling based on the min-max method. The goal was to bring each attribute into the 0–1 domain so that, when finding clusters (calculating distances between observations), each variable had the same impact on the calculated distance.
- 4.
- Conversion of 3-dimensional space into a 2-dimensional space using the PCA method and visualization of the observed distribution.
- 5.
- Clustering into segments using three selected algorithms: K-means, Gaussian mixture models (GMM), and Agglomerative Clustering (AC). Each algorithm divides the dataset into 2 to 10 clusters.
- (a)
- Assessment of cluster cohesion using the described indicators.
- i.
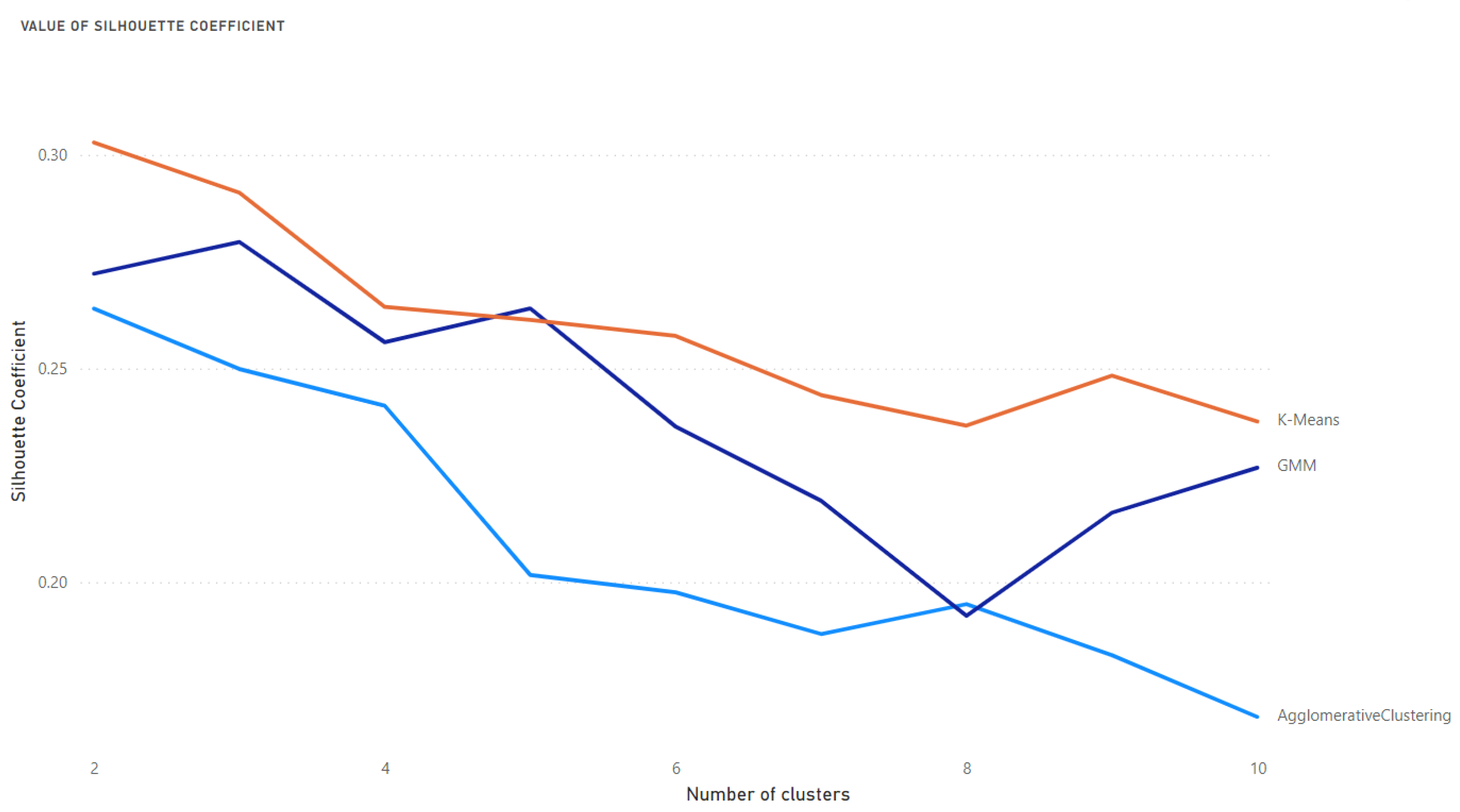
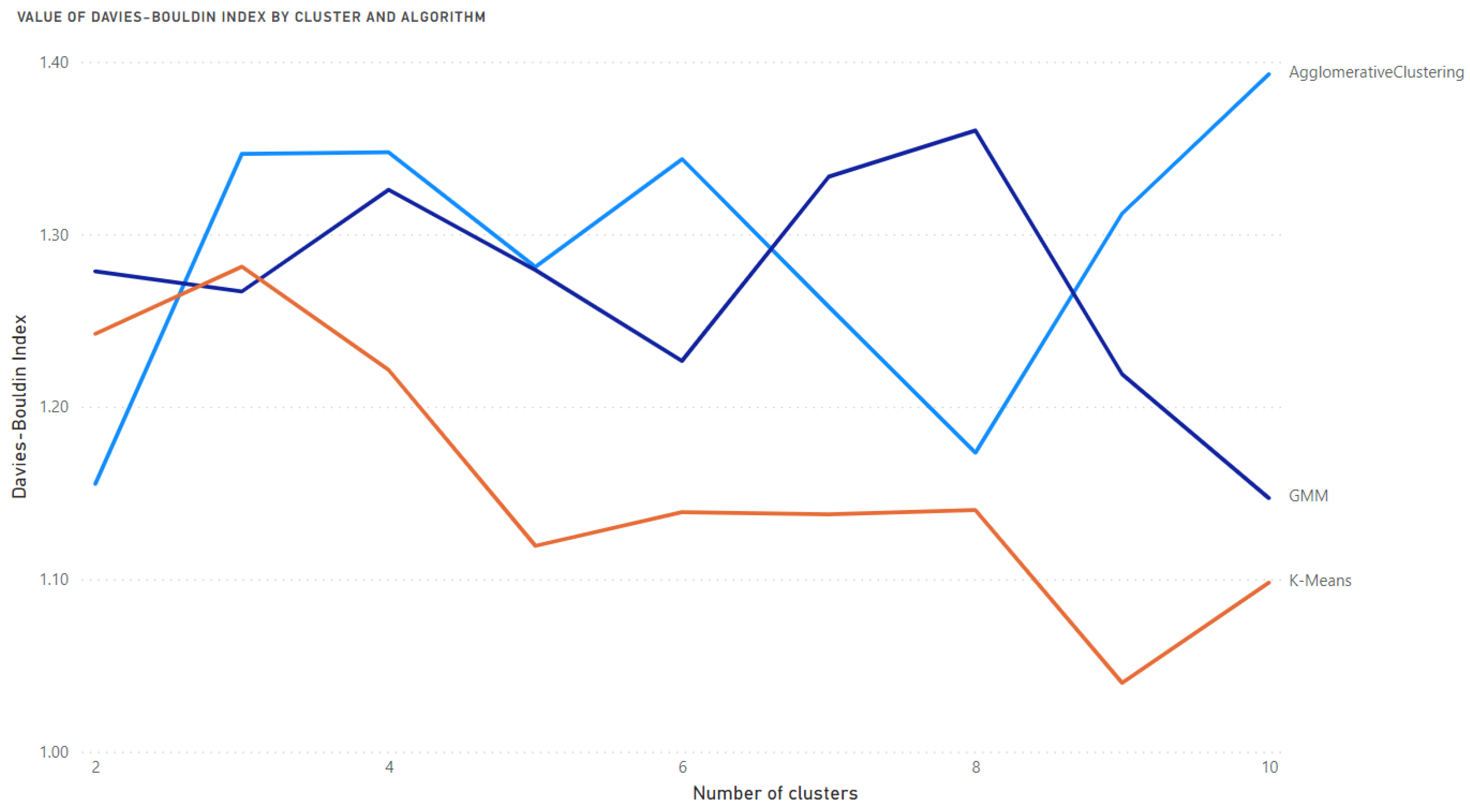
- The Davies-Bouldin Index—the lowest value means the best clustering.
- ii.
- The Silhouette Coefficient—the highest value means the best clustering.
- (b)
- Selection of the optimal number of clusters and best algorithm using the Davies-Bouldin and Silhouette Coefficient indices.
- 6.
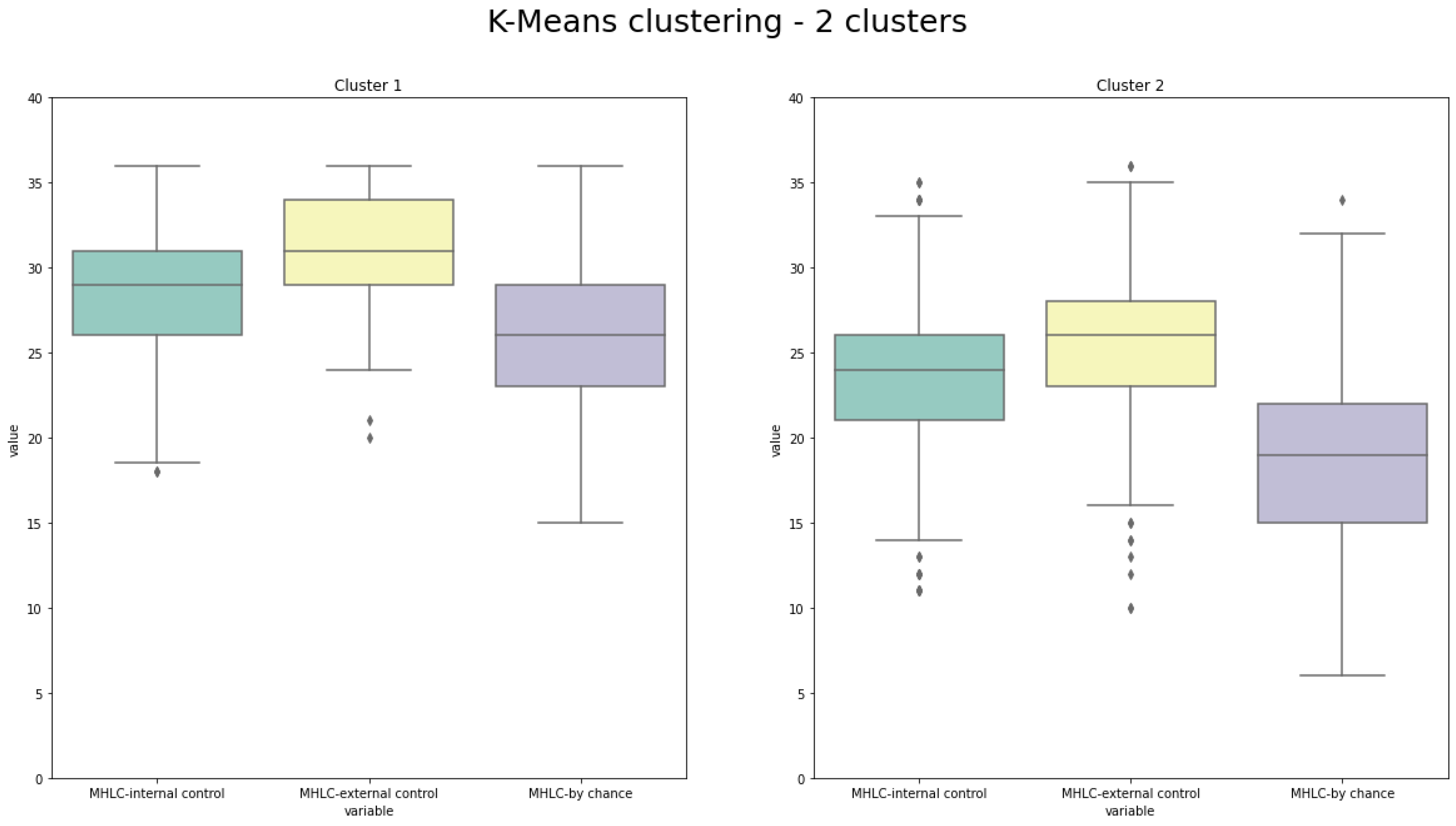
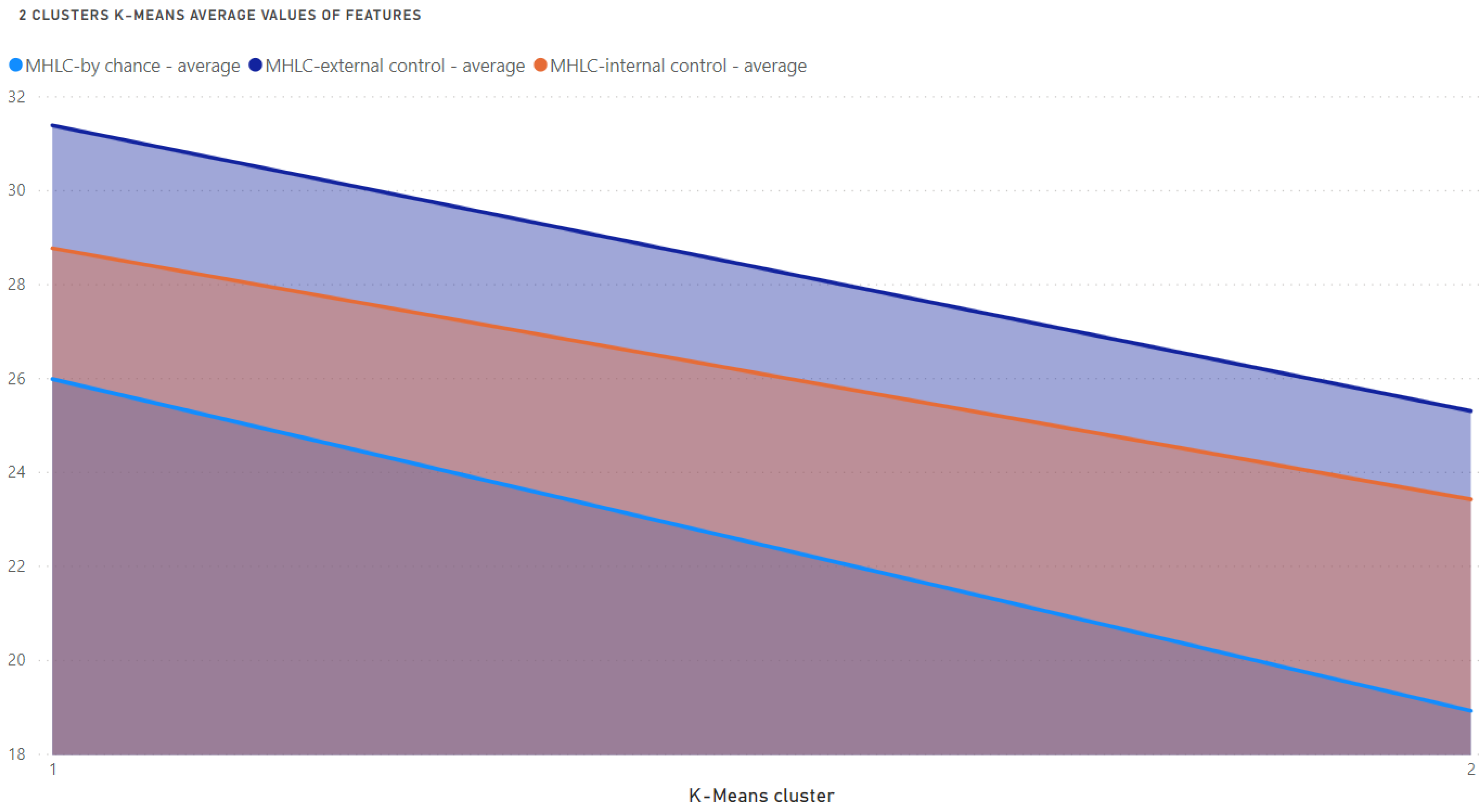
- The best clustering outcomes were evaluated and interpreted using the box plots providing visualization of MHLC measures within the individual cluster and the number of patients in the clusters.
3. Results
Results of Clustering
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Bower, A.B.; Taylor, V.A. Increasing Intention to Comply with Pharmaceutical Product Instructions: An Exploratory Study Investigating the Rolesof Frame and Plain Language. J. Health Commun. 2010, 8, 145–156. [Google Scholar] [CrossRef]
- Barello, S.; Graffigna, G.; Vegni, E.; Savarese, M.; Lombardi, F.; Bosio, A.C. ‘Engage me in taking care of my heart’: A grounded theory study on patient-cardiologist relationship in the hospital management of heart failure. BMJ Open 2015, 5, e005582. [Google Scholar] [CrossRef]
- Farmer, S.A.; Magasi, S.; Block, P.; Whelen, M.J.; Hansen, L.O.; Bonow, R.O.; Schmidt, P.; Shah, A.; Grady, K.L. Patient, Caregiver, and Physician Work in Heart Failure Disease Management. Mayo Clin. Proc. 2016, 91, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, A. The crucial role of patient education in heart failure. Eur. J. Heart Fail. 2005, 7, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Colonna, P.; Sorino, M.; D’Agostino, C.; Bovenzi, F.; De Luca, L.; Arrigo, F. Nonpharmacologic care of heart failure: Counseling, dietary restriction, rehabilitation, treatment of sleep apnea, and ultrafiltration. Am. J. Cardiol. 2003, 91, 41–50. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, X.; Ma, L.-L.; Sun, T.-W.; Bishop, L.; Gardiner, F.W.; Wang, L. A nurse-led structured education program improves self-management skills and reduces hospital readmissions in patients with chronic heart failure: A randomized and controlled trial in China. Rural Remote Health 2019, 19, 5270. [Google Scholar] [CrossRef]
- Salam, A.M.; Sulaiman, K.; Alsheikh-Ali, A.A.; Singh, R.; AlHabib, K.F.; Al-Zakwani, I.; Asaad, N.; Al-Qahtani, A.; Al-Jarallah, M.; AlMahmeed, W.; et al. Precipitating Factors for Hospitalization with Heart Failure: Prevalence and Clinical Impact Observations from the Gulf CARE (Gulf aCute heArt failuRe rEgistry). Med. Princ. Pract. 2020, 29, 270–278. [Google Scholar] [CrossRef]
- Ruppar, T.M.; Cooper, P.S.; Mehr, D.R.; Delgado, J.M.; Dunbar-Jacob, J.M. Medication Adherence Interventions Improve Heart Failure Mortality and Readmission Rates: Systematic Review and Meta-Analysis of Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002606. [Google Scholar] [CrossRef]
- Ambardekar, A.V.; Fonarow, G.C.; Hernandez, A.F.; Pan, W.; Yancy, C.W.; Krantz, M.J. Characteristics and in-hospital outcomes for nonadherent patients with heart failure: Findings from Get With The Guidelines-Heart Failure (GWTG-HF). Am. Heart J. 2009, 158, 644–652. [Google Scholar] [CrossRef]
- Ponikowski, P.; Jankowska, E.A. Pathogenesis and Clinical Presentation of Acute Heart Failure. Rev. Española De Cardiol. (Engl. Ed.) 2015, 68, 331–337. [Google Scholar] [CrossRef]
- Shah, A.; Clayman, M.L.; Glass, S.; Kandula, N.R. Protect Your Heart: A Culture-Specific Multimedia Cardiovascular Health Education Program. J. Health Commun. 2015, 20, 424–430. [Google Scholar] [CrossRef]
- Siennicka, A.; Stromberg, A.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Psychological aspects of heart failure–beyond depression, anxiety and quality of life. Health Psychol. Rep. 2015, 2, 99–114. [Google Scholar] [CrossRef]
- Jaarsma, T.; Cameron, J.; Riegel, B.; Stromberg, A. Factors Related to Self-Care in Heart Failure Patients According to the Middle-Range Theory of Self-Care of Chronic Illness: A Literature Update. Curr. Heart Fail. Rep. 2017, 14, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Kim, C. The mediating effect of resilience on the relationship between Type D personality and self-care behavior in patients with heart failure. Jpn. J. Nurs. Sci. 2020, 17, e12359. [Google Scholar] [CrossRef]
- Bundgaard, J.S.; Østergaard, L.; Gislason, G.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Olesen, L.L.; Thøgersen, A.M.; Torp-Pedersen, C.; et al. Association between Type D personality and outcomes in patients with non-ischemic heart failure. Qual. Life Res. 2019, 28, 2901–2908. [Google Scholar] [CrossRef]
- Siennicka, A.E.; Gościńska-Bis, K.; Wilczek, J.; Wójcik, M.; Błaszczyk, R.; Szymański, F.M.; Nadrowski, P.; Michalski, B.; Mizia-Stec, K.; Ptaszyńska-Kopczyńska, K.; et al. Perception of health control and self-efficacy in heart failure. Kardiol. Polska 2016, 74, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Kessing, D.; Denollet, J.; Widdershoven, J.; Kupper, N. Psychological Determinants of Heart Failure Self-Care: Systematic Review and Meta-Analysis, Psychosomatic Medicine. Psychosom. Med. 2016, 78, 412–431. [Google Scholar] [CrossRef]
- Riegel, B.; Lee, C.; Albert, N.; Lennie, T.; Chung, M.; Song, E.K.; Bentley, B.; Heo, S.; Worrall-Carter, L.; Moser, D.K. From Novice to Expert: Confidence and activity status determine heart failure self-care performance. Nurs. Res. 2011, 60, 132–138. [Google Scholar] [CrossRef]
- Holmes, E.A.; Hughes, D.A.; Morrison, V.L. Predicting Adherence to Medications Using Health Psychology Theories: A Systematic Review of 20 Years of Empirical Research. Value Health 2014, 17, 863–876. [Google Scholar] [CrossRef]
- Pekonen, A.; Eloranta, S.; Stolt, M.; Virolainen, P.; Leino-Kilpi, H. Measuring patient empowerment—A systematic review. Patient Educ. Couns. 2020, 103, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Marijanovic, I.; Pavlekovic, G.; Buhovac, T.; Martinac, M. The Relationship between Health Locus of Control, Depression, and Sociodemographic Factors and Amount of Time Breast Cancer Patients Wait before Seeking Diagnosis and Treatment. Psychiatr. Danub. 2017, 29, 330–344. [Google Scholar] [CrossRef]
- West, L.M.; Theuma, R.B.; Cordina, M. Health locus of control: Its relationship with medication adherence and medication wastage. Res. Soc. Adm. Pharm. 2018, 14, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Bąk-Sosnowska, M.; Wyszomirska, J.; Daniel-Sielańczyk, A. Selected psychological factors and medication adherence in patients with rheumatoid arthritis. Rheumatology 2021, 59, 90–97. [Google Scholar] [CrossRef]
- Lima, M.P.; Machado, W.D.L.; Irigaray, T. Predictive factors of treatment adherence in cancer outpatients. Psycho-Oncology 2018, 27, 2823–2828. [Google Scholar] [CrossRef] [PubMed]
- Gerland, H.-M.E.; Prell, T. Association Between the Health Locus of Control and Medication Adherence: An Observational, Cross-Sectional Study in Primary Care. Front. Med. (Lausanne) 2021, 8, 705202. [Google Scholar] [CrossRef]
- Wallston, K.A.; Wallston, B.S.; DeVellis, R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ. Monogr. 1978, 6, 160–170. [Google Scholar] [CrossRef]
- Luszczynska, A.; Schwarzer, R. Multidimensional Health Locus of Control: Comments on the Construct and its Measurement. J. Health Psychol. 2005, 10, 633–642. [Google Scholar] [CrossRef]
- Lin, C.; Dracup, K.; Pelter, M.M.; Biddle, M.J.; Moser, D.K. Association of psychological distress with reasons for delay in seeking medical care in rural patients with worsening heart failure symptoms. J. Rural Health 2021, 38, 713–720. [Google Scholar] [CrossRef]
- Wiśnicka, A.; Uchmanowicz, I.; Dyjakon, D.; Cichoń, E.; Szczepanowski, R.; A Jankowska, E. Acceptance of the disease and sexual functions of patients with heart failure. Eur. J. Cardiovasc. Nurs. 2020, 20, 220–230. [Google Scholar] [CrossRef]
- Urban, S.; Błaziak, M.; Jura, M.; Iwanek, G.; Zdanowicz, A.; Guzik, M.; Borkowski, A.; Gajewski, P.; Biegus, J.; Siennicka, A.; et al. Novel Phenotyping for Acute Heart Failure—Unsupervised Machine Learning-Based Approach. Biomedicines 2022, 10, 1514. [Google Scholar] [CrossRef]
- Conner, M.; Norman, P. Comprehensive Clinical Psychology. In Health Behavior; American Psychological Association: Washington, DC, USA, 1998; pp. 1–37. [Google Scholar] [CrossRef]
- Stenström, U.; Einarson, S.; Jacobsson, B.; Lindmark, U.; Wenander, Å.; Hugoson, A. The importance of psychological factors in the maintenance of oral health: A study of Swedish university students. Oral Health Prev. Dent. 2009, 7, 225–22533. [Google Scholar] [CrossRef]
- Slopieck, A.; Chrapek, M. Relationship between health related locus of control and health behaviour among university students. Sleep Med. Disord. Int. J. 2019, 3, 10–15. [Google Scholar] [CrossRef]
- Smoleń, E.; Dobrowolska, B. The health locus of control and chosen health behaviors of nurses from the Lublin and Podkarpackie voivodeships. Nurs. Probl./Probl. Pielęgniarstwa 2019, 26, 290–299. [Google Scholar] [CrossRef]
- Nazareth, M.; Richards, J.; Javalkar, K.; Haberman, C.; Zhong, Y.; Rak, E.; Jain, N.; Ferris, M.; van Tilburg, M.A. Relating Health Locus of Control to Health Care Use, Adherence, and Transition Readiness Among Youths With Chronic Conditions, North Carolina, 2015. Prev. Chronic Dis. 2019, 13, E93. [Google Scholar] [CrossRef]
- Kretchy, I.A.; Owusu-Daaku, F.; Danquah, S. Locus of control and anti-hypertensive medication adherence in Ghana. Pan Afr. Med. J. 2014, 17, 13. [Google Scholar] [CrossRef]
- Ozolins, A.R.; Stenström, U. Validation of health locus of control patterns in Swedish adolescents. Adolescence 2003, 38, 651–657. [Google Scholar]
- Alashwal, H.; El Halaby, M.; Crouse, J.J.; Abdalla, A.; Moustafa, A.A. The Application of Unsupervised Clustering Methods to Alzheimer’s Disease. Front. Comput. Neurosci. 2019, 13, 31. [Google Scholar] [CrossRef]
- Eick, C.; Zeidat, N.; Zhao, Z. Supervised clustering-algorithms and benefits. In Proceedings of the 16th IEEE International Conference on Tools with Artificial Intelligence, Baca Raton, FL, USA, 15–17 November 2004. [Google Scholar] [CrossRef]
- Pondel, M.; Korczak, J. Collective clustering of marketing data—Recommendation system Upsaily. In Proceedings of the 2018 Federated Conference on Computer Science and Information Systems (FedCSIS), Poznan, Polan, 9–12 September 2018; Volume 15, pp. 801–810. [Google Scholar] [CrossRef]
- Pondel, M.; Korczak, J. Recommendations Based on Collective Intelligence—Case of Customer Segmentation. In Information Technology for Management: Emerging Research and Applications; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Davies, D.L.; Bouldin, D.W. A Cluster Separation Measure. IEEE Trans. Pattern Anal. Mach. Intell. 1979, PAMI-1, 224–227. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996; Volume 1, pp. 10–1037. [Google Scholar]
- Náfrádi, L.; Nakamoto, K.; Schulz, P.J. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS ONE 2017, 12, e0186458. [Google Scholar] [CrossRef]
- Potdar, S.; Lakshminarayan, N.; Reddy, S.G. Relationship of Locus of control with Plaque and Gingival status before and after Oral Health Education in a group of college students-An experimental study. Int. J. Dent. Hyg. 2015, 13, 42–48. [Google Scholar] [CrossRef]
- Lima, M.P.; Moret-Tatay, C.; Irigaray, T.Q. Locus of control, personality and depression symptoms in cancer: Testing a moderated mediation model. Clin. Psychol. Psychother. 2021, 29, 489–500. [Google Scholar] [CrossRef]
- Edyta Kobiela. Intelligent Recommendation Systems (pol. Inteligentne Systemy Rekomendacji-Network Magazyn). 2011. Available online: https://networkmagazyn.pl/inteligentne-systemy-rekomendacji/ (accessed on 7 April 2021).
- Burke, R. Hybrid Recommender Systems: Survey and Experiments. User Model. User-Adapted Interact. 2002, 12, 331–370. [Google Scholar] [CrossRef]
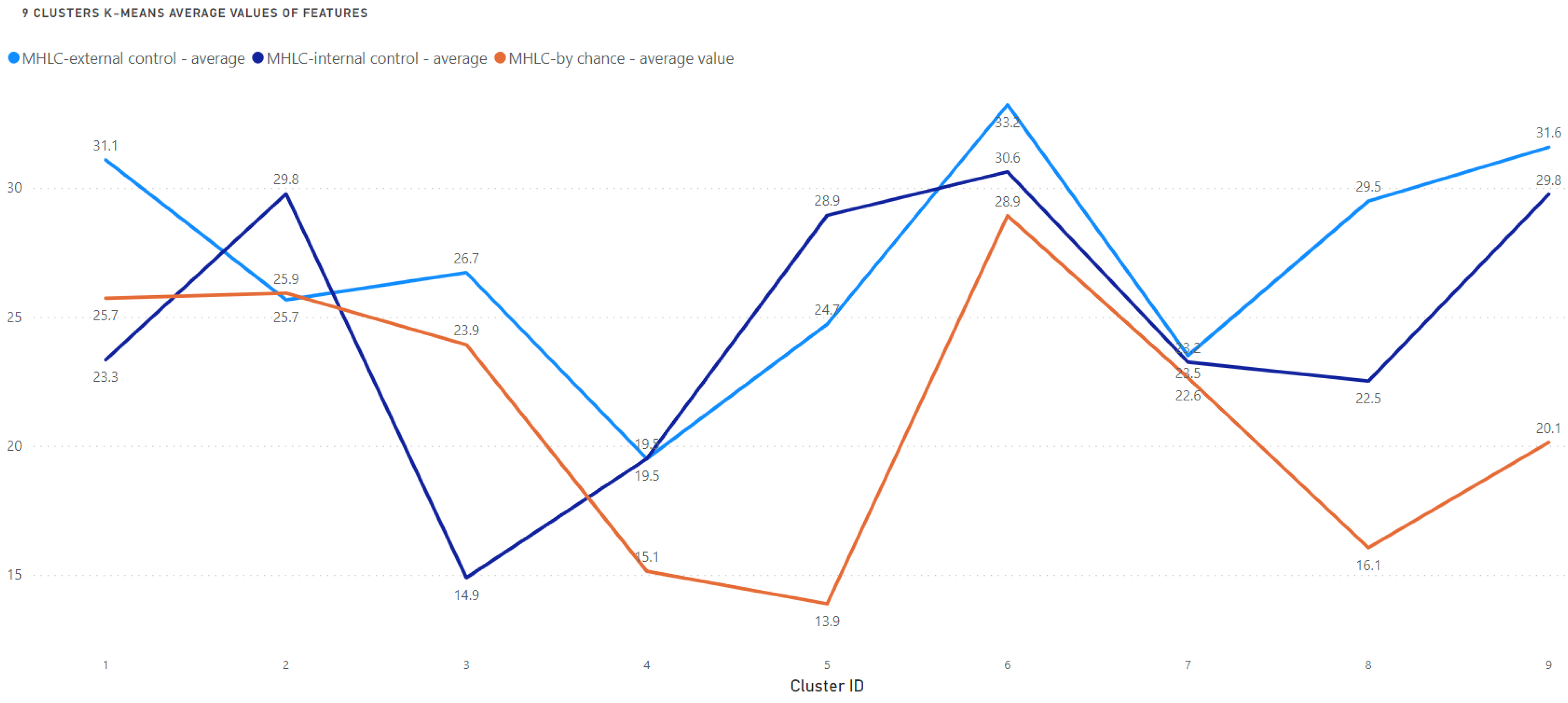
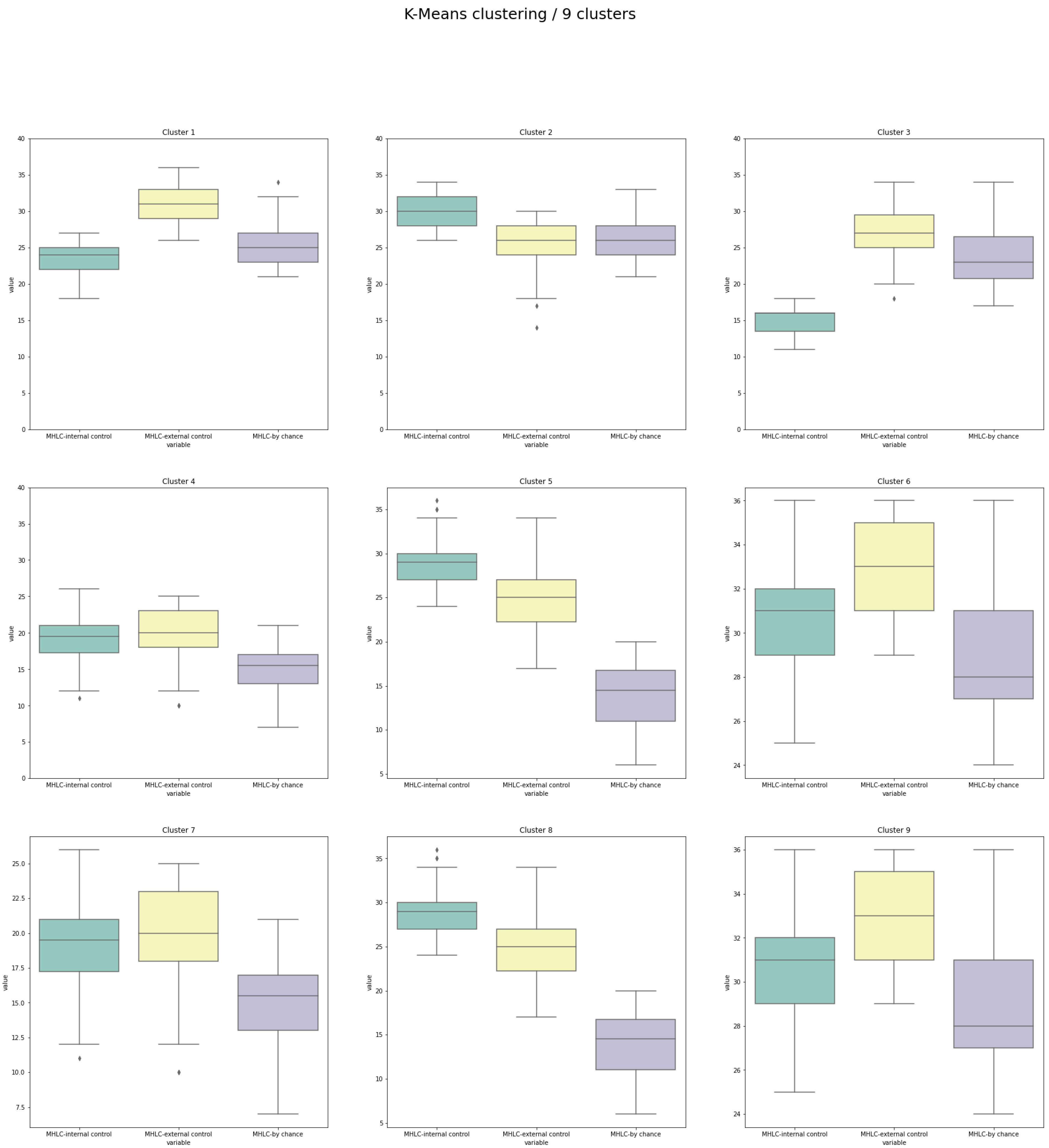
| Cluster | n | Internal (I) * | Others (O) * | Chance (C) * | Predicted Attitude to Education on HF Management | Observations That Should Be Encountered While Planning Educational or Motivational Interventions |
|---|---|---|---|---|---|---|
| 1 | 111 | 3 | 1 | 2 | Rather good | Risk of underestimating their role in HF management (beliefs in chance higher than internal control) |
| 2 | 76 | 1 | 2 | 2 | Problematic | Risk of ignoring professional recommendations (beliefs in chance equal to beliefs in others) |
| 3 | 31 | 3 | 1 | 2 | Very problematic | High risk of underestimating their role in HF management (the lowest internal control among all clusters) |
| 4 | 42 | 2 | 1 | 3 | Very good | Probably the most promising structure of beliefs (beliefs in others and internal control, not in chance) |
| 5 | 74 | 1 | 2 | 3 | Rather good | Risk of relying on personal, not professional recommendations (internal beliefs are the highest) |
| 6 | 129 | 2 | 1 | 3 | Rather good | The big difference between beliefs in others vs. internal control: may require very precise recommendations, risk of underestimating their role in HF management |
| 7 | 118 | 2 | 1 | 3 | Rather good | The big difference between beliefs in others vs. internal control: may require very precise recommendations, risk of underestimating their role in HF management |
| 8 | 79 | 1 | 2 | 3 | Rather good | Risk of relying on personal, not professional recommendations (internal beliefs are the highest) |
| 9 | 98 | 2 | 1 | 3 | Rather good | The big difference between beliefs in others vs. internal control: may require very precise recommendations, risk of underestimating their role in HF management |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siennicka, A.; Pondel, M.; Urban, S.; Jankowska, E.A.; Ponikowska, B.; Uchmanowicz, I. Patterns of Locus of Control in People Suffering from Heart Failure: An Approach by Clustering Method. Medicina 2022, 58, 1542. https://doi.org/10.3390/medicina58111542
Siennicka A, Pondel M, Urban S, Jankowska EA, Ponikowska B, Uchmanowicz I. Patterns of Locus of Control in People Suffering from Heart Failure: An Approach by Clustering Method. Medicina. 2022; 58(11):1542. https://doi.org/10.3390/medicina58111542
Chicago/Turabian StyleSiennicka, Agnieszka, Maciej Pondel, Szymon Urban, Ewa Anita Jankowska, Beata Ponikowska, and Izabella Uchmanowicz. 2022. "Patterns of Locus of Control in People Suffering from Heart Failure: An Approach by Clustering Method" Medicina 58, no. 11: 1542. https://doi.org/10.3390/medicina58111542
APA StyleSiennicka, A., Pondel, M., Urban, S., Jankowska, E. A., Ponikowska, B., & Uchmanowicz, I. (2022). Patterns of Locus of Control in People Suffering from Heart Failure: An Approach by Clustering Method. Medicina, 58(11), 1542. https://doi.org/10.3390/medicina58111542






