The Prevalence of Iron Deficiency in Atrial Fibrillation: Low Hanging Fruit?
Abstract
1. Introduction
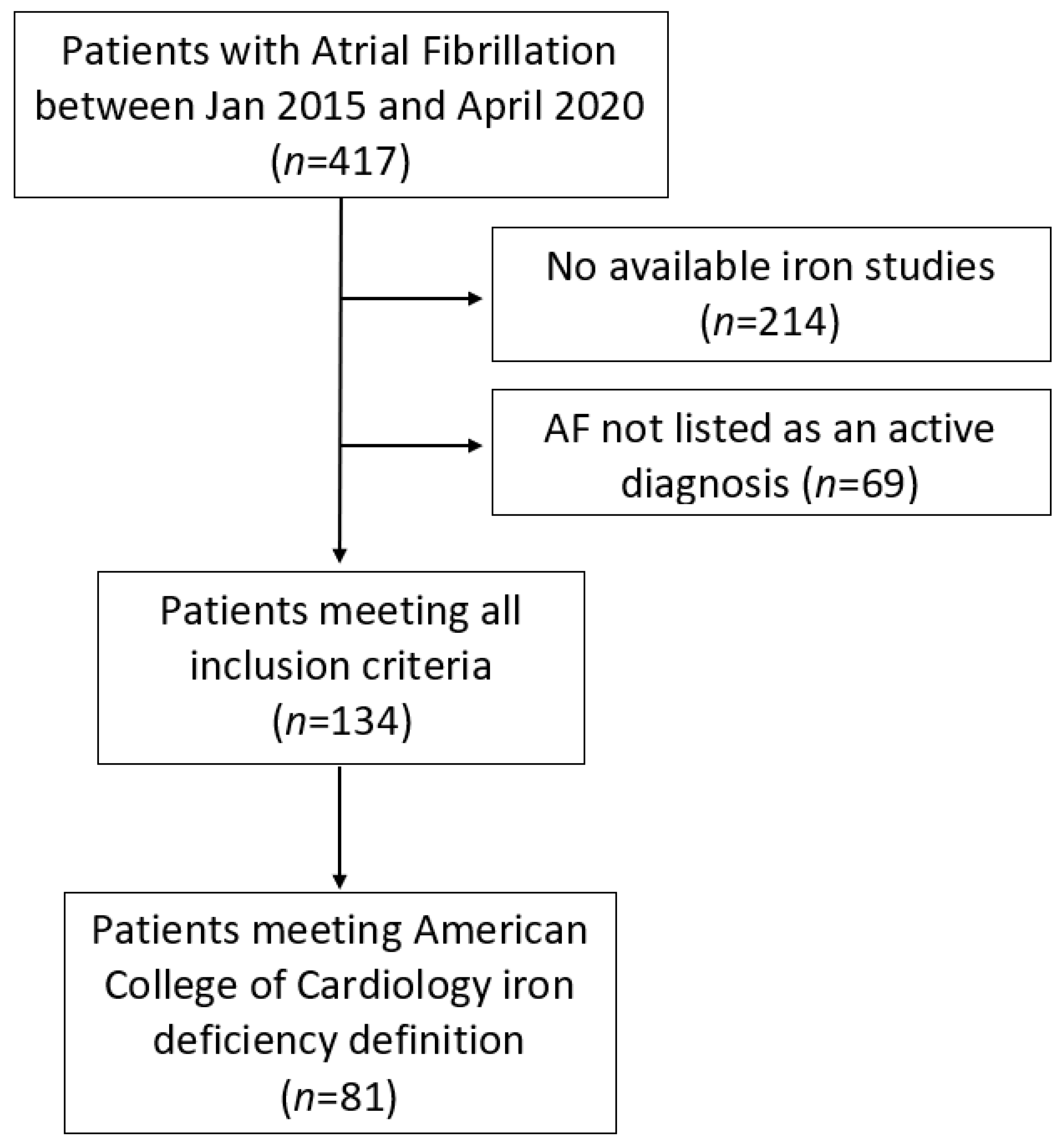
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Michaud, G.F.; Stevenson, W.G. Atrial Fibrillation. In Harrison’s Principles of Internal Medicine, 20e; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Graham, F.J.; Pellicori, P.; Ford, I.; Petrie, M.C.; Kalra, P.R.; Cleland, J.G.F. Intravenous iron for heart failure with evidence of iron deficiency: A meta-analysis of randomised trials. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Kirwan, B.A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Lüscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: An individual patient data meta-analysis. Eur. J. Heart Fail. 2018, 20, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Cardillo, M.T.; Caroli, A.; Marini, M.G.; Sonnino, C.; Narducci, M.L.; Biasucci, L.M. Inflammation and C-reactive protein in atrial fibrillation: Cause or effect? Tex. Heart Inst. J. 2014, 41, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dudley, S.C., Jr. Evidence for Inflammation as a Driver of Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.; Walkey, D.; Hogarth, K.; Gregory, Y.H.L. Optimizing Atrial Fibrillation Management: From ICU and Beyond. Chest 2015, 148, 859–864. [Google Scholar] [CrossRef]
- Lee, W.H.; Hsu, P.C.; Chu, C.Y.; Lee, H.H.; Lee, M.K.; Lee, C.S.; Yen, H.W.; Lin, T.H.; Voon, W.C.; Lai, W.T.; et al. Anemia as an Independent Predictor of Adverse Cardiac Outcomes in Patients with Atrial Fibrillation. Int. J. Med. Sci. 2015, 12, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Adamsson Eryd, S.; Borné, Y.; Melander, O.; Persson, M.; Smith, J.G.; Hedblad, B.; Engström, G. Red blood cell distribution width is associated with incidence of atrial fibrillation. J. Intern. Med. 2014, 275, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Simsek, H.; Gunes, Y.; Demir, C.; Sahin, M.; Gumrukcuoglu, H.A.; Tuncer, M. The effects of iron deficiency anemia on p wave duration and dispersion. Clinics 2010, 65, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Masini, G.; Graham, F.J.; Pellicori, P.; Cleland, J.G.F.; Cuthbert, J.J.; Kazmi, S.; Inciardi, R.M.; Clark, A.L. Criteria for Iron Deficiency in Patients with Heart Failure. J. Am. Coll. Cardiol. 2022, 79, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Ural, D.; Altay, S.; Argan, O.; Börklü, E.B.; Kozan, Ö. Iron deficiency and hematinic deficiencies in atrial fibrillation: A new insight into comorbidities. Turk. Kardiyol. Dern. Ars. 2018, 46, 103–110. [Google Scholar] [PubMed]
- Punj, S.; Ghafourian, K.; Ardehali, H. Iron deficiency and supplementation in heart failure and chronic kidney disease. Mol. Asp. Med. 2020, 75, 100873. [Google Scholar] [CrossRef]
| Baseline Characteristics | |
|---|---|
| Age | 73.3 (±11.5) |
| Gender (Male:Female) | 76:58 |
| Body Mass Index | 30.0 (±6.6) |
| Chads-Vasc Score | 3.7 (±1.8) |
| HAS-BLED Score | 2.8 (±1.2) |
| Charlson Comorbidity Index | 5.6 (±2.6) |
| Comorbidities | |
| Hypertension % (n) | 53.4% (72) |
| Heart Failure % (n) | 35.1% (47) |
| Ischaemic Heart Disease % (n) | 26.9% (36) |
| Dyslipidemia % (n) | 28.4% (38) |
| Diabetes Mellitus % (n) | 29.9% (40) |
| Chronic Kidney Disease % (n) | 33.6% (45) |
| Prior Ischemic Stroke/TIA % (n) | 23.1% (31) |
| Echocardiographic Characteristics | |
| LV Ejection Fraction | 49.7 (±15.6) |
| LV Wall Thickness (mm) | 11.9 (±2.4) |
| Left Atrial Area (cm2) | 28.8 (±9.3) |
| Serological Characteristics | |
| Haemoglobin (g/L) | 122 (±25.4) |
| Creatinine (umol/L) | 130 (±142) |
| Ferritin (ug/L) | 240 (±288) |
| Transferrin (g/L) | 2.9 (±6.2) |
| Transferrin Saturation (%) | 16.7 (±11.0) |
| Variable | Iron Deficient Group | Iron Non-Deficient Group | p-Value |
|---|---|---|---|
| Baseline demographics and clinical characteristics | |||
| Age (years) | 74.3 (±1.3) | 71.95 (±1.5) | 0.258 |
| CHA2DS2-VASc Score | 4.2 (±0.20) | 2.9 (±0.24) | <0.0001 |
| HAS-BLED Score | 3.0 (±0.14) | 2.6 (±0.16) | 0.068 |
| CCI | 6.1 (±0.30) | 4.8 (±0.31) | 0.007 |
| Hypertension % (n) | 55.5% (45) | 50.1% (27) | 0.60 |
| Heart Failure % (n) | 39.5% (32) | 28.3% (15) | 0.18 |
| Ischaemic Heart Disease % (n) | 28.4% (23) | 24.5% (13) | 0.62 |
| Diabetes Mellitus % (n) | 40.7% (33) | 13.2% (7) | 0.0006 |
| Chronic Kidney Disease % (n) | 35.8% (29) | 30.2% (16) | 0.50 |
| Prior Ischemic Stroke/TIA % (n) | 32.1% (26) | 11.3% (6) | 0.006 |
| History of Major Bleeding % (n) | 11.1% (9) | 5.6% (3) | 0.36 |
| Oral Anticoagulation % (n) | 54.2% (44) | 39.6% (21) | 0.096 |
| Anaemia % (n) | 55.5% (45) | 41.5% (22) | 0.11 |
| Length of Stay (Days) | 11.5 (±1.8) | 18.6 (±6.6) | 0.297 |
| Readmission at Follow-up % (n) | 80% (65) | 85% (45) | 0.49 |
| 1-year Mortality % (n) | 7.4% (6) | 9.4% (5) | 0.68 |
| Echocardiographic characteristics | |||
| Ejection fraction (%) | 48.7 (±2.2) | 51.0 (±2.8) | 0.506 |
| LV Wall Thickness (mm) | 11.7 (±2.7) | 12.2 (±1.8) | 0.422 |
| LA area (cm2) | 28.5 (±1.) | 29.3 (±2.4) | 0.778 |
| Serological results | |||
| Haemoglobin (g/L) | 118.0 (±2.6) | 128.4 (±3.8) | 0.022 |
| Ferritin (ug/L) | 98.3 (±8.6) | 457.0 (±48.9) | <0.0001 |
| Transferrin Saturation (%) | 11.9 (±0.8) | 23.9 (±1.7) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdullah, B.; Ferreira, D.; Bourke, E.; Kamalanathan, H.; Elashri, I.; Porwal, K.; Tiller, M.J.; Gadre, P.H.; Jones, S.; McGee, M. The Prevalence of Iron Deficiency in Atrial Fibrillation: Low Hanging Fruit? Medicina 2022, 58, 1492. https://doi.org/10.3390/medicina58101492
Alabdullah B, Ferreira D, Bourke E, Kamalanathan H, Elashri I, Porwal K, Tiller MJ, Gadre PH, Jones S, McGee M. The Prevalence of Iron Deficiency in Atrial Fibrillation: Low Hanging Fruit? Medicina. 2022; 58(10):1492. https://doi.org/10.3390/medicina58101492
Chicago/Turabian StyleAlabdullah, Bachar, David Ferreira, Erin Bourke, Harish Kamalanathan, Ibrahim Elashri, Kushal Porwal, Michael J. Tiller, Payal H. Gadre, Sarah Jones, and Michael McGee. 2022. "The Prevalence of Iron Deficiency in Atrial Fibrillation: Low Hanging Fruit?" Medicina 58, no. 10: 1492. https://doi.org/10.3390/medicina58101492
APA StyleAlabdullah, B., Ferreira, D., Bourke, E., Kamalanathan, H., Elashri, I., Porwal, K., Tiller, M. J., Gadre, P. H., Jones, S., & McGee, M. (2022). The Prevalence of Iron Deficiency in Atrial Fibrillation: Low Hanging Fruit? Medicina, 58(10), 1492. https://doi.org/10.3390/medicina58101492





