Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A Monocentric Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
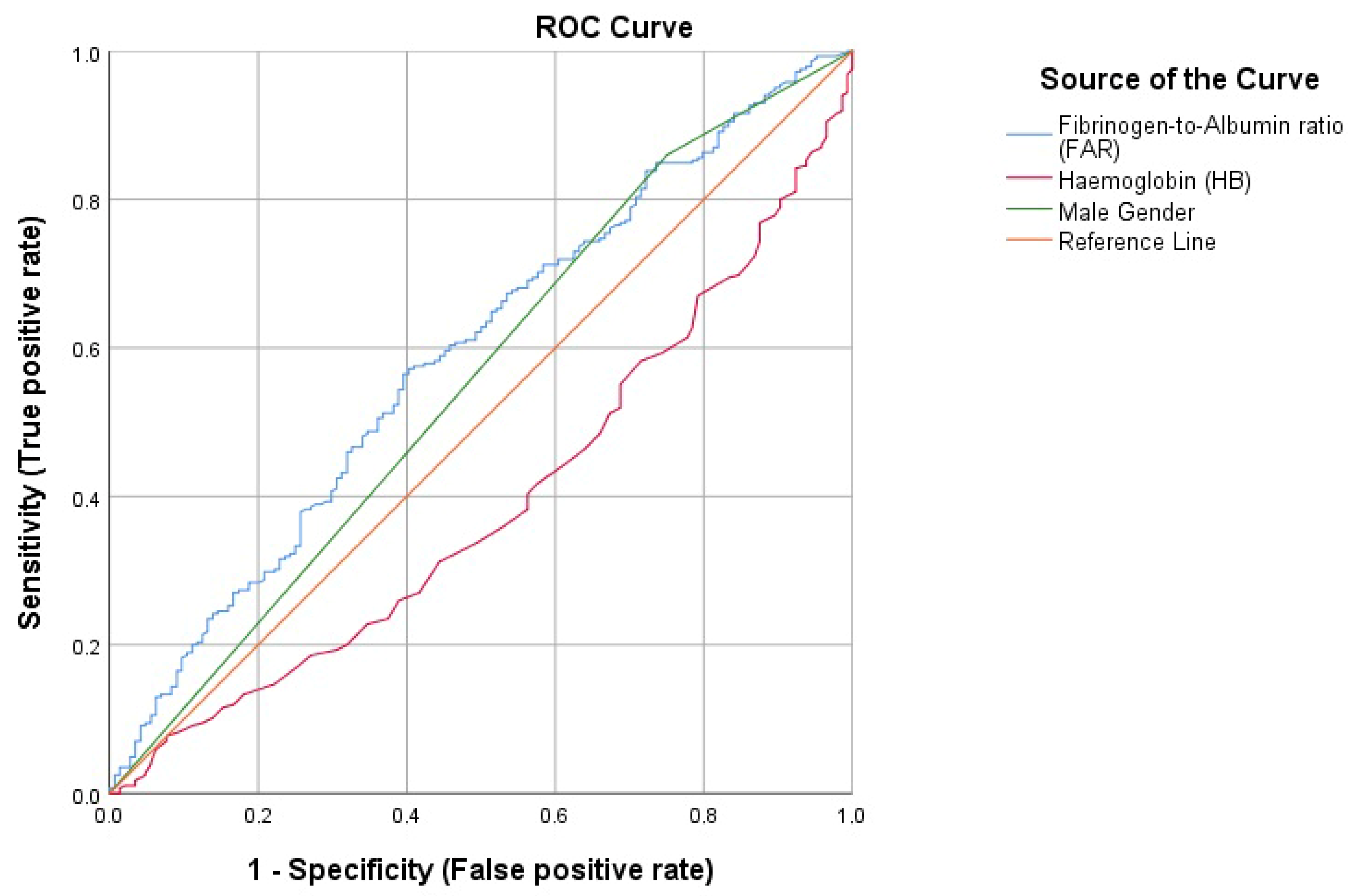
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khadhouri, S.; Gallagher, K.M.; MacKenzie, K.R.; Shah, T.T.; Gao, C.; Moore, S.; Zimmermann, E.F.; Edison, E.; Jefferies, M.; Nambiar, A.; et al. The IDENTIFY study: The investigation and detection of urological neoplasia in patients referred with suspected urinary tract cancer—A multicentre observational study. BJU Int. 2021, 128, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and Risk Factors of Urothelial Bladder Cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Tarantino, G.; Crocetto, F.; Di Vito, C.; Creta, M.; Martino, R.; Pandolfo, S.D.; Pesce, S.; Napolitano, L.; Capone, D.; Imbimbo, C. Association of NAFLD and Insulin Resistance with Non Metastatic Bladder Cancer Patients: A Cross-Sectional Retrospective Study. J. Clin. Med. 2021, 10, 346. [Google Scholar] [CrossRef]
- Ferro, M.; Chiujdea, S.; Musi, G.; Lucarelli, G.; Del Giudice, F.; Hurle, R.; Damiano, R.; Cantiello, F.; Mari, A.; Minervini, A.; et al. Impact of Age on Outcomes of Patients With Pure Carcinoma In Situ of the Bladder: Multi-Institutional Cohort Analysis. Clin. Genitourin. Cancer 2021, 20, e166–e172. [Google Scholar] [CrossRef]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Prim. 2017, 3, 17022. [Google Scholar] [CrossRef]
- Van de Putte, E.E.F.; Bosschieter, J.; van der Kwast, T.H.; Bertz, S.; Denzinger, S.; Manach, Q.; Compérat, E.M.; Boormans, J.L.; Jewett, M.A.S.; Stoehr, R.; et al. The World Health Organization 1973 classification system for grade is an important prognosticator in T1 non-muscle-invasive bladder cancer. BJU Int. 2018, 122, 978–985. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ). Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2020, 79, 82–104. [Google Scholar] [CrossRef]
- Sossenheimer, C.; Cizik, A.; Lenherr, S.O.; Neil, B.; Dechet, C.B.; Sanchez, A.; Tward, J.D. The effect of neoadjuvant chemotherapy on quality of life for patients with muscle invasive bladder cancer (MIBC) undergoing cystectomy. J. Clin. Oncol. 2021, 39, e18614. [Google Scholar] [CrossRef]
- Miller, G.J.; Bauer, K.A.; Howarth, D.J.; Cooper, J.A.; Humphries, S.E.; Rosenberg, R.D. Increased incidence of neoplasia of the digestive tract in men with persistent activation of the coagulant pathway. J. Thromb. Haemost. 2004, 2, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- He, S.-S.; Wang, Y.; Yang, L.; Chen, H.-Y.; Liang, S.-B.; Lu, L.-X.; Chen, Y. Plasma Fibrinogen Correlates with Metastasis and is Associated with Prognosis in Human Nasopharyngeal Carcinoma. J. Cancer 2017, 8, 403–409. [Google Scholar] [CrossRef]
- Ghezzi, F.; Cromi, A.; Siesto, G.; Giudici, S.; Serati, M.; Formenti, G.; Franchi, M. Prognostic significance of preoperative plasma fibrinogen in endometrial cancer. Gynecol. Oncol. 2010, 119, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- An, Q.; Liu, W.; Yang, Y.; Yang, B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer 2020, 20, 691. [Google Scholar] [CrossRef]
- Hwang, K.-T.; Chung, J.K.; Roh, E.Y.; Kim, J.; Oh, S.A.; Kim, Y.; Rhu, J.; Kim, S. Prognostic Influence of Preoperative Fibrinogen to Albumin Ratio for Breast Cancer. J. Breast Cancer 2017, 20, 254–263. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Ren, H.; Du, Y.-X.; Wang, C.-F. Prognostic value of the preoperative fibrinogen-to-albumin ratio in pancreatic ductal adenocarcinoma patients undergoing R0 resection. World J. Gastroenterol. 2020, 26, 7382–7404. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.-H.; Knowles, M.; Bivalacqua, T.J. Urothelial carcinoma. Adv. Urol. 2012, 2012, 461370. [Google Scholar] [CrossRef] [PubMed]
- Matulay, J.T.; Kamat, A.M. Advances in risk stratification of bladder cancer to guide personalized medicine. F1000Research 2018, 7, 1137. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Babă, D.-F.; de Cobelli, O.; Musi, G.; Lucarelli, G.; Terracciano, D.; Porreca, A.; Busetto, G.M.; Del Giudice, F.; Soria, F.; et al. Neutrophil percentage-to-albumin ratio predicts mortality in bladder cancer patients treated with neoadjuvant chemotherapy followed by radical cystectomy. Futur. Sci. OA 2021, 7, FSO709. [Google Scholar] [CrossRef]
- Ferro, M.; Caputo, V.F.; Barone, B.; Imbimbo, C.; de Cobelli, O.; Crocetto, F. Lymphocyte to Monocyte Ratio: A New Independent Prognostic Factor in Bladder Cancer Progression? Front. Oncol. 2021, 11, 754649. [Google Scholar] [CrossRef]
- Crocetto, F.; Pandolfo, S.D.; Aveta, A.; Martino, R.; Trama, F.; Caputo, V.F.; Barone, B.; Abate, M.; Sicignano, E.; Cilio, S.; et al. A Comparative Study of the Triglycerides/HDL Ratio and Pseudocholinesterase Levels in Patients with Bladder Cancer. Diagnostics 2022, 12, 431. [Google Scholar] [CrossRef]
- Crocetto, F.; Barone, B.; Ferro, M.; Busetto, G.M.; La Civita, E.; Buonerba, C.; Di Lorenzo, G.; Terracciano, D.; Schalken, J.A. Liquid biopsy in bladder cancer: State of the art and future perspectives. Crit. Rev. Oncol. 2022, 170, 103577. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Sun, P.; Wang, Z.Q.; Wang, D.S.; Zhang, D.S.; Wang, F.H.; Fu, J.H.; Xu, R.H.; Li, Y.H. Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 2413–2424. [Google Scholar] [CrossRef]
- Qin, W.-Z.; Li, Q.; Chen, W.-F.; Xu, M.-D.; Zhang, Y.-Q.; Zhong, Y.-S.; Ma, L.-L.; Hu, J.-W.; Cai, M.-Y.; He, M.-J.; et al. Overexpression of fibrinogen-like protein 2 induces epithelial-to-mesenchymal transition and promotes tumor progression in colorectal carcinoma. Med. Oncol. 2014, 31, 181. [Google Scholar] [CrossRef]
- Artigas, A.; Wernerman, J.; Arroyo, V.; Vincent, J.-L.; Levy, M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J. Crit. Care 2015, 33, 62–70. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-Y.; Zhang, H.-H.; Xiong, J.-P.; Yang, X.-B.; Bai, Y.; Lin, J.-Z.; Long, J.-Y.; Zheng, Y.-C.; Zhao, H.-T.; Sang, X.-T. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J. Gastroenterol. 2018, 24, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hao, L.; Zhang, S.; Zhang, Y.; Dong, B.; Zhang, Q.; Han, C. Preoperative Fibrinogen–Albumin Ratio, Potential Prognostic Factors for Bladder Cancer Patients Undergoing Radical Cystectomy: A Two-Center Study. Cancer Manag. Res. 2021, 13, 3181–3192. [Google Scholar] [CrossRef]
- Claps, F.; Rai, S.; Mir, M.C.; van Rhijn, B.W.; Mazzon, G.; Davis, L.E.; Valadon, C.L.; Silvestri, T.; Rizzo, M.; Ankem, M.; et al. Prognostic value of preoperative albumin-to-fibrinogen ratio (AFR) in patients with bladder cancer treated with radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 835.e9–835.e17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, D.; Zeng, S.; Wu, T.; Wang, Y.; Zhang, H.; Wang, B.; Hu, X. Prognostic Value of Preoperative Albumin-to-Fibrinogen Ratio in Patients with Bladder Cancer. J. Cancer 2021, 12, 5864–5873. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, W.; Li, J.; Lu, X. Different patterns in the prognostic value of age for bladder cancer-specific survival depending on tumor stages. Am. J. Cancer Res. 2015, 5, 2090–2097. [Google Scholar] [PubMed]
- Soria, F.; Moschini, M.; Abufaraj, M.; Wirth, G.J.; Foerster, B.; Gust, K.M.; Özsoy, M.; Briganti, A.; Gontero, P.; Mathieu, R.; et al. Preoperative anemia is associated with disease recurrence and progression in patients with non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 113.e9–113.e14. [Google Scholar] [CrossRef]
- Luo, F.; Wang, Y.S.; Su, Y.H.; Zhang, Z.H.; Sun, H.H.; Li, J. Prognostic impact of preoperative anemia on non-muscle-invasive bladder carcinoma treated with GreenLight laser vaporization. Lasers Med. Sci. 2017, 32, 397–403. [Google Scholar] [CrossRef]
- Claps, F.; Mir, C.; Rubio-Briones, J. Optimization of the different therapeutic strategies in muscle invasive bladder cancer using biomarkers. Arch. Esp. Urol. 2022, 75, 144–155. [Google Scholar]
- Claps, F.; Pavan, N.; Umari, P.; Rizzo, M.; Barbone, F.; Giangreco, M.; Liguori, G.; Mir, C.M.; Bussani, R.; Trombetta, C. Incidence, predictive factors and survival outcomes of incidental prostate cancer in patients who underwent radical cystectomy for bladder cancer. Minerva Urol. Nephrol. 2021, 73, 349–356. [Google Scholar] [CrossRef]
| Median | IQR | |
|---|---|---|
| Age (years) | 73 | 14 |
| Hemoglobin (g/dL) | 14 | 2.2 |
| Creatinine (mg/dL) | 0.9 | 0.3 |
| Albumin (g/dL) | 4.3 | 0.5 |
| Uric acid (mg/dL) | 5.5 | 1.7 |
| Total Cholesterol (mg/dL) | 175 | 57 |
| Triglycerides (mg/dL) | 106 | 57 |
| LDL (mg/dL) | 109 | 52 |
| HDL (mg/dL) | 45 | 17 |
| AST (mU/mL) | 19 | 7 |
| ALT (mU/mL) | 17 | 13 |
| Fibrinogen (mg/dL) | 325 | 109.4 |
| Count | Percentage | |
| Gender | ||
| Male | 413 | 81 |
| Female | 97 | 19 |
| Diabetes | ||
| Yes | 78 | 15.3 |
| No | 432 | 84.7 |
| Chronic Kidney Disease | ||
| Yes | 58 | 11.4 |
| No | 452 | 88.7 |
| Cancer | ||
| Yes | 340 | 66.7 |
| No | 170 | 33.3 |
| Grade | ||
| Negative | 170 | 33.3 |
| Low-grade | 152 | 29.8 |
| High-grade | 188 | 36.9 |
| Muscle-Invasive Bladder Cancer | ||
| Yes | 59 | 17.8 |
| No | 272 | 82.2 |
| Normal Range | Negative | Low-Grade | High-Grade | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |||
| Age (years) | N.A | 70 | 15 | 72 | 15 | 75 | 13 | <0.0001 |
| Hemoglobin (g/dL) | 12–17.5 | 14.5 | 2.13 | 14 | 2.3 | 13.6 | 2.32 | <0.0001 |
| Creatinine (mg/dL) | 0.7–1.2 | 0.9 | 0.33 | 0.9 | 0.3 | 0.92 | 0.36 | 0.008 |
| Albumin (g/dL) | 3.2–4.6 | 4.4 | 0.5 | 4.4 | 0.4 | 4.3 | 0.5 | 0.021 |
| Uric acid (mg/dL) | 3.5–7.2 | 5.3 | 1.8 | 5.8 | 1.8 | 5.7 | 2 | 0.072 |
| Total Cholesterol (mg/dL) | <200 | 178 | 48 | 176 | 63 | 167.5 | 60 | 0.344 |
| Triglycerides (mg/dL) | <150 | 95.5 | 58 | 110 | 51 | 107 | 66 | 0.218 |
| LDL (mg/dL) | <100 | 113 | 45 | 105 | 63 | 108 | 54 | 0.572 |
| HDL (mg/dL) | >40 | 47 | 15 | 45 | 21 | 44 | 16 | 0.118 |
| AST (mU/mL) | 0–34 | 19 | 7 | 19 | 7 | 19 | 8 | 0.852 |
| ALT (mU/mL) | 0–55 | 19 | 12 | 17 | 12 | 16 | 10 | 0.004 |
| Fibrinogen (mg/dL) | 160–350 | 311 | 108.5 | 338 | 108 | 326 | 121.3 | 0.089 |
| Fibrinogen-to-Albumin Ratio (FAR) | N.A | 70.55 | 28.19 | 78.2 | 25.87 | 81.01 | 31.43 | 0.006 |
| Count | Percentage | Count | Percentage | Count | Percentage | |||
| Gender (male) | N.A | 127 | 74.7 | 124 | 81.6 | 162 | 86.2 | 0.022 |
| Diabetes (yes) | N.A | 20 | 13.1 | 19 | 13.9 | 39 | 23.2 | 0.027 |
| Chronic Kidney Disease (yes) | N.A | 15 | 19.5 | 15 | 19.7 | 28 | 30.1 | 0.170 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | 1.040 (1.022–1.058) | <0.0001 | 1.034 (1.014–1.055) | 0.001 |
| Hemoglobin (g/dL) | 0.826 (0.740–0.923) | 0.001 | 0.814 (0.701–0.944) | 0.006 |
| Creatinine (mg/dL) | 0.957 (0.866–1.058) | 0.392 | 0.906 (0.740–1.109) | 0.906 |
| Albumin (g/dL) | 0.481 (0.288–0.803) | 0.005 | 1.165 (0.594–2.285) | 0.658 |
| ALT (mU/mL) | 0.997 (0.980–1.013) | 0.674 | 1.011 (0.991–1.032) | 0.264 |
| FAR continuous | 1.013 (1.004–1.022) | 0.004 | 1.001 (0.988–1.013) | 0.928 |
| FAR categorical | ||||
| <76 | Ref. | - | Ref. | - |
| >76 | 2.062 (1.378–3.084) | <0.0001 | 1.657 (0.895–3.066) | 0.108 |
| Gender | ||||
| Female | Ref. | - | Ref. | - |
| Male | 1.640 (1.045–2.572) | 0.031 | 2.151 (1.253–3.694) | 0.005 |
| Diabetes | ||||
| No | Ref. | - | Ref. | - |
| Yes | 1.171 (0.988–2.963) | 0.055 | 1.471 (0.793–2.728) | 0.221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, B.; Napolitano, L.; Reccia, P.; De Luca, L.; Morra, S.; Turco, C.; Melchionna, A.; Caputo, V.F.; Cirillo, L.; Fusco, G.M.; et al. Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A Monocentric Retrospective Study. Medicina 2022, 58, 1490. https://doi.org/10.3390/medicina58101490
Barone B, Napolitano L, Reccia P, De Luca L, Morra S, Turco C, Melchionna A, Caputo VF, Cirillo L, Fusco GM, et al. Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A Monocentric Retrospective Study. Medicina. 2022; 58(10):1490. https://doi.org/10.3390/medicina58101490
Chicago/Turabian StyleBarone, Biagio, Luigi Napolitano, Pasquale Reccia, Luigi De Luca, Simone Morra, Carmine Turco, Alberto Melchionna, Vincenzo Francesco Caputo, Luigi Cirillo, Giovanni Maria Fusco, and et al. 2022. "Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A Monocentric Retrospective Study" Medicina 58, no. 10: 1490. https://doi.org/10.3390/medicina58101490
APA StyleBarone, B., Napolitano, L., Reccia, P., De Luca, L., Morra, S., Turco, C., Melchionna, A., Caputo, V. F., Cirillo, L., Fusco, G. M., Mastrangelo, F., Calace, F. P., Amicuzi, U., Morgera, V., Romano, L., Trivellato, M., Mattiello, G., Sicignano, E., Passaro, F., ... Crocetto, F. (2022). Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A Monocentric Retrospective Study. Medicina, 58(10), 1490. https://doi.org/10.3390/medicina58101490










