An Update on the Current and Emerging Use of Thiazolidinediones for Type 2 Diabetes
Abstract
1. Introduction
2. Current Approaches in the Management of T2DM
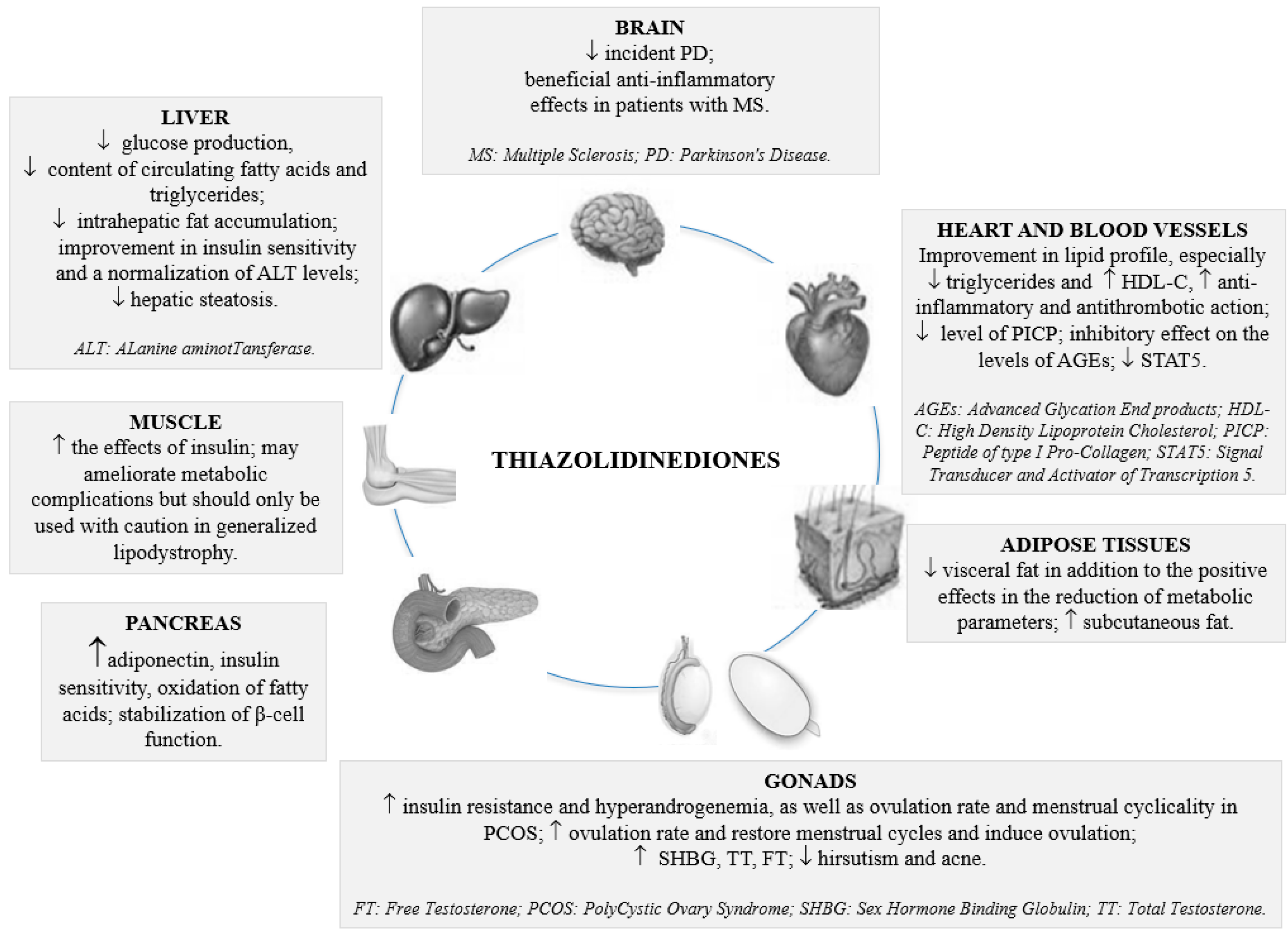
3. Current and Emerging Role of Thiazolidinediones
| Trial Name and Acronym | Short Description of Methods | Subjects and Duration | Results |
|---|---|---|---|
| Pioglitazone vs. Vitamin E vs. Placebo for Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis (PIVENS) ref. [30] | Determine if therapy with pioglitazone or vitamin E will lead to an improvement in liver histology in non-diabetic adult patients with NASH | A total of 247 participants with improvement in NAFLD activity defined by change in standardized scoring of liver biopsies at baseline and after 96 weeks of treatment | Significant reduction in ALT, AST, fatty liver and lobular inflammation in patients with NASH, possibly due to an improvement in small dense lipoproteins |
| PROspective pioglitAzone Clinical Trial In macroVascular Events (PROACTIVE) ref. [51] | Determine whether pioglitazone, once daily, can delay the time to death, heart attack, acute coronary syndrome, heart bypass surgery, stroke, leg bypass surgery or amputation in patients with type 2 diabetes | A total of 4373 participants treated with pioglitazone in combination with other medications for glycemic management; pioglitazone might reduce the incidence of macrovascular events associated with this disease compared with placebo; 4 years | Significant reduction was observed with pioglitazone in the main secondary composite endpoint of all-cause mortality, non-fatal MI and stroke; the number of deaths from heart failure was similar in both the pioglitazone and the placebo groups |
| Thiazolidinediones Or Sulphonylureas and Cardiovascular Accidents Intervention Trial (TOSCA.IT) ref. [52] | Evaluate the effects of add-on pioglitazone as compared with add-on a SU on the incidence of cardiovascular events in T2DM patients inadequately controlled with metformin; compare the two treatments in terms of glycemic control, safety, and economic costs | A total of 3371 T2DM patients; primary composite endpoint of all-cause mortality, non-fatal MI (including silent MI), non-fatal stroke, and unplanned coronary revascularization; secondary outcomes. Principal secondary outcome: a composite ischemic endpoint of sudden death, fatal and non-fatal acute MI (including silent MI), fatal and non-fatal stroke, major amputations (above ankle), endovascular or surgical intervention on the coronary, leg or carotid arteries | The incidence of cardiovascular events was similar with sulfonylureas and pioglitazone as add-on treatments to metformin but with fewer hypoglycemic events in the latter |
| Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycemia in Diabetes (RECORD) study ref. [53] | The study is being performed to monitor the incidence of cancer and bone fractures in RECORD patients for a period of 4 years after the end of the main RECORD study (2008–2012) | In this study, patients inadequately controlled on background metformin will be randomized to receive, in addition to metformin, either rosiglitazone or a SU in a ratio of 1:1; patients inadequately controlled on background SU will be randomized to receive, in addition to SU, either rosiglitazone or metformin in a ratio of 1:1; equal numbers of patients receiving background metformin and SU at entry will be entered into the study | The primary outcome of cardiovascular death or cardiovascular hospitalization was similar in both groups and there was no significant difference for cardiovascular death, MI and stroke; there was a significant increase in the rate of heart failure that resulted in hospitalization or death with rosiglitazone compared to the active controls |
| Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) study ref. [61] | Find out whether treatment with rosiglitazone improves the state of the liver and related blood markers in patients with NASH | A total of 64 patients with biopsy proven NASH will be randomized to receive either rosiglitazone 8 mg/day or placebo for one year; after one year of treatment patients will undergo a liver biopsy then a 4-month follow off treatment | Improvement in insulin sensitivity and a normalization of ALT levels that were four times greater than the placebo group; a significant 30% decrease in hepatic steatosis was observed; there was no significant histological improvement in the rosiglitazone group compared to the placebo group |
| A Randomized Trial to Slow the Progression of Diabetes (TRIPOD) ref. [68] | Test whether an evidence-based, low-cost mobile DMP, with or without an incentive program grounded in economic theory (M-POWER Rewards), can effectively and cost-effectively improve health outcomes for adults with type 2 diabetes | A total of 262 participants; a 52-week, three-arm randomized controlled trial to evaluate whether an evidence-based, low-cost mobile DMP, with or without an incentive program grounded in economic theory, can effectively and cost-effectively improve outcomes for adults with diabetes | Insulin resistance was improved during the thiazolidinedione treatment periods |
| Monotherapy | Combination Treatment with Other Antidiabetic Agents | Use in T2DM and Atherosclerotic Disease |
|---|---|---|
| Advantages
|
|
4. The Clinical Efficacy of Thiazolidinediones
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Biomath, D.; Devins, T.; Johansen, O.E.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Doyle-Delgado, K.; Chamberlain, J.J.; Shubrook, J.H.; Skolnik, N.; Trujillo, J. Pharmacologic approaches to glycemic treatment of type 2 diabetes: Synopsis of the 2020 American diabetes association’s standards of medical care in diabetes clinical guideline. Ann. Intern. Med. 2020, 173, 813–821. [Google Scholar] [CrossRef]
- Ahmad, L.A.; Crandall, J.P. Type 2 diabetes prevention: A review. Clin. Diabetes 2010, 28, 53–95. [Google Scholar] [CrossRef]
- Quesada, I.; Tudurí, E.; Ripoll, C.; Nadal, A. Physiology of the pancreatic α-cell and glucagon secretion: Role in glucose homeostasis and diabetes. J. Endocrinol. 2008, 199, 5–19. [Google Scholar] [CrossRef]
- De Fronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 Diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Firneisz, G. Non-alcoholic fatty liver disease and type 2 Diabetes mellitus: The liver disease of our age? World J. Gastroenterol. 2014, 20, 9072–9089. [Google Scholar]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.J.; Ismail, M. Stress and type 2 diabetes: A review of how stress contributes to the development of type 2 diabetes. Annu. Rev. Public Health 2015, 36, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.A.; Steptoe, A. Type 2 Diabetes mellitus and psychological stress: A modifiable risk factor. Nat. Rev. Endocrinol. 2017, 13, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Hackett, R.A.; Lazzarino, A.I.; Bostock, S.; La Marca, R.; Carvalho, L.A.; Hamer, M. Disruption of multisystem responses to stress in type 2 diabetes: Investigating the dynamics of allostatic load. Proc. Natl. Acad. Sci. USA 2014, 111, 15693–15698. [Google Scholar] [CrossRef]
- Vasanth, R.; Ganesh, A.; Shanker, R. Impact of stress on type 2 Diabetes mellitus management. Psychiatr. Danub. 2017, 29, 416–421. [Google Scholar]
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S111–S124. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Janez, A.; Muzurovic, E.; Stoian, A.P.; Haluzik, M.; Guja, C.; Czupryniak, L.; Duvnjak, L.; Lalic, N.; Tankova, T.; Bogdanski, P.; et al. Translating results from the cardiovascular outcomes trials with glucagon-like peptide-1 receptor agonists into clinical practice: Recommendations from a Eastern and Southern Europe diabetes expert group. Int. J. Cardiol. 2022, 365, 8–18. [Google Scholar] [CrossRef]
- Banach, M.; Gaita, D.; Haluzik, M. Cardio-Metabolic Academy Europe East. Adoption of the ADA/EASD guidelines in 10 Eastern and Southern European countries: Physician survey and good clinical practice recommendations from an international expert panel. Diabetes Res. Clin. Pract. 2021, 172, 108535. [Google Scholar] [CrossRef]
- Rizzo, M.; Nauck, M.A.; Mantzoros, C.S. Incretin-based therapies in 2021—Current status and perspectives for the future. Metabolism 2021, 122, 154843. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Linhart, A.; Vrablik, M.; Liberopoulos, E.; Rizzo, M. Safety and benefit of incretin-based therapies in patients with type 2 diabetes: Learnings and reflections. Expert Opin Drug Saf. 2022, 21, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; Giglio, R.V.; Rizvi, A.A.; Patti, A.M.; Montalto, G.; Maranta, F.; Cianflone, D.; Stoian, A.P.; Rizzo, M. Liraglutide Reduces Carotid Intima-Media Thickness by Reducing Small Dense Low-Density Lipoproteins in a Real-World Setting of Patients with Type 2 Diabetes: A Novel Anti-Atherogenic Effect. Diabetes Ther. 2021, 12, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Nikolic, D.; Patti, A.M.; Mannina, C.; Montalto, G.; McAdams, B.S.; Rizvi, A.A.; Cosentino, F. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2814–2821. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K. The clinical relevance of low-density-lipoproteins size modulation by statins. Cardiovasc. Drugs Ther. 2006, 20, 205–217. [Google Scholar] [CrossRef]
- Corrado, E.; Rizzo, M.; Coppola, G.; Muratori, I.; Carella, M.; Novo, S. Endothelial dysfunction and carotid lesions are strong predictors of clinical events in patients with early stages of atherosclerosis: A 24-month follow-up study. Coron. Artery Dis. 2008, 19, 139–144. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K. Should we measure routinely the LDL peak particle size? Int. J. Cardiol. 2006, 107, 166–170. [Google Scholar] [CrossRef]
- Brown, J.D.; Plutzky, J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007, 115, 518–533. [Google Scholar] [CrossRef]
- Hsiao, A.; Worrall, D.S.; Olefsky, J.M.; Subramaniam, S. Variance-modeled posterior inference of microarray data: Detecting gene-expression changes in 3T3-L1 adipocytes. Bioinformatics 2004, 20, 3108–3127. [Google Scholar] [CrossRef]
- Corey, K.E.; Wilson, L.A.; Altinbas, A.; Yates, K.P.; Kleiner, D.E.; Chung, R.T.; Krauss, R.M.; Chalasani, N.; NASH Clinical Research Network; the NASH Clinical Research Network. Relationship between resolution of non-alcoholic steatohepatitis and changes in lipoprotein sub-fractions: A post-hoc analysis of the PIVENS trial. Aliment. Pharmacol. Ther. 2019, 49, 1205–1213. [Google Scholar] [CrossRef]
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 Diabetes mellitus: A randomized trial. Ann. Intern. Med. 2016, 165, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Berneis, K.; Rizzo, M.; Stettler, C.; Chappuis, B.; Braun, M.; Diem, P.; Christ, E.R. Comparative effects of rosiglitazone and pioglitazone on fasting and postprandial low-density lipoprotein size and subclasses in patients with Type 2 diabetes. Expert. Opin. Pharmacother. 2008, 9, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Christ, E.R.; Rini, G.B.; Spinas, G.A.; Berneis, K. The differential effects of thiazolidindiones on atherogenic dyslipidemia in type 2 diabetes: What is the clinical significance? Expert. Opin. Pharmacother. 2008, 9, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Vekic, J.; Koulouris, S.; Zeljkovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V.; Rini, G.B.; Sakellariou, D.; Pastromas, S.; Mikhailidis, D.P.; et al. Effects of rosiglitazone on fasting and postprandial low- and high-density lipoproteins size and subclasses in type 2 diabetes. Angiology 2010, 61, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Kendall, D.M.; Deeg, M.A.; Buse, J.B.; Zagar, A.J.; Pinaire, J.A.; Tan, M.H.; Khan, M.A.; Perez, A.T.; Jacober, S.J.; et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005, 28, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Barylski, M.; Toth, P.P.; Nikolic, D.; Banach, M.; Rizzo, M.; Montalto, G. Emerging therapies for raising high-density lipoprotein cholesterol (HDL-C) and augmenting HDL particle functionality. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 453–461. [Google Scholar] [CrossRef]
- Toth, P.P.; Barylski, M.; Nikolic, D.; Rizzo, M.; Montalto, G.; Banach, M. Should low high-density lipoprotein cholesterol (HDL-C) be treated? Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 353–368. [Google Scholar] [CrossRef]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Ž.; Mancini, J.; Rizzo, M.; Mitchenko, O.; et al. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef]
- Rizzo, M.; Spinas, G.A.; Cesur, M.; Ozbalkan, Z.; Rini, G.B.; Berneis, K. Atherogenic lipoprotein phenotype and LDL size and subclasses in drug-naïve patients with early rheumatoid arthritis. Atherosclerosis 2009, 207, 502–506. [Google Scholar] [CrossRef]
- Rizzo, M.; Rini, G.; Berneis, K. The clinical relevance of LDL size and subclasses modulation in patients with type-2 diabetes. Exp. Clin. Endocrinol. Diabetes 2007, 115, 477–482. [Google Scholar] [CrossRef]
- Superko, H.; Garrett, B. Small Dense LDL: Scientific Background, Clinical Relevance, and Recent Evidence Still a Risk Even with ‘Normal’ LDL-C Levels. Biomedicines 2022, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Nicholls, S.J.; Wolski, K.; Nesto, R.; Kupfer, S.; Perez, A.; Jure, H.; Larochellière, R.D.; Staniloae, C.S.; Mavromatis, K.; et al. PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: The PERISCOPE randomized controlled trial. JAMA 2008, 299, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, T.; Meyer, P.M.; Feinstein, S.B.; Davidson, M.H.; Kondos, G.T.; D’Agostino, R.B.; Perez, A.; Provost, J.-C.; Haffner, S.M. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: A randomized trial. JAMA 2006, 296, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.J.; Sun, Y.; Muo, C.H.; Chen, R.C.; Chen, P.C.; Hsu, C.Y. Risk of stroke with thiazolidinediones: A ten-year nationwide population-based cohort study. Cerebrovasc. Dis. 2013, 36, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H. Do thiazolidinediones cause heart failure? A critical review. Clevel. Clin. J. Med. 2006, 73, 390–397. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H. The Low-Dose (7.5 mg/day) Pioglitazone Therapy. J. Clin. Med. Res. 2017, 9, 821–825. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Bakris, G.L. Protection of the kidney by thiazolidinediones: An assessment from bench to bedside. Kidney Int. 2006, 70, 1223–1233. [Google Scholar] [CrossRef]
- Nicholas, S.B.; Kawano, Y.; Wakino, S.; Collins, A.R.; Hsueh, W.A. Expression and function of peroxisome proliferator-activated receptor-gamma in mesangial cells. Hypertension 2001, 37, 722–727. [Google Scholar] [CrossRef][Green Version]
- Guo, B.; Koya, D.; Isono, M.; Sugimoto, T.; Kashiwagi, A.; Haneda, M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes 2004, 53, 200–208. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Lasaridis, A.N. Insulin resistance and endothelin: Another pathway for renal injury in patients with the cardiometabolic syndrome? J. Cardiometab. Syndr. 2008, 3, 183–187. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macrovascular events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Vaccaro, O.; Masulli, M.; Nicolucci, A.; Bonora, E.; Del Prato, S.; Maggioni, A.P.; Rivellese, A.A.; Squatrito, S.; Giorda, C.B.; Sesti, G.; et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): A randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017, 5, 887–897. [Google Scholar] [CrossRef]
- Home, P.D.; Pocock, S.J.; Beck-Nielsen, H.; Curtis, P.S.; Gomis, R.; Hanefeld, M.; Jones, N.P.; Komajda, M.; McMurray, J.J. RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet 2009, 373, 2125–2135. [Google Scholar] [CrossRef]
- Komajda, M.; McMurray, J.J.; Beck-Nielsen, H.; Gomis, R.; Hanefeld, M.; Pocock, S.J.; Curtis, P.S.; Jones, N.P.; Home, P.D. Heart failure events with rosiglitazone in type 2 diabetes: Data from the RECORD clinical trial. Eur. Heart J. 2010, 31, 824–831. [Google Scholar] [CrossRef]
- Savage, D.B.; Tan, G.D.; Acerini, C.L.; Jebb, S.A.; Agostini, M.; Gurnell, M.; Williams, R.L.; Umpleby, A.M.; Thomas, E.L.; Bell, J.D.; et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes 2003, 52, 910.e7. [Google Scholar] [CrossRef] [PubMed]
- Dumasia, R.; Eagle, K.A.; Kline-Rogers, E.; May, N.; Cho, L.; Mukherjee, D. Role of PPAR- gamma agonist thiazolidinediones in treatment of pre-diabetic and diabetic individuals: A cardiovascular perspective. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 377–386. [Google Scholar] [CrossRef]
- Van Wagner, L.B.; Rinella, M.E. The role of insulin-sensitizing agents in the treatment of nonalcoholic steatohepatitis. Ther. Adv. Gastroenterol. 2011, 4, 249–263. [Google Scholar] [CrossRef]
- Chang, E.; Park, C.Y.; Park, S.W. Role of thiazolidinediones, insulin sensitizers, in non-alcoholic fatty liver disease. J. Diabetes Investig. 2013, 4, 517–524. [Google Scholar]
- Boettcher, E.; Csako, G.; Pucino, F.; Wesley, R.; Loomba, R. Meta-analysis: Pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2012, 35, 66–75. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef]
- Ratziu, V.; Giral, P.; Jacqueminet, S.; Charlotte, F.; Hartemann–Heurtier, A.; Serfaty, L.; Podevin, P.; Lacorte, J.M.; Bernhardt, C.; Bruckert, E.; et al. Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology 2008, 135, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Goyal, O.; Nohria, S.; Goyal, P.; Kaur, J.; Sharma, S.; Sood, A.; Chhina, R.S. Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: A prospective, observational, real world study. Sci. Rep. 2020, 10, 21117. [Google Scholar] [CrossRef]
- Patti, A.M.; Giglio, R.V.; Pafili, K.; Rizzo, M.; Papanas, N. Pharmacotherapy for gestational diabetes. Expert. Opin. Pharmacother. 2018, 19, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Pafili, K.; Papanas, N.; Rizzo, M. Metabolic disorders during pregnancy and postpartum cardiometabolic risk. Endocr. Connect. 2018, 7, E1–E4. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H. Gestational Diabetes mellitus. J. Clin. Investig. 2005, 115, 485–491. [Google Scholar] [CrossRef]
- Kahn, S.E.; Zinman, B.; Lachin, J.M.; Haffner, S.M.; Herman, W.H.; Holman, R.R.; Kravitz, B.G.; Yu, D.; Heise, M.A.; Aftring, R.P.; et al. Rosiglitazone-associated fractures in type 2 diabetes: An Analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 2008, 31, 845–851. [Google Scholar] [CrossRef]
- Xiang, A.H.; Peters, R.K.; Kjos, S.L.; Marroquin, A.; Goico, J.; Ochoa, C.; Kawakubo, M.; Buchanan, T.A. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006, 55, 517–522. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Xing, C.; Li, C.; He, B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J. Clin. Endocrinol. Metab. 2020, 105, 2950–2963. [Google Scholar] [CrossRef]
- Wilding, J. Thiazolidinediones, insulin resistance and obesity: Finding a balance. Int. J. Clin. Pract. 2006, 60, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Efficacy of metformin and pioglitazone metformin in the treatment of obese polycystic ovary syndrome. J. Matern. Child Health Care China 2017, 32, 3887–3889. [Google Scholar]
- Brannian, J.D.; Eyster, K.M.; Weber, M.; Diggins, M. Pioglitazone administration alters ovarian gene expression in aging obese lethal yellow mice. Reprod. Biol. Endocrinol. 2008, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Yu, Y.X.; Liu, C.Q.; Zhang, W.; Zhang, H.J.; Yan, B.; Wang, L.Y.; Yang, S.Y.; Zhang, S.H. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: A meta-analysis. Clin. Endocrinol. 2011, 74, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Zhang, Z.M. Effects of metformin and pioglitazone on sexual hormones and metabolic level in patients with polycystic ovary syndrome. J. Guangxi Med. J. 2019, 160, 148–152. [Google Scholar]
- Garg, A. Clinical review: Lipodystrophies: Genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab. 2011, 96, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Vilar, D.; Santini, F. Diagnosis and treatment of lipodystrophy: A step-by-step approach. J. Endocrinol. Investig. 2019, 42, 61–73. [Google Scholar] [CrossRef]
- Foss-Freitas, M.C.; Akinci, B.; Luo, Y.; Stratton, A.; Oral, E.A. Diagnostic strategies and clinical management of lipodystrophy. Expert Rev. Endocrinol. Metab. 2020, 15, 95–114. [Google Scholar] [CrossRef]
- Agostini, M.; Schoenmakers, E.; Beig, J.; Fairall, L.; Szatmari, I.; Rajanayagam, O.; Muskett, F.W.; Adams, C.; Marais, A.D.; O’Rahilly, S.; et al. A pharmacogenetic approach to the treatment of patients with PPARG mutations. Diabetes 2018, 67, 1086–1092. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.C.; Mikhailidis, D.P.; Toth, P.P.; Muntner, P.; Ursoniu, S.; Mosterou, S.; Glasser, S.; Martin, S.S.; Jones, S.R.; et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: A systematic review and meta-analysis. Pharmacol. Res. 2016, 103, 236–252. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Obradovic, M.; Montalto, G.; Rysz, J.; Mikhailidis, D.P.; Isenovic, E.R. PCSK9 inhibition—A novel mechanism to treat lipid disorders? Curr. Pharm. Des. 2013, 19, 3869–3877. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, A.; Boschmann, M.; Colpe, C.; Engeli, S.; Adams, F.; Birkenfeld, A.L.; Haufe, S.; Rahn, G.; Luft, F.C.; Schmidt, H.H.-J.; et al. Thiazolidinedione response in familial lipodystrophy patients with LMNA mutations: A case series. Horm. Metab. Res. 2012, 44, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Wang, G. Beneficial effects of pioglitazone on retardation of persistent atrial fibrillation progression in Diabetes mellitus patients. Int. Heart J. 2014, 55, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Wang, Q.X.; Guo, M.; Liu, F.; Song, Z.P.; Zhang, D.D. Beneficial effects of pioglitazone on atrial structural and electrical remodeling in vitro cellular models. J. Mol. Cell. Cardiol. 2013, 65, 1–8. [Google Scholar] [CrossRef]
- Xu, D.; Murakoshi, N.; Igarashi, M.; Hirayama, A.; Ito, Y.; Seo, Y.; Tada, H.; Aonuma, K. PPAR-gamma activator pioglitazone prevents age-related atrial fibrillation susceptibility by improving antioxidant capacity and reducing apoptosis in a rat model. J. Cardiovasc. Electrophysiol. 2012, 23, 209–217. [Google Scholar] [CrossRef]
- Brakedal, B.; Flønes, I.; Reiter, S.F.; Torkildsen, Ø.; Dölle, C.; Assmus, J.; Haugarvoll, K.; Tzoulis, C. Glitazone use associated with reduced risk of Parkinson’s disease. Mov. Disord. 2017, 32, 1594–1599. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Carta, A.R.; Frau, L.; Pisanu, A.; Wardas, J.; Spiga, S.; Carboni, E. Rosiglitazone decreases peroxisome proliferator receptor-gamma levels in microglia and inhibits TNF-alpha production: New evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience 2011, 194, 250–261. [Google Scholar] [CrossRef]
- Prost, S.; Relouzat, F.; Spentchian, M.; Ouzegdouh, Y.; Saliba, J.; Massonnet, G.; Beressi, J.-P.; Verhoeyen, E.; Raggueneau, V.; Maneglier, B.; et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature 2015, 525, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Mrowka, P.; Glodkowska-Mrowka, E. PPARγ Agonists in Combination Cancer Therapies. Curr. Cancer Drug Targets 2020, 20, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Negrotto, L.; Farez, M.F.; Correale, J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 2016, 73, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.S.; Manzano, A.; Olivar, L.C.; Nava, M.; Salazar, J.; D’Marco, L.; Ortiz, R.; Chacín, M.; Guerrero-Wyss, M.; de Bravo, M.C.; et al. The Role of the α Cell in the Pathogenesis of Diabetes: A World beyond the Mirror. Int. J. Mol. Sci. 2021, 22, 9504. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; Puckett, C.; Adams, J.; Khattab, A.; Baskoy, G.; Cersosimo, E.; Triplitt, C.; DeFronzo, R.A. Durability of Triple Combination Therapy Versus Stepwise Addition Therapy in Patients with New-Onset T2DM: 3-Year Follow-up of EDICT. Diabetes Care 2021, 44, 433–439. [Google Scholar] [CrossRef]
- DeRosa, G.; D’Angelo, A.; Salvadeo, S.A.; Ferrari, I.; Fogari, E.; Gravina, A.; Mereu, R.; Palumbo, I.; Maffioli, P.; Randazzo, S.; et al. Modulation of adipokines and vascular remodelling markers during OGTT with acarbose or pioglitazone treatment. Biomed. Pharmacother. 2009, 63, 723–733. [Google Scholar] [CrossRef]
- Abate, N.; Sallam, H.S.; Rizzo, M.; Nikolic, D.; Obradovic, M.; Bjelogrlic, P.; Isenovic, E.R. Resistin: An Inflammatory Cytokine. Role in Cardiovascular Diseases, Diabetes and the Metabolic Syndrome. Curr. Pharm. Des. 2014, 20, 4961–4969. [Google Scholar] [CrossRef]
- Ceriello, A.; Stoian, A.P.; Rizzo, M. COVID-19 and diabetes management: What should be considered? Diabetes Res. Clin. Pract 2020, 163, 108151. [Google Scholar] [CrossRef]
- Al Mahmeed, W.; Al-Rasadi, K.; Banerjee, Y.; Ceriello, A.; Cosentino, F.; Galia, M.; Goh, S.-Y.; Kempler, P.; Lessan, N.; Papanas, N.; et al. CArdiometabolic Panel of International experts on Syndemic COVID-19 (CAPISCO). Promoting a Syndemic Approach for Cardiometabolic Disease Management during COVID-19: The CAPISCO International Expert Panel. Front. Cardiovasc. Med. 2021, 8, 787761. [Google Scholar] [CrossRef]
- Colca, J.R.; McDonald, W.G.; Waldon, D.J.; Leone, J.W.; Lull, J.M.; Bannow, C.A.; Lund, E.T.; Mathews, W.R. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E252–E260. [Google Scholar] [CrossRef]
- Paddock, M.L.; Wiley, S.E.; Axelrod, H.L.; Cohen, A.E.; Roy, M.; Abresch, E.C.; Capraro, D.; Murphy, A.N.; Nechushtai, R.; Dixon, J.E.; et al. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc. Natl. Acad. Sci. USA 2007, 104, 14342–14347. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giglio, R.V.; Papanas, N.; Rizvi, A.A.; Ciaccio, M.; Patti, A.M.; Ilias, I.; Pantea Stoian, A.; Sahebkar, A.; Janez, A.; Rizzo, M. An Update on the Current and Emerging Use of Thiazolidinediones for Type 2 Diabetes. Medicina 2022, 58, 1475. https://doi.org/10.3390/medicina58101475
Giglio RV, Papanas N, Rizvi AA, Ciaccio M, Patti AM, Ilias I, Pantea Stoian A, Sahebkar A, Janez A, Rizzo M. An Update on the Current and Emerging Use of Thiazolidinediones for Type 2 Diabetes. Medicina. 2022; 58(10):1475. https://doi.org/10.3390/medicina58101475
Chicago/Turabian StyleGiglio, Rosaria Vincenza, Nikolaos Papanas, Ali Abbas Rizvi, Marcello Ciaccio, Angelo Maria Patti, Ioannis Ilias, Anca Pantea Stoian, Amirhossein Sahebkar, Andrej Janez, and Manfredi Rizzo. 2022. "An Update on the Current and Emerging Use of Thiazolidinediones for Type 2 Diabetes" Medicina 58, no. 10: 1475. https://doi.org/10.3390/medicina58101475
APA StyleGiglio, R. V., Papanas, N., Rizvi, A. A., Ciaccio, M., Patti, A. M., Ilias, I., Pantea Stoian, A., Sahebkar, A., Janez, A., & Rizzo, M. (2022). An Update on the Current and Emerging Use of Thiazolidinediones for Type 2 Diabetes. Medicina, 58(10), 1475. https://doi.org/10.3390/medicina58101475













