Application Value of Systemic Inflammatory Indexes in the Clinical Evaluation of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF)
Abstract
1. Introduction
2. Method
2.1. Study Population
2.2. Blood Indexes
2.3. Echocardiography
2.4. Evaluation of NYHA Functional Classification
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison of SII Levels
3.3. Regression Analysis
3.3.1. Regression Analysis for HF
3.3.2. Regression Analysis for Increased NYHA Functional Classification
3.4. Correlation Analysis
3.4.1. Correlation Analysis between SII and NYHA Functional Classification
3.4.2. Correlation Analysis between SII and Echocardiography Indexes
3.5. Diagnosis of HF
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L., Jr. Heart failure with reduced ejection fraction: A review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, Z.; Zhao, S. Baseline levels of serum high sensitivity C reactive protein and lipids in predicting the residual risk of cardiovascular events in Chinese population with stable coronary artery disease: A prospective cohort study. Lipids Health Dis. 2018, 17, 273. [Google Scholar] [CrossRef]
- Haybar, H.; Pezeshki, S.M.S.; Saki, N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Lipids Health Dis. 2019, 110, 104267. [Google Scholar] [CrossRef]
- Duan, Z.; Luo, C.; Fu, B.; Han, D. Association between fibrinogen-to-albumin ratio and the presence and severity of coronary artery disease in patients with acute coronary syndrome. BMC Cardiovasc. Disord. 2021, 21, 588. [Google Scholar] [CrossRef]
- Yano, M.; Nishino, M.; Ukita, K.; Kawamura, A.; Nakamura, H.; Matsuhiro, Y.; Yasumoto, K.; Tsuda, M.; Okamoto, N.; Tanaka, A.; et al. High density lipoprotein cholesterol/C reactive protein ratio in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef]
- Tromp, J.; Khan, M.A.; Klip, I.T.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J. Am. Heart Assoc. 2017, 6, e003989. [Google Scholar] [CrossRef]
- Tromp, J.; Westenbrink, B.D.; Ouwerkerk, W.; van Veldhuisen, D.J.; Samani, N.J.; Ponikowski, P.; Metra, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; et al. Identifying Pathophysiological Mechanisms in Heart Failure With Reduced Versus Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2018, 72, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Summary of Prices of Basic Medical Services in Public Medical Institutions in Guangzhou (2021 Version). Available online: https://www.gy120.net/newsshow.asp?articleid=6440 (accessed on 7 September 2022).
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Abernethy, A.; Raza, S.; Sun, J.L.; Anstrom, K.J.; Tracy, R.; Steiner, J.; VanBuren, P.; LeWinter, M.M. Pro-inflammatory biomarkers in stable versus acutely decompensated heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2018, 7, e007385. [Google Scholar] [CrossRef]
- Iqbal, N.; Wentworth, B.; Choudhary, R.; Landa Ade, L.; Kipper, B.; Fard, A.; Maisel, A.S. Cardiac biomarkers: New tools for heart failure management. Cardiovasc. Diagn. Ther. 2012, 2, 147–164. [Google Scholar] [CrossRef]
- Silva, N.; Bettencourt, P.; Guimarães, J.T. The lymphocyte-to-monocyte ratio: An added value for death prediction in heart failure. Nutr. Metab. Cardiovasc. 2015, 25, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Horgan, S.; Baugh, J.A. Monocyte and macrophage subsets along the continuum to heart failure: Misguided heroes or targetable villains? J. Mol. Cell. Cardiol. 2015, 89, 136–145. [Google Scholar] [CrossRef]
- Gary, T.; Pichler, M.; Belaj, K.; Eller, P.; Hafner, F.; Gerger, A.; Brodmann, M. Lymphocyte-to-monocyte ratio: A novel marker for critical limb ischemia in PAOD patients. Int. J. Clin. Pract. 2014, 68, 1483–1487. [Google Scholar] [CrossRef]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Parissis, J.T.; Kremastinos, D.T. A glossary of circulating cytokines in chronic heart failure. Eur. J. Heart Fail. 2001, 3, 517–526. [Google Scholar] [CrossRef]
- Wei, L. Immunological aspect of cardiac remodeling: T lymphocyte subsets in inflammation-mediated cardiac fibrosis. Exp. Mol. Pathol. 2011, 90, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Damås, J.K.; Oie, E.; Ueland, T.; Gullestad, L.; Aukrust, P. Systemic inflammation in heart failure--the whys and wherefores. Heart Fail. Rev. 2006, 11, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, L.; Hare, J.M. Inflammatory cytokines in heart failure: Roles in aetiology and utility as biomarkers. Eur. Heart J. 2010, 31, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D. Cytokine-induced modulation of cardiac function. Circ. Res. 2004, 95, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Korantzopoulos, P.; Kolettis, T.; Siogas, K.; Goudevenos, J. Atrial fibrillation and electrical remodeling: The potential role of inflammation and oxidative stress. Med. Sci. Monit. 2003, 9, Ra225–Ra229. [Google Scholar] [PubMed]
- Wrigley, B.J.; Lip, G.Y.; Shantsila, E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur. J. Heart Fail. 2011, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, S.; Lip, G.Y.; Shantsila, E. Monocytes in heart failure: Relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc. Res. 2010, 85, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, F.; Rauch, P.J.; Ueno, T.; Gorbatov, R.; Marinelli, B.; Lee, W.W.; Dutta, P.; Wei, Y.; Robbins, C.; Iwamoto, Y.; et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 2012, 209, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lee, K.; Li, N.; Corbett, D.; Mendoza, L.; Frangogiannis, N.G. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem. Cell Biol. 2009, 131, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, F.; Kai, H.; Tokuda, K.; Takeya, M.; Takeshita, A.; Egashira, K.; Imaizumi, T. Hypertensive myocardial fibrosis and diastolic dysfunction: Another model of inflammation? Hypertension 2004, 43, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Kuwahara, F.; Tokuda, K.; Imaizumi, T. Diastolic dysfunction in hypertensive hearts: Roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens. Res. 2005, 28, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Voon, V.; Watson, C.; Horgan, S.; McDonald, K.; Ledwidge, M.; Baugh, J. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: Evidence of M2 macrophage activation in disease pathogenesis. J. Card. Fail. 2015, 21, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Sager, H.B.; Roh, J.D.; Valero-Muñoz, M.; Houstis, N.E.; Iwamoto, Y.; Sun, Y.; Wilson, R.M.; Wojtkiewicz, G.; Tricot, B.; et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018, 215, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef]
- Núñez, J.; Núñez, E.; Miñana, G.; Sanchis, J.; Bodí, V.; Rumiz, E.; Palau, P.; Olivares, M.; Merlos, P.; Bonanad, C.; et al. Effectiveness of the relative lymphocyte count to predict one-year mortality in patients with acute heart failure. Am. J. Cardiol. 2011, 107, 1034–1039. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Ambrosy, A.P.; Greene, S.J.; Mentz, R.J.; Subacius, H.P.; Maggioni, A.P.; Swedberg, K.; Nodari, S.; Zannad, F.; Konstam, M.A.; et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: Insights from the EVEREST trial. Circ. Heart Fail. 2012, 5, 750–758. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef]
- Shantsila, E.; Bialiuk, N.; Navitski, D.; Pyrochkin, A.; Gill, P.S.; Pyrochkin, V.; Snezhitskiy, V.; Lip, G.Y. Blood leukocytes in heart failure with preserved ejection fraction: Impact on prognosis. Int. J. Cardiol. 2012, 155, 337–338. [Google Scholar] [CrossRef]
- Dixon, D.L.; Griggs, K.M.; Bersten, A.D.; De Pasquale, C.G. Systemic inflammation and cell activation reflects morbidity in chronic heart failure. Cytokine 2011, 56, 593–599. [Google Scholar] [CrossRef]
- Van de Wouw, J.; Broekhuizen, M.; Sorop, O.; Joles, J.A.; Verhaar, M.C.; Duncker, D.J.; Danser, A.H.J.; Merkus, D. Chronic kidney disease as a risk factor for heart failure with preserved ejection fraction: A focus on microcirculatory factors and therapeutic targets. Front. Physiol. 2019, 10, 1108. [Google Scholar] [CrossRef]
- Schiller, G.J.; Berkman, S.A. Hematologic aspects of renal insufficiency. Blood Rev. 1989, 3, 141–146. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.S.; Fayyaz, A.U.; de Denus, S.; Felker, G.M.; Borlaug, B.A.; Dasari, S.; Carter, R.E.; Redfield, M.M. Obese-inflammatory phenotypes in heart failure with preserved ejection fraction. Circ. Heart Fail. 2020, 13, e006414. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J. Epidemiology, pathophysiology, diagnosis and treatment of heart failure in diabetes. Diabetes Metab. J. 2021, 45, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tan, C.; Liu, X.; Chen, Y. Association between the neutrophil-to-lymphocyte ratio and diabetes secondary to exocrine pancreatic disorders. Front. Endocrinol. 2022, 13, 957129. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | NYHA I (n = 48) | NYHA II (n = 53) | NYHA III (n = 56) | NYHA IV (n = 32) | p | NYHA II–IV (n = 141) | p |
|---|---|---|---|---|---|---|---|
| Age (years) | 59.02 ± 10.89 | 66.87 ± 11.38 | 72.64 ± 13.57 | 69.77 ± 14.37 | 0.000 * | 69.82 ± 13.12 | 0.000 * |
| Male, n (%) | 34 (71) | 28 (53) | 27 (48) | 17 (53) | 0.097 | 72 (51) | 0.017 * |
| BMI (kg/m2) | 24.90 ± 3.21 | 25.02 ± 3.40 | 24.23 ± 5.00 | 24.42 ± 10.05 | 0.831 | 24.59 ± 5.85 | 0.239 |
| Smoker, n (%) | 6 (13) | 9 (17) | 6 (11) | 4 (13) | 0.802 | 19 (13) | 0.863 |
| heart rate, (n/min) | 74.52 ± 10.87 | 79.38 ± 16.45 | 75.29 ± 13.00 | 87.09 ± 19.13 | 0.001 * | 79.51 ± 16.38 | 0.051 |
| Alcohol drinker, n (%) | 2 (4) | 7 (13) | 0 (0) | 3 (9) | 0.031 * | 10 (7) | 0.473 |
| WBC (×109/L) | 6.73 ± 1.53 | 6.91 ± 2.04 | 6.82 ± 2.22 | 7.81 ± 3.09 | 0.344 | 7.08 ± 2.40 | 0.809 |
| LYM (×109/L) | 1.97 ± 0.68 | 1.74 ± 0.62 | 1.30 ± 0.49 | 1.37 ± 0.49 | 0.000 * | 1.48 ± 0.58 | 0.000 * |
| MONO (×109/L) | 0.46 ± 0.15 | 0.46 ± 0.16 | 0.54 ± 0.20 | 0.88 ± 1.33 | 0.029 * | 0.59 ± 0.67 | 0.182 |
| NEUT (×109/L) | 4.07 ± 1.25 | 4.53 ± 1.78 | 4.76 ± 2.03 | 5.49 ± 2.68 | 0.019 * | 4.84 ± 2.13 | 0.066 |
| PLT (×109/L) | 228.28 ± 52.40 | 222.69 ± 54.53 | 200.82 ± 58.36 | 224.19 ± 110.98 | 0.077 | 214.29 ± 72.85 | 0.222 |
| CRP (mg/dL) | 5.17 ± 11.78 | 4.89 ± 8.28 | 7.49 ± 13.31 | 33.59 ± 45.86 | 0.076 | 12.51 ± 26.33 | 0.158 |
| PLT volume (fL) | 9.22 ± 0.84 | 8.99 ± 1.17 | 9.41 ± 1.45 | 9.37 ± 1.36 | 0.307 | 9.24 ± 1.33 | 0.740 |
| ALB (g/L) | 40.58 ± 6.98 | 40.85 ± 3.75 | 36.57 ± 4.62 | 36.29 ± 4.48 | 0.000 * | 38.17 ± 4.75 | 0.010 * |
| γ-GGT (U/L) | 34.00 ± 38.53 | 28.66 ± 19.68 | 34.50 ± 26.03 | 78.59 ± 75.17 | 0.007 * | 41.63 ± 44.35 | 0.308 |
| HDL-c (mmol/L) | 1.16 ± 0.39 | 1.15 ± 0.28 | 1.07 ± 0.35 | 1.13 ± 0.29 | 0.384 | 1.11 ± 0.31 | 0.429 |
| FIB (g/L) | 3.22 ± 0.81 | 3.35 ± 0.62 | 3.55 ± 1.16 | 4.18 ± 1.33 | 0.007 * | 3.61 ± 1.07 | 0.035 * |
| Scr (umol/L) | 79.42 ± 23.85 | 79.30 ± 26.41 | 112.64 ± 61.76 | 112.24 ± 49.02 | 0.000 * | 99.84 ± 50.29 | 0.015 * |
| AST (U/L) | 23.44 ± 5.87 | 23.62 ± 9.82 | 24.82 ± 11.73 | 46.45 ± 51.97 | 0.103 | 29.19 ± 27.64 | 0.952 |
| CKMB (U/L) | 20.39 ± 37.39 | 18.67 ± 6.89 | 16.96 ± 10.57 | 23.94 ± 19.55 | 0.523 | 19.21 ± 12.37 | 0.750 |
| CTNI (ng/L) | 0.13 ± 0.74 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.37 ± 0.91 | 0.317 | 0.06 ± 0.35 | 0.482 |
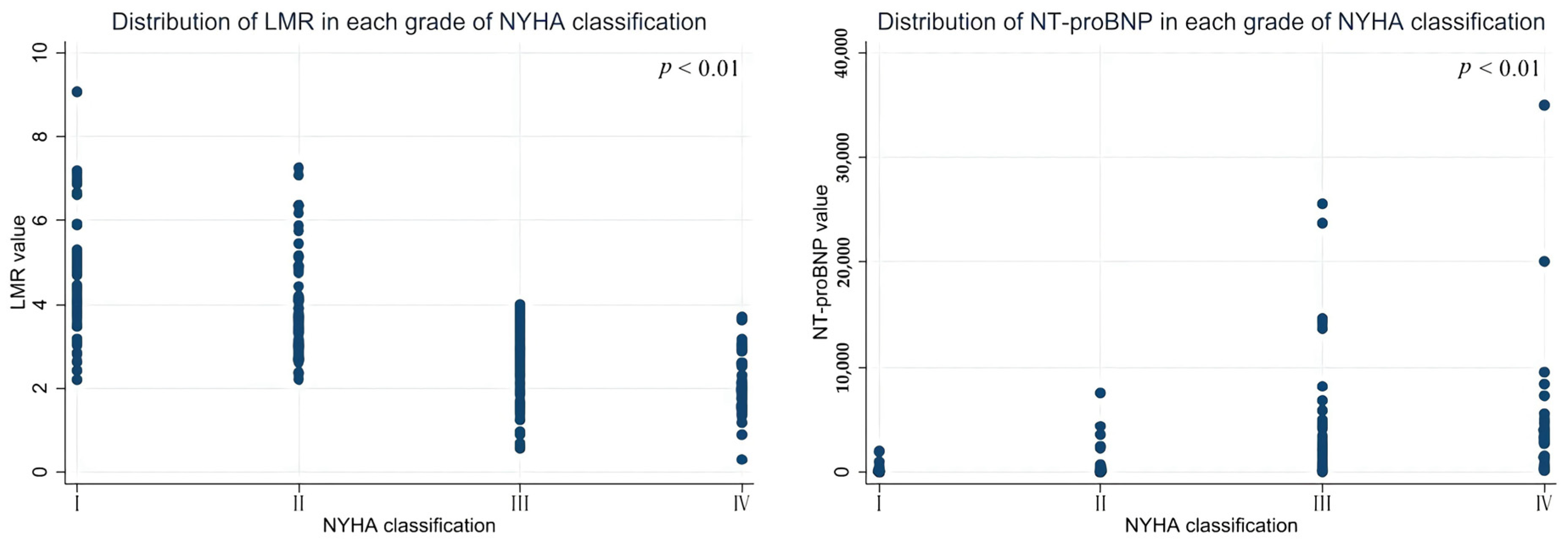
| NT-proBNP | 159.10 ± 321.23 | 572.56 ± 1371.97 | 3376.93 ± 5440.12 | 4880.40 ± 6863.44 | 0.000 * | 2715.09 ± 5115.04 | 0.000 * |
| LAD (mm) | 35.31 ± 4.86 | 37.36 ± 7.47 | 44.77 ± 10.99 | 46.50 ± 10.77 | 0.000 * | 42.38 ± 10.47 | 0.000 * |
| LVDd (mm) | 44.15 ± 3.80 | 43.92 ± 4.97 | 46.64 ± 6.47 | 48.31 ± 9.22 | 0.010 * | 46.00 ± 6.89 | 0.254 |
| LVPWd (mm) | 8.88 ± 1.53 | 9.15 ± 1.57 | 9.59 ± 1.78 | 9.97 ± 2.24 | 0.028 * | 9.51 ± 1.84 | 0.032 * |
| LVEF | 62.00 ± 2.79 | 62.34 ± 4.44 | 61.55 ± 5.67 | 62.25 ± 8.20 | 0.921 | 62.01 ± 7.79 | 0.553 |
| A/E (>1) | 36 (75) | 39 (74) | 42 (75) | 28 (88) | 0.467 | 109 (77) | 0.744 |
| CHD, n (%) | 31 (65) | 41 (77) | 37 (66) | 18 (56) | 0.221 | 96 (68) | 0.655 |
| Hypertension, n (%) | 24 (50) | 36 (68) | 36 (64) | 22 (69) | 0.215 | 94 (67) | 0.039 * |
| Diabetes, n (%) | 6 (13) | 20 (38) | 22 (39) | 8 (25) | 0.010 * | 50 (35) | 0.003 * |
| Hyperlipidemia, n (%) | 4 (8) | 9 (17) | 6 (11) | 1 (3) | 0.218 | 16 (11) | 0.558 |
| Af, n (%) | 1 (2) | 4 (8) | 15 (27) | 11 (36) | 0.000 * | 30 (21) | 0.002 * |
| Af with A/E (>1) | 1 (2) | 3 (6) | 13 (23) | 10 (31) | 0.000 * | 26 (18) | 0.005 * |
| ACEI or ARB, n (%) | 18 (38) | 17 (32) | 13 (23) | 10 (31) | 0.463 | 40 (28) | 0.236 |
| Beta-blocker, n (%) | 16 (33) | 12 (23) | 14 (42) | 10 (22) | 0.605 | 36 (26) | 0.296 |
| Diuretics, n (%) | 1 (2) | 1 (2) | 3 (5) | 6 (19) | 0.006 * | 10 (7) | 0.200 |
| Aldosterone Antagonist, n (%) | 0 (0) | 0 (0) | 2 (4) | 2 (6) | 0.145 | 4 (3) | 0.238 |
| Statin, n (%) | 6 (13) | 8 (15) | 7 (13) | 7 (22) | 0.635 | 22 (16) | 0.601 |
| Metformin, n (%) | 6 (13) | 13 (25) | 6 (11) | 3 (9) | 0.129 | 22 (16) | 0.601 |
| Index | NYHA I | NYHA II | NYHA III | NYHA IV | p | NYHA II–IV | p |
|---|---|---|---|---|---|---|---|
| NLR NLR | 2.389 ± 1.532 | 3.086 ± 2.937 | 4.235 ± 2.856 | 4.341 ± 2.344 | 0.000 * | 3.827 ± 2.821 | 0.000 * |
| PLR | 131.370 ± 55.964 | 144.834 ± 71.566 | 175.866 ± 81.772 | 169.395 ± 69.441 | 0.007 * | 162.861 ± 76.157 | 0.010 * |
| LMR | 4.484 ± 1.447 | 3.946 ± 1.247 | 2.592 ± 0.908 | 2.087 ± 0.768 | 0.000 * | 2.986 ± 1.276 | 0.000 * |
| FAR | 0.096 ± 0.115 | 0.083 ± 0.188 | 0.097 ± 0.034 | 0.118 ± 0.046 | 0.001 * | 0.096 ± 0.035 | 0.967 |
| AGR | 1.769 ± 0.920 | 2.083 ± 1.224 | 1.609 ± 0.916 | 0.934 ± 0.772 | 0.000 * | 1.650 ± 1.104 | 0.521 |
| MHR | 0.444 ± 0.217 | 0.436 ± 0.219 | 0.561 ± 0.289 | 0.819 ± 1.129 | 0.021 * | 0.5711 ± 0.591 | 0.151 |
| WMR | 0.731 ± 0.182 | 0.791 ± 0.290 | 0.741 ± 0.270 | 0.871 ± 0.423 | 0.257 | 0.789 ± 0.319 | 0.739 |
| HCR | 5.012 ± 11.924 | 4.779 ± 8.788 | 6.298 ± 8.628 | 30.145 ± 41.964 | 0.105 | 11.219 ± 23.563 | 0.186 |
| LVEF | 62.000 ± 2.791 | 62.340 ± 4.437 | 61.545 ± 5.666 | 62.250 ± 8.195 | 0.921 | 62.007 ± 7.791 | 0.553 |
| NT-proBNP | 159.119 ± 321.226 | 572.555 ± 1371.969 | 3376.926 ± 5440.117 | 4880.400 ± 6863.435 | 0.000 * | 2715.085 ± 5115.039 | 0.000 * |
| Index | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.917 | 1.038–1.100 | 0.000 * | |||
| Male | 1.917 | 1.150–4.708 | 0.019 * | |||
| Hypertension | 1.917 | 0.980–3.748 | 0.057 | |||
| Diabetes | 3.846 | 1.529–9.674 | 0.004 * | |||
| Scr | 1.015 | 1.004–1.027 | 0.010 * | |||
| NLR | 1.703 | 1.248–2.325 | 0.001 * | 1.388 | 1.031–1.870 | 0.031 * |
| PLR | 1.008 | 1.002–1.014 | 0.012 * | 1.005 | 0.998–1.012 | 0.138 |
| LMR | 0.463 | 0.348–0.617 | 0.000 * | 0.463 | 0.348–0.617 | 0.000 * |
| NT-proBNP | 1.002 | 1.001–1.004 | 0.004 * | 1.002 | 1.000–1.003 | 0.008 * |
| Index | OR | 95% CI | p |
|---|---|---|---|
| NLR | 0.848 | 0.751–0.957 | 0.007 * |
| PLR | 0.996 | 0.993–1.000 | 0.079 |
| LMR | 2.630 | 2.016–3.435 | 0.000 * |
| FAR | 0.002 | 0.000–0.237 | 0.011 * |
| AGR | 1.629 | 1.219–2.175 | 0.001 * |
| MHR | 0.090 | 0.028–0.291 | 0.000 * |
| NT-proBNP | 1.000 | 1.000–1.000 | 0.003 * |
| Spearman | NLR | PLR | LMR | FAR | AGR | MHR | WMR | CHR | LVEF | NT-proBNP |
|---|---|---|---|---|---|---|---|---|---|---|
| r | 0.459 | 0.275 | −0.667 | 0.376 | −0.291 | 0.251 | 0.016 | 0.413 | −0.104 | 0.681 |
| p | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.001 * | 0.832 | 0.000 * | 0.156 | 0.000 * |
| Index | LADs | LVDd | LVPWd | Pearson |
|---|---|---|---|---|
| LMR | −0.359 | −0.213 | −0.180 | r |
| 0.000 * | 0.003 * | 0.013 * | p | |
| FAR | 0.154 | r | ||
| 0.045 * | p | |||
| AGR | −0.283 | r | ||
| 0.000 * | p | |||
| NT-proBNP | 0.315 | 0.279 | 0.156 | r |
| 0.000 * | 0.000 * | 0.041 * | p |
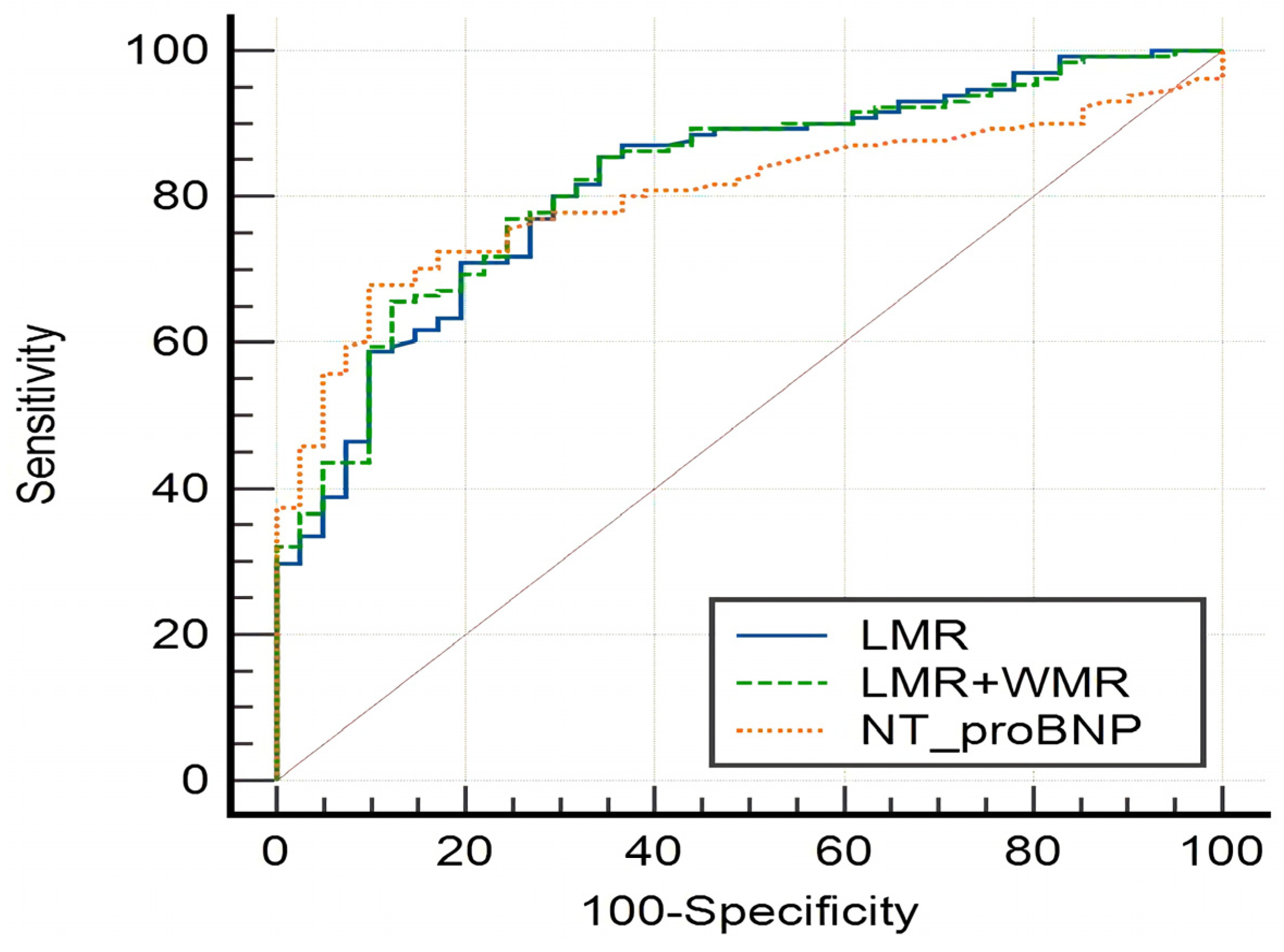
| Index | Youden | Critical Value | Sensitivity | Specificity | AUC | p | 95% CI |
|---|---|---|---|---|---|---|---|
| NLR | 0.4681 | 2.1563 | 78.72 | 68.09 | 0.753 | 0.0001 * | 0.685–0.813 |
| PLR | 0.3054 | 123.1707 | 65.96 | 64.58 | 0.648 | 0.0017 * | 0.575–0.715 |
| LMR | 0.4867 | 3.4516 | 69.50 | 79.17 | 0.803 | 0.0001 * | 0.729–0.849 |
| FAR | 0.2842 | 0.0807 | 57.69 | 70.73 | 0.667 | 0.0009 * | 0.591–0.737 |
| AGR | 0.1985 | 0.7521 | 26.67 | 93.18 | 0.562 | 0.1762 | 0.486–0.636 |
| MHR | 0.2184 | 0.2529 | 89.93 | 31.91 | 0.589 | 0.0737 | 0.514–0.660 |
| WMR | 0.1986 | 0.9519 | 30.50 | 89.36 | 0.516 | 0.7093 | 0.442–0.590 |
| CHR | 0.3581 | 1.5248 | 67.95 | 67.86 | 0.654 | 0.0085 * | 0.556–0.744 |
| LVEF | 0.1999 | 59 | 26.24 | 93.75 | 0.528 | 0.5002 | 0.455–0.601 |
| NT-proBNP | 0.5842 | 219 | 67.94 | 90.48 | 0.805 | 0.0001 * | 0.738–0.861 |
| LMR+WMR | 0.815 | 0.0351 * | 0.752–0.868 | ||||
| LMR+NT-proBNP | 0.841 | 0.0001 * | 0.778–0.892 |
| Index | AUC | Difference | SE | 95% CI | z | p |
|---|---|---|---|---|---|---|
| LMR/NT-proBNP | 0.811/0.805 | 0.00618 | 0.0430 | −0.0781–0.0905 | 0.144 | 0.8858 |
| LMR/LMR+WMR | 0.803/0.815 | 0.00407 | 0.0054 | −0.00651–0.0147 | 0.755 | 0.4505 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wu, J.; Ye, H.; Zhang, X.; Wang, L. Application Value of Systemic Inflammatory Indexes in the Clinical Evaluation of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF). Medicina 2022, 58, 1473. https://doi.org/10.3390/medicina58101473
Wang R, Wu J, Ye H, Zhang X, Wang L. Application Value of Systemic Inflammatory Indexes in the Clinical Evaluation of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF). Medicina. 2022; 58(10):1473. https://doi.org/10.3390/medicina58101473
Chicago/Turabian StyleWang, Ruxin, Juan Wu, Haowen Ye, Xiaofang Zhang, and Lihong Wang. 2022. "Application Value of Systemic Inflammatory Indexes in the Clinical Evaluation of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF)" Medicina 58, no. 10: 1473. https://doi.org/10.3390/medicina58101473
APA StyleWang, R., Wu, J., Ye, H., Zhang, X., & Wang, L. (2022). Application Value of Systemic Inflammatory Indexes in the Clinical Evaluation of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF). Medicina, 58(10), 1473. https://doi.org/10.3390/medicina58101473






