Relationship between Facial Color Changes and Psychological Problems Associated with Lower Back Pain
Abstract
1. Introduction
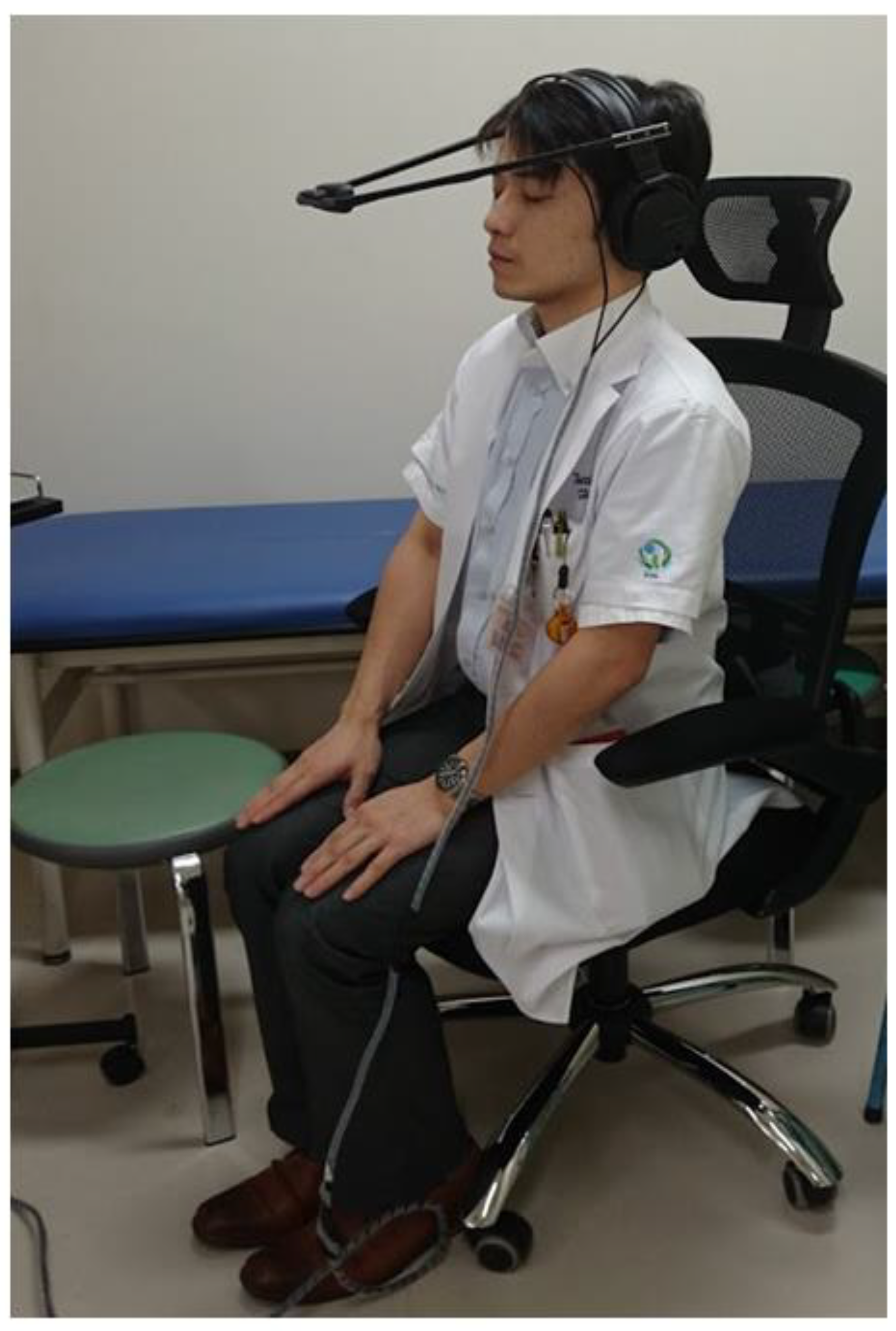
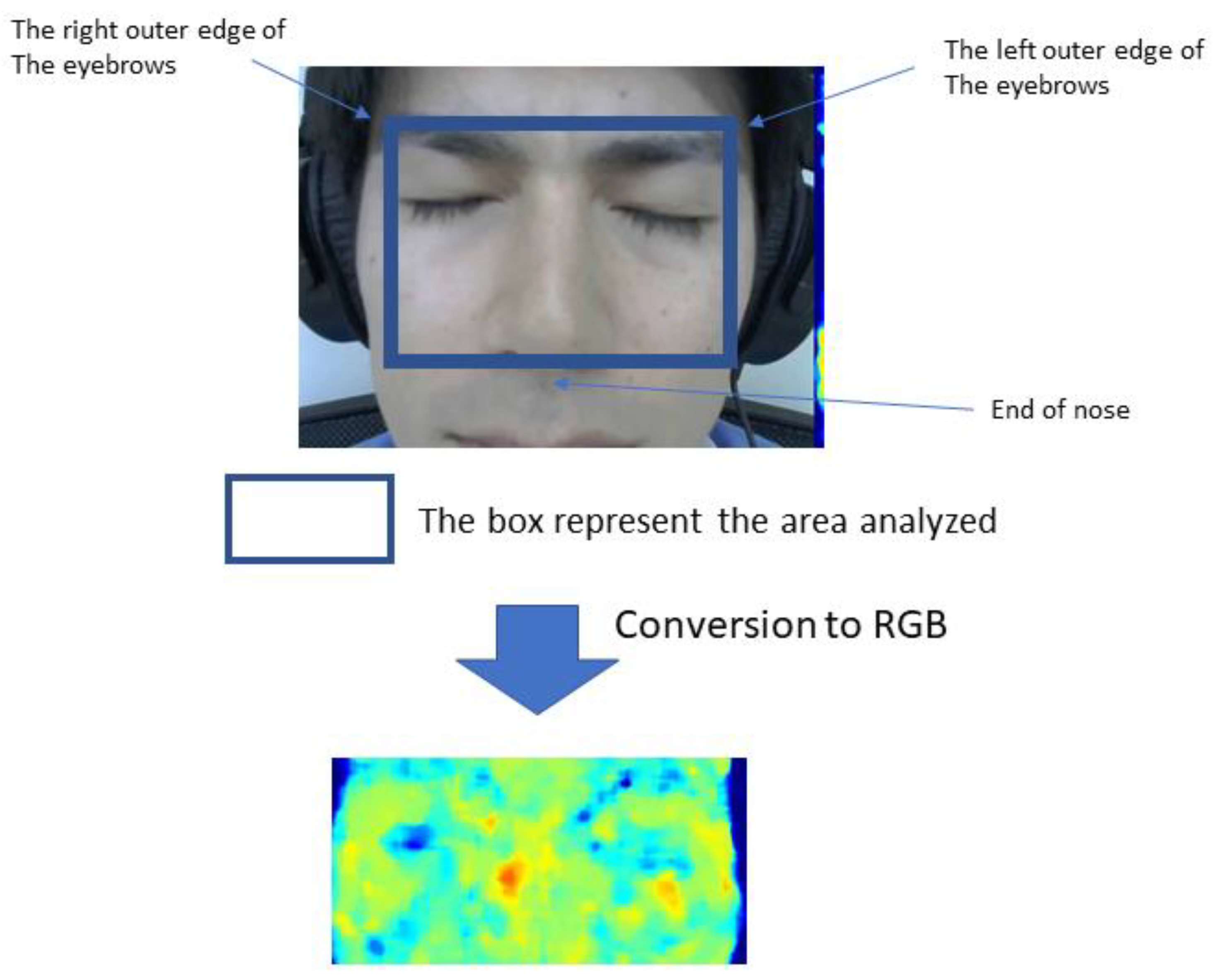
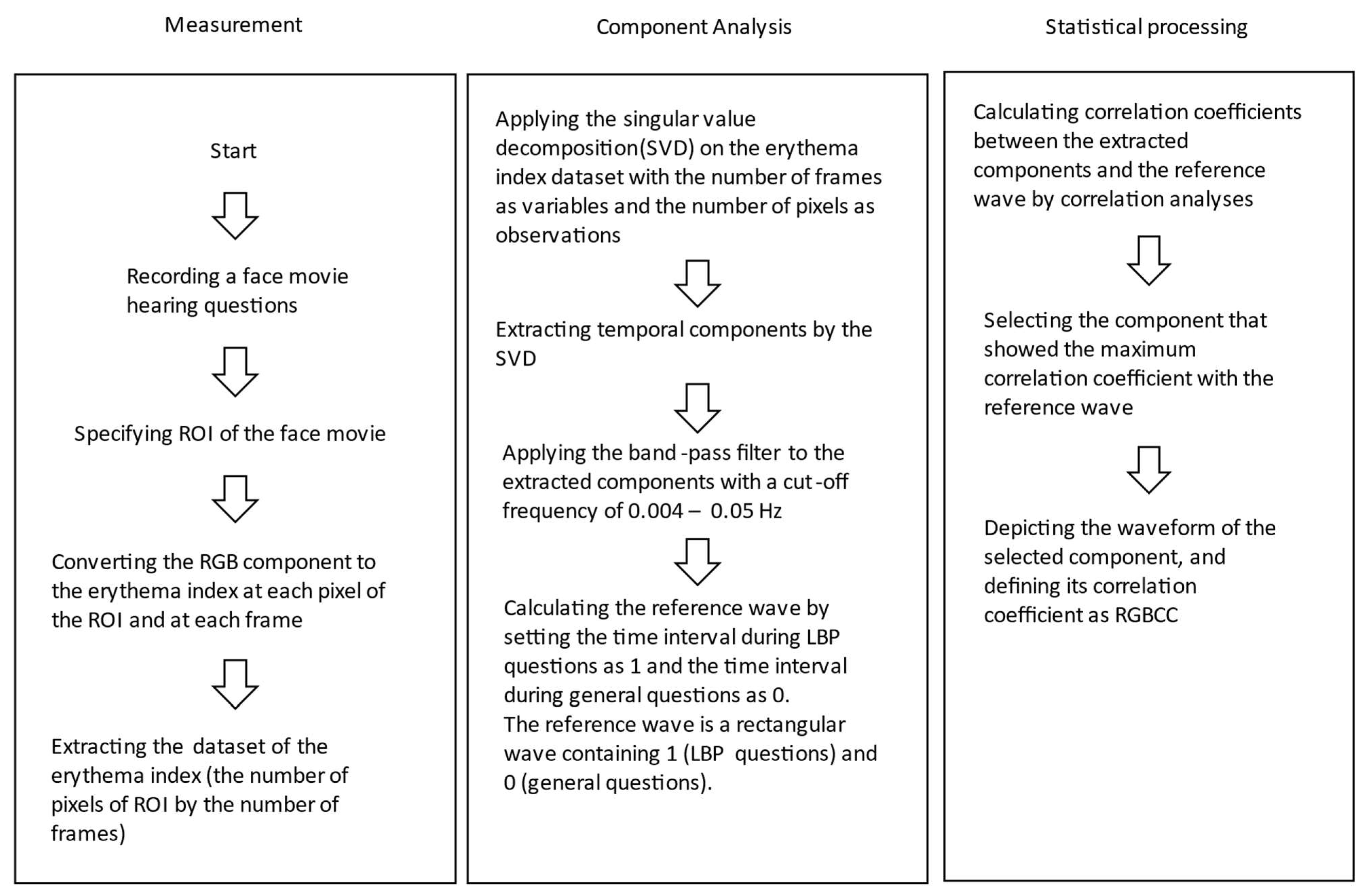
2. Materials and Methods
2.1. Questionnaires
2.2. Statistical Analysis
3. Results
3.1. Sample Characteristics of Chronic Lower Back Pain Patients
3.2. Sample Characteristics of Control Subjects
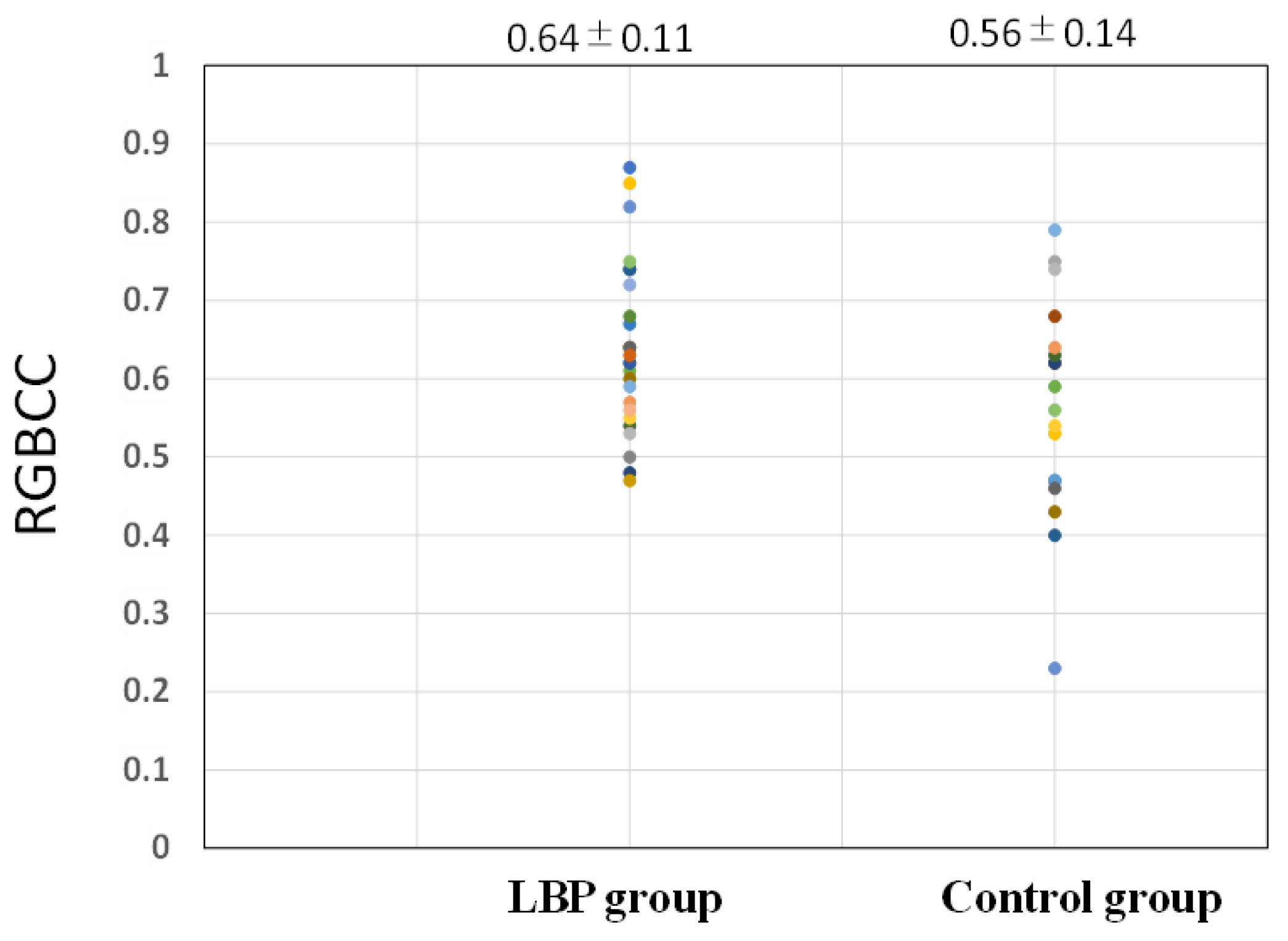
3.3. Comparison of RGBCC between the LBP and Control Groups
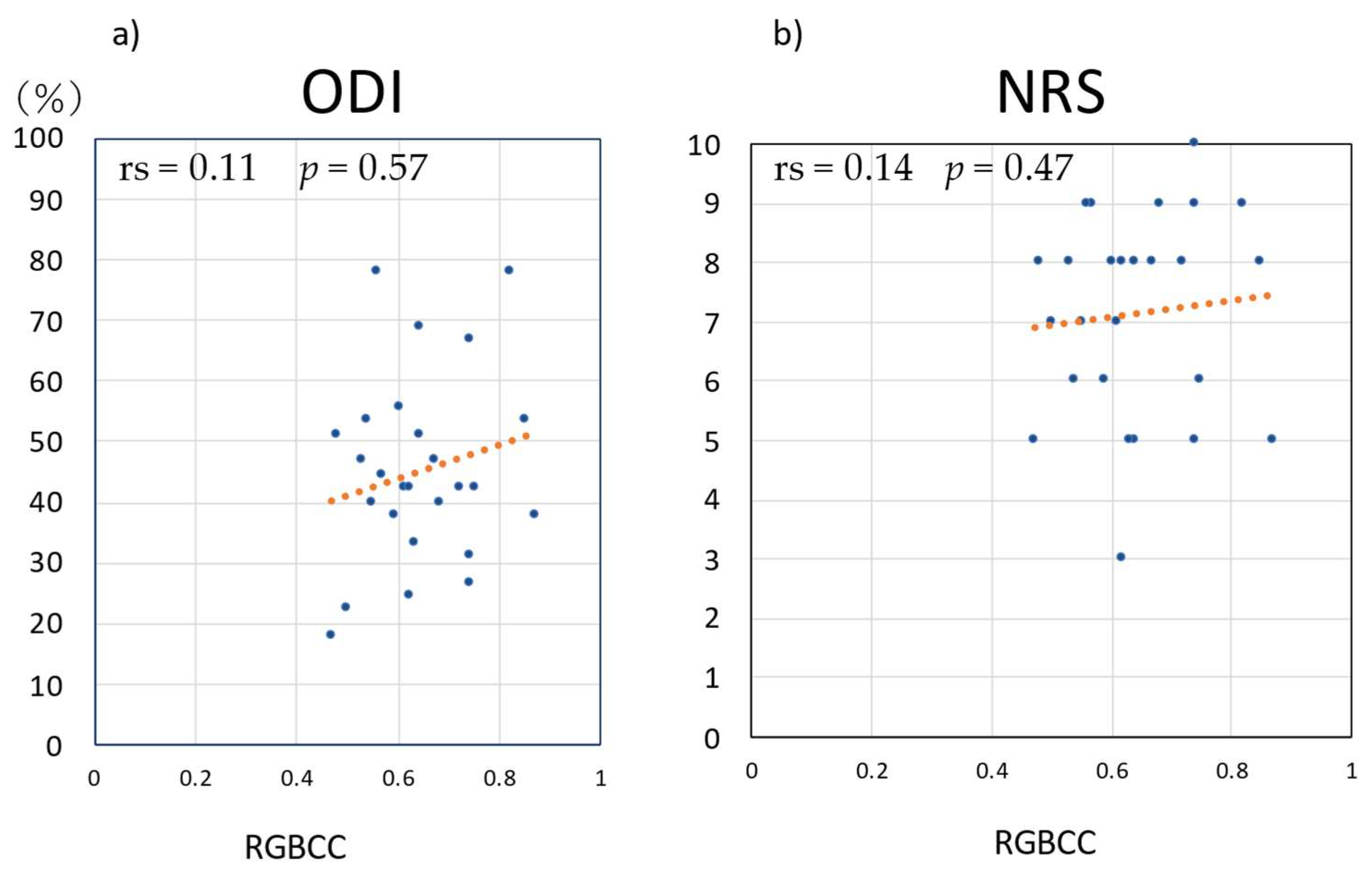
3.4. Correlations of RGBCC with ODI and NRS Score in the LBP Group
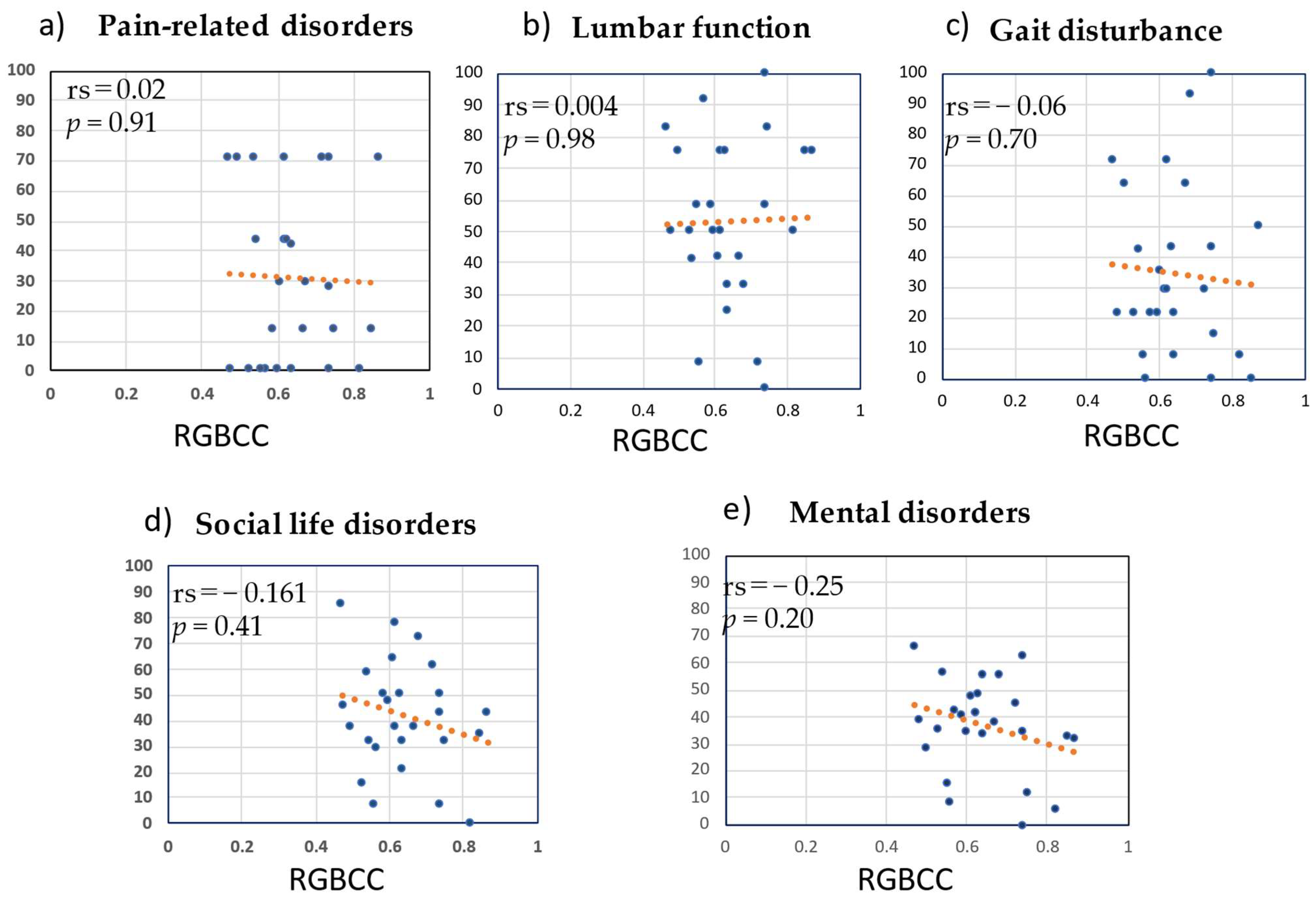
3.5. Correlation between RGBCC and JOABPEQ Score in the LBP Group
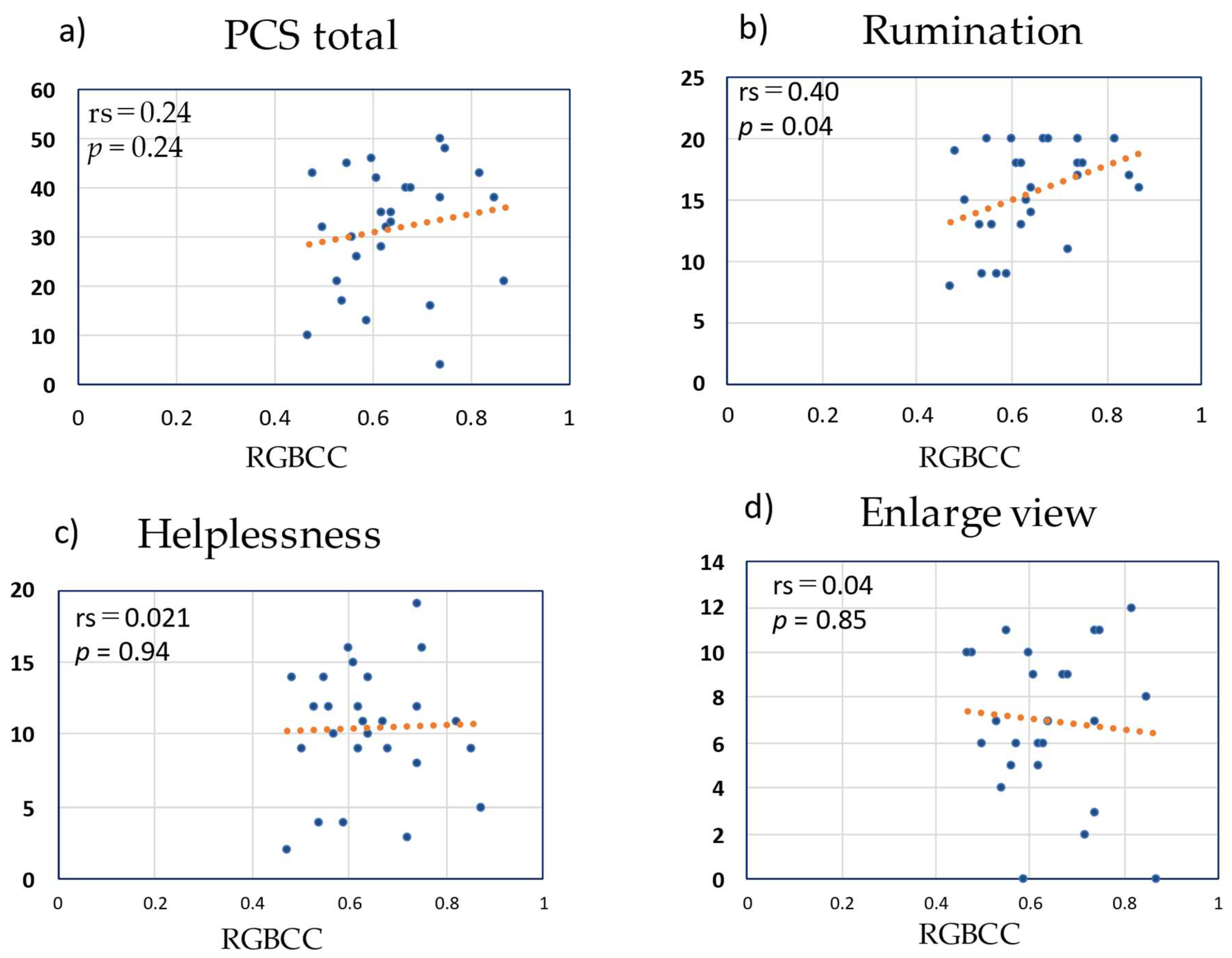
3.6. Correlation between RGBCC and PCS Score in the LBP Group
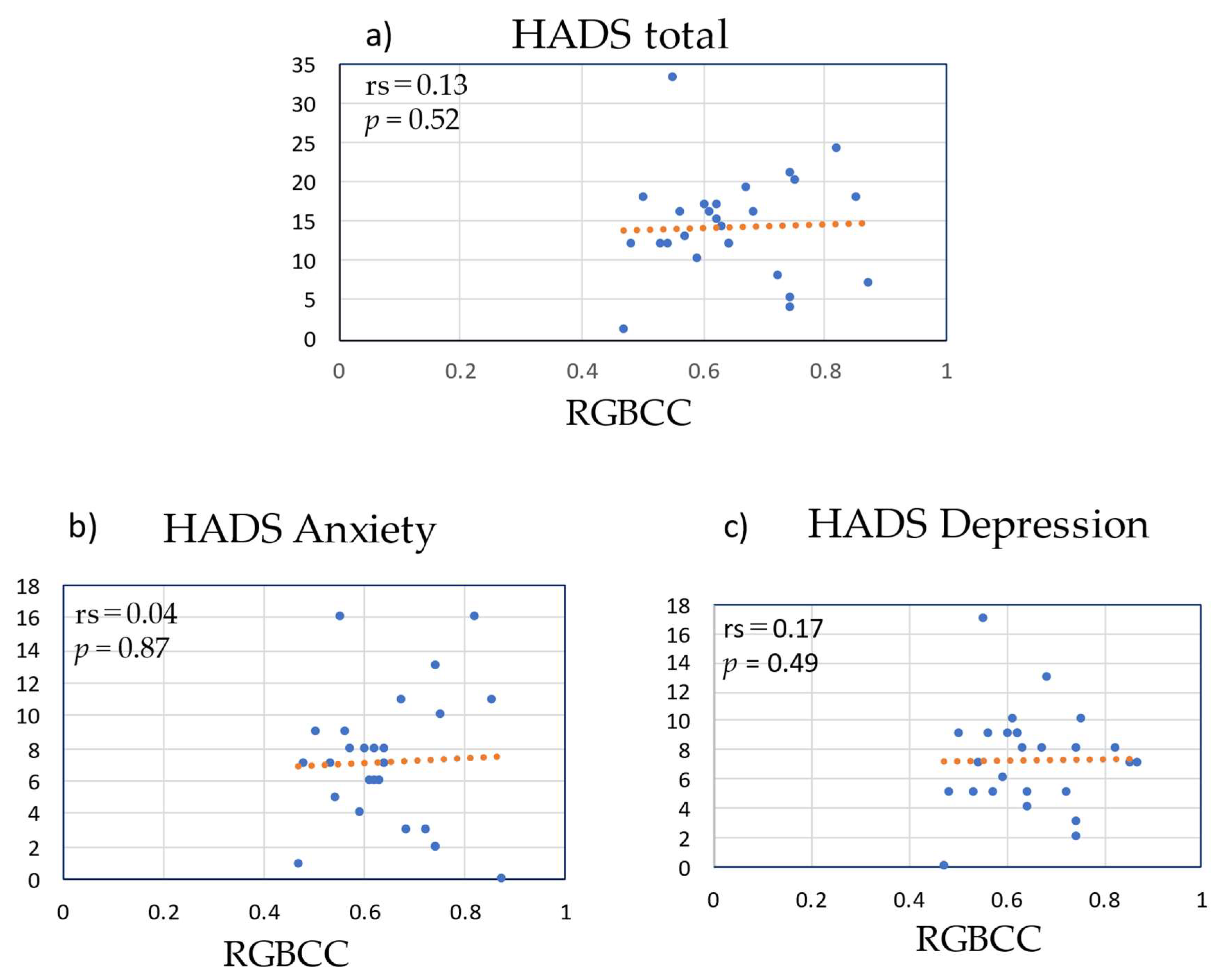
3.7. Correlation between RGBCC and HADS Score in the LBP Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McWilliams, L.A.; Cox, B.J.; Enns, M.W. Mood and anxiety disorders associated with chronic pain: An examination in a nationally representative sample. Pain 2003, 106, 127–133. [Google Scholar] [CrossRef]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Ramond, A.; Bouton, C.; Richard, I.; Roquelaure, Y.; Baufreton, C.; Legrand, E.; Huez, J.-F. Psychosocial risk factors for chronic low back pain in primary care—A systematic review. Fam. Pr. 2011, 28, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wertli, M.M.; Eugster, R.; Held, U.; Steurer, J.; Kofmehl, R.; Weiser, S. Catastrophizing—A prognostic factor for outcome in patients with low back pain: A systematic review. Spine J. 2014, 14, 2639–2657. [Google Scholar] [CrossRef]
- Wertli, M.M.; Burgstaller, J.M.; Weiser, S.; Steurer, J.; Kofmehl, R.; Held, U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: A systematic review. Spine 2014, 39, 263–273. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Tetreault, P.; Petre, B.; Huang, L.; Berger, S.E.; Torbey, S.; Baria, A.T.; Mansour, A.R.; Hashmi, J.A.; Griffith, J.W.; et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain J. Neurol. 2016, 139, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.A.; Baliki, M.N.; Huang, L.; Baria, A.T.; Torbey, S.; Hermann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain J. Neurol. 2013, 136, 2751–2768. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Cedraschi, C.; Duplan, B.; Bazin, T.; Duquesnoy, B. Information and low back pain management: A systematic review. Spine 2006, 31, E326–E334. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Waddell, G.; Newton, M.; Henderson, I.; Somerville, D.; Main, C.J. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993, 52, 157–168. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Fukui, M.; Chiba, K.; Kawakami, M.; Kikuchi, S.; Konno, S.; Miyamoto, M.; Seichi, A.; Shimamura, T.; Shirado, O.; Taguchi, T.; et al. JOA Back Pain Evaluation Questionnaire (JOABPEQ)/JOA Cervical Myelopathy Evaluation Questionnaire (JOACMEQ). The report on the development of revised versions. April 16, 2007. The Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on Low Back Pain and Cervical Myelopathy Evaluation. J. Orthop. Sci. 2009, 14, 348–365. [Google Scholar]
- Heiss, W.D.; Zaro Weber, O. Validation of MRI Determination of the Penumbra by PET Measurements in Ischemic Stroke. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 187–193. [Google Scholar] [CrossRef]
- Kanno, I.; Lassen, N.A. Two methods for calculating regional cerebral blood flow from emission computed tomography of inert gas concentrations. J. Comput. Assist. Tomogr. 1979, 3, 71–76. [Google Scholar] [CrossRef]
- Koike, S.; Nishimura, Y.; Takizawa, R.; Yahata, N.; Kasai, K. Near-infrared spectroscopy in schizophrenia: A possible biomarker for predicting clinical outcome and treatment response. Front. Psychiatry 2013, 4, 145. [Google Scholar] [CrossRef]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A multi-modal parcellation of human cerebral cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Linares, I.M.; Trzesniak, C.; Chagas, M.H.; Hallak, J.E.; Nardi, A.E.; Crippa, J.A. Neuroimaging in specific phobia disorder: A systematic review of the literature. Braz. J. Psychiatry 2012, 34, 101–111. [Google Scholar] [CrossRef]
- Fredrikson, M.; Faria, V. Neuroimaging in anxiety disorders. Mod. Trends Pharm. 2013, 29, 47–66. [Google Scholar]
- Fujii, M.; Ueki, M.; Uehara, K.; Yashima, K.; Kawaguchi, K.; Ikebuchi, Y.; Kinoshita, H.; Arai, J.; Matsubara, A.; Goto, T.; et al. Pain Evaluation during Colonoscopy by the Erythema Index of the Facial Image. Yonago Acta Med. 2019, 62, 100–108. [Google Scholar] [CrossRef]
- White, M.D.; Greiner, J.G.; McDonald, P.L. Point: Humans do demonstrate selective brain cooling during hyperthermia. J. Appl. Physiol. 2011, 110, 569–571; discussion 81–82. [Google Scholar] [CrossRef]
- McDuff, D.J.B.E.; Estepp, J.R. The impact of video compression on remote cardiac pulse measurement using imaging photoplethysmography. In Proceedings of the 2017 12th IEEE International Conference on Automatic Face & Gesture Recognition (FG 2017), Washington, DC, USA, 30 May–3 June 2017; pp. 63–70. [Google Scholar]
- Daikin Industries, Ltd.; Tokyo Institute of Technology. The Publication of Japanese Patent, Brain activity estimator. No. JP 6189486 B2, 5 January 2017. [Google Scholar]
- Tomoko Murakami, K.K.; Yamashita, T. The Quantification of Skin State from Image by microscope. In Proceedings of the 27th Annual Conference of the Japanese Society for Artificial Intelligence, Toyama, Japan, 4–7 June 2013. [Google Scholar]
- Hartrick, C.T.; Kovan, J.P.; Shapiro, S. The numeric rating scale for clinical pain measurement: A ratio measure? Pain Pr. 2003, 3, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952; discussion 52. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Niioka, T.; Sudo, E.; Izumi, H. Evidence for parasympathetic vasodilator fibres in the rat masseter muscle. J. Physiol. 2005, 569, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Shimo, K.; Ueno, T.; Younger, J.; Nishihara, M.; Inoue, S.; Ikemoto, T.; Taniguchi, S.; Ushida, T. Visualization of painful experiences believed to trigger the activation of affective and emotional brain regions in subjects with low back pain. PLoS ONE 2011, 6, e26681. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.; Bentley, D.E.; Elliott, R.; Julyan, P.J.; Boger, E.; Watson, A.; Boyle, Y.; El-Deredy, W.; Jones, A. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007, 56, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Hornik, R.; Kikut, A.; Jesch, E.; Woko, C.; Siegel, L.; Kim, K. Association of COVID-19 Misinformation with Face Mask Wearing and Social Distancing in a Nationally Representative US Sample. Health Commun. 2021, 36, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Takahashi, R.; Morihara, Y.; Kin, I.; Ogawa-Ochiai, K.; Tsumura, N. Development of a camera-based remote diagnostic system focused on color reproduction using color charts. Artif. Life Robot. 2020, 25, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; Chen, X.; Shen, Y.Q.; Li, L.; Chen, N.X.; Zhu, Z.C.; Castellanos, F.X.; Yan, C.-G. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. Neuroimage 2020, 206, 116287. [Google Scholar] [CrossRef]
- Wada, T.; Tanishima, S.; Osaki, M.; Nagashima, H.; Hagino, H. Relationship between sarcopenia and pain catastrophizing in patients with lumbar spinal stenosis: A cross-sectional study. Osteoporos. Sarcopenia 2019, 5, 132–136. [Google Scholar] [CrossRef] [PubMed]
 ): component without band-pass filtering, (
): component without band-pass filtering, ( ): regressor (wave showing questions related to lower back pain and questions unrelated to lower back pain-).
): regressor (wave showing questions related to lower back pain and questions unrelated to lower back pain-).
 ): component without band-pass filtering, (
): component without band-pass filtering, ( ): regressor (wave showing questions related to lower back pain and questions unrelated to lower back pain-).
): regressor (wave showing questions related to lower back pain and questions unrelated to lower back pain-).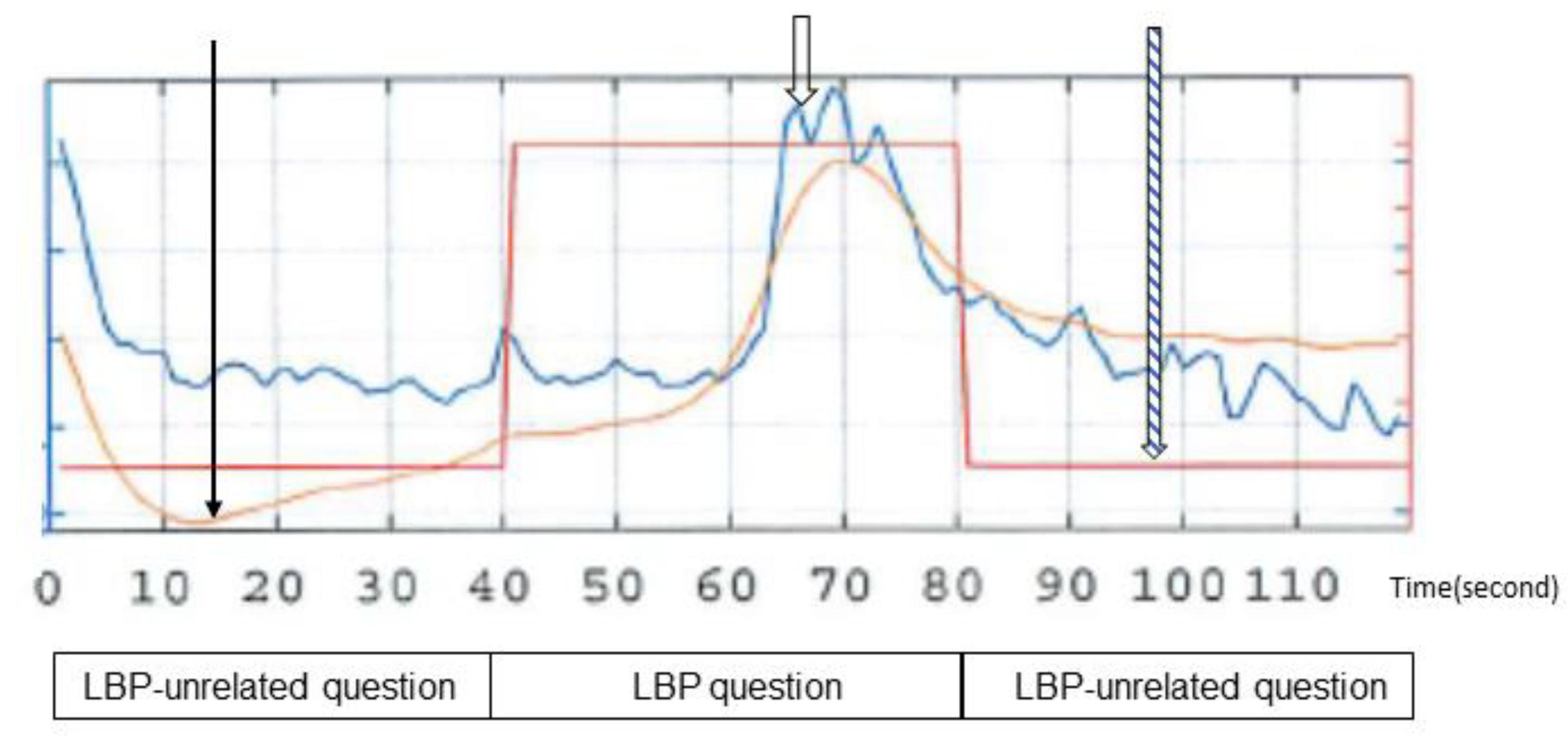
| General questions unrelated to lower back pain | Are you over 20 years of age? |
| What part of Japan are you from? | |
| Do you have siblings? | |
| What color(s) do you like? | |
| Questions related to lower back pain | What would you like to have (or do) if you did not have lower back pain? |
| Would you like to take medications to relieve the lower back pain? | |
| What do you think is the prognosis of the lower back pain? | |
| In your opinion, how did your doctor’s approach to diagnosis and treatment affect your lower back pain? | |
| General questions unrelated to lower back pain | Do you like reading books? |
| Do you like apples? | |
| Is the weather good today? | |
| Is it a hot day today? |
| Characteristic (n = 26) | Value * |
|---|---|
| Age (mean, SD) | 68.0 (13.9) |
| Gender (Male: Female) | 9:17 |
| Diseases causing back pain | |
| Lumbar spinal stenosis | 13 |
| Lumbar spondylosis | 2 |
| Lumbar vertebral fracture | 2 |
| Lumbar kyphoscoliosis | 2 |
| Lumbar spondylosis deformans | 2 |
| Sacroiliac arthritis | 2 |
| Lumbar disc herniation | 3 |
| Cases with previous lumbar spine surgery | 12 |
| Disease period (month) | 43.5 (35.4) |
| RGBCC | 0.64 (0.11) |
| ODI | 45.1 (15.3) |
| JOABPEQ | |
| Lower back pain | 31.2 (28.7) |
| Lumbar function | 53.4 (26.0) |
| Walking ability | 34.7 (28.2) |
| Social life function | 1.7 (21.4) |
| Mental health | 37.0 (17.2) |
| PCS (Total score) | 28.5 (15.9) |
| Ruminations | 13.1 (6.8) |
| Helplessness | 9.4 (5.1) |
| Magnification | 6.0 (4.0) |
| HADS (Total score) | 12.3 (8.3) |
| Anxiety | 6.2 (4.7) |
| Depression | 5.9 (4.1) |
| Lumbar NRS (at study) | 7.1 (1.7) |
| Characteristic | Value * |
|---|---|
| Age (mean, SD) | 60.8 (16.1) |
| Gender (Male:Female) | 12:6 |
| Diseases | |
| Knee osteoarthritis | 2 |
| Shoulder osteoarthritis | 1 |
| Cervical spondylotic myelopathy | 2 |
| Rotator cuff injury | 1 |
| Hip osteoarthritis | 2 |
| Arteriosclerosis obliterans | 1 |
| Bruise on the lower leg | 1 |
| No disease (healthy subjects) | 8 |
| RGBCC | 0.56 (0.14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanishima, S.; Kotani, Y.; Takeda, C.; Mihara, T.; Ogawa, S.; Matsubara, A.; Goto, T.; Hirayama, T.; Hashizume, H.; Arai, J.; et al. Relationship between Facial Color Changes and Psychological Problems Associated with Lower Back Pain. Medicina 2022, 58, 1471. https://doi.org/10.3390/medicina58101471
Tanishima S, Kotani Y, Takeda C, Mihara T, Ogawa S, Matsubara A, Goto T, Hirayama T, Hashizume H, Arai J, et al. Relationship between Facial Color Changes and Psychological Problems Associated with Lower Back Pain. Medicina. 2022; 58(10):1471. https://doi.org/10.3390/medicina58101471
Chicago/Turabian StyleTanishima, Shinji, Yasunori Kotani, Chikako Takeda, Tokumitsu Mihara, Shinya Ogawa, Akira Matsubara, Takashi Goto, Takahiro Hirayama, Hideki Hashizume, Junichiro Arai, and et al. 2022. "Relationship between Facial Color Changes and Psychological Problems Associated with Lower Back Pain" Medicina 58, no. 10: 1471. https://doi.org/10.3390/medicina58101471
APA StyleTanishima, S., Kotani, Y., Takeda, C., Mihara, T., Ogawa, S., Matsubara, A., Goto, T., Hirayama, T., Hashizume, H., Arai, J., Mukunoki, D., & Nagashima, H. (2022). Relationship between Facial Color Changes and Psychological Problems Associated with Lower Back Pain. Medicina, 58(10), 1471. https://doi.org/10.3390/medicina58101471







