The Outcomes of Robotic Rehabilitation Assisted Devices Following Spinal Cord Injury and the Prevention of Secondary Associated Complications
Abstract
1. Introduction
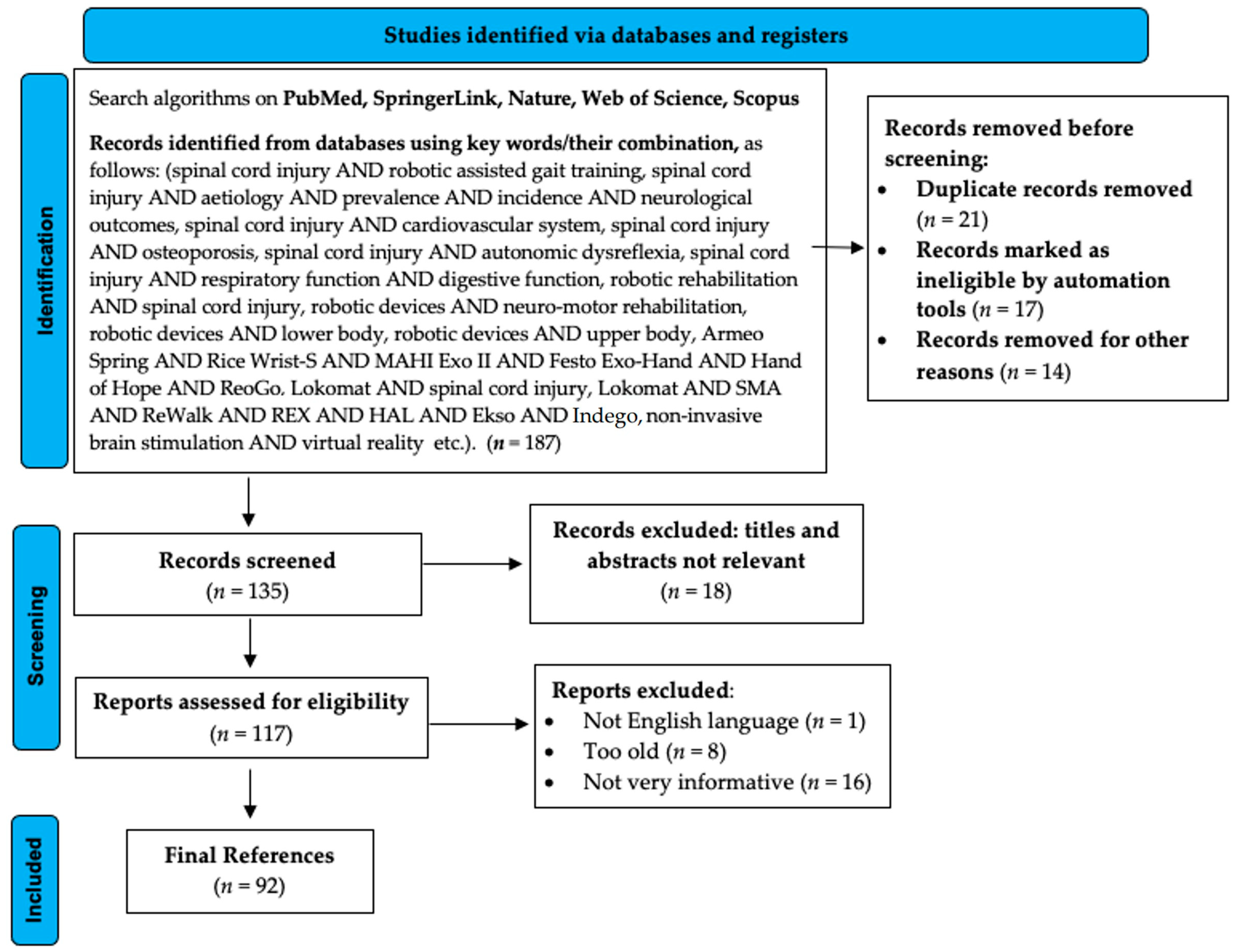
2. Methodology
3. Spinal Cord Injury: Etiology, Neurological Outcomes, and Treatment
3.1. Etiology
3.2. Neurological Outcomes of SCI
3.3. Treatment
4. Robotic Devices Used in Neuro-Motor Rehabilitation
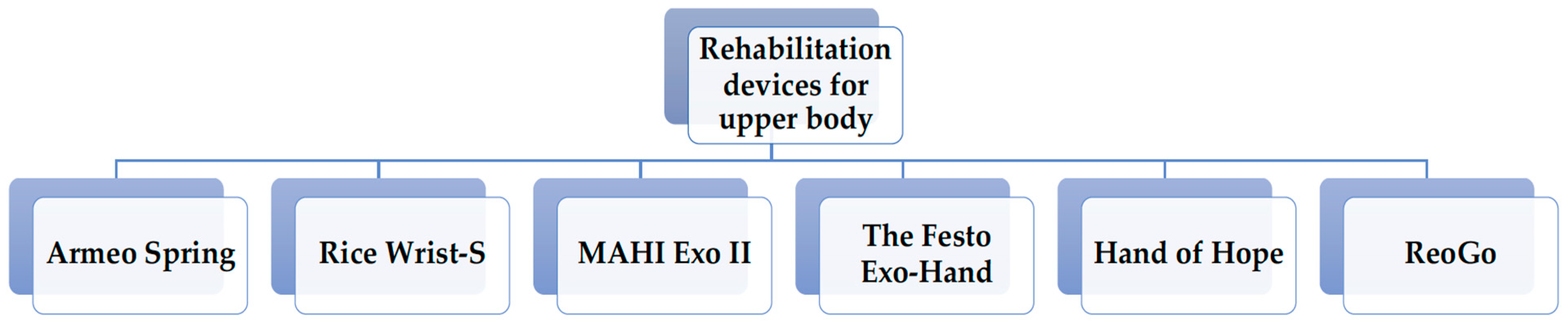
4.1. Rehabilitation Devices for Upper Body
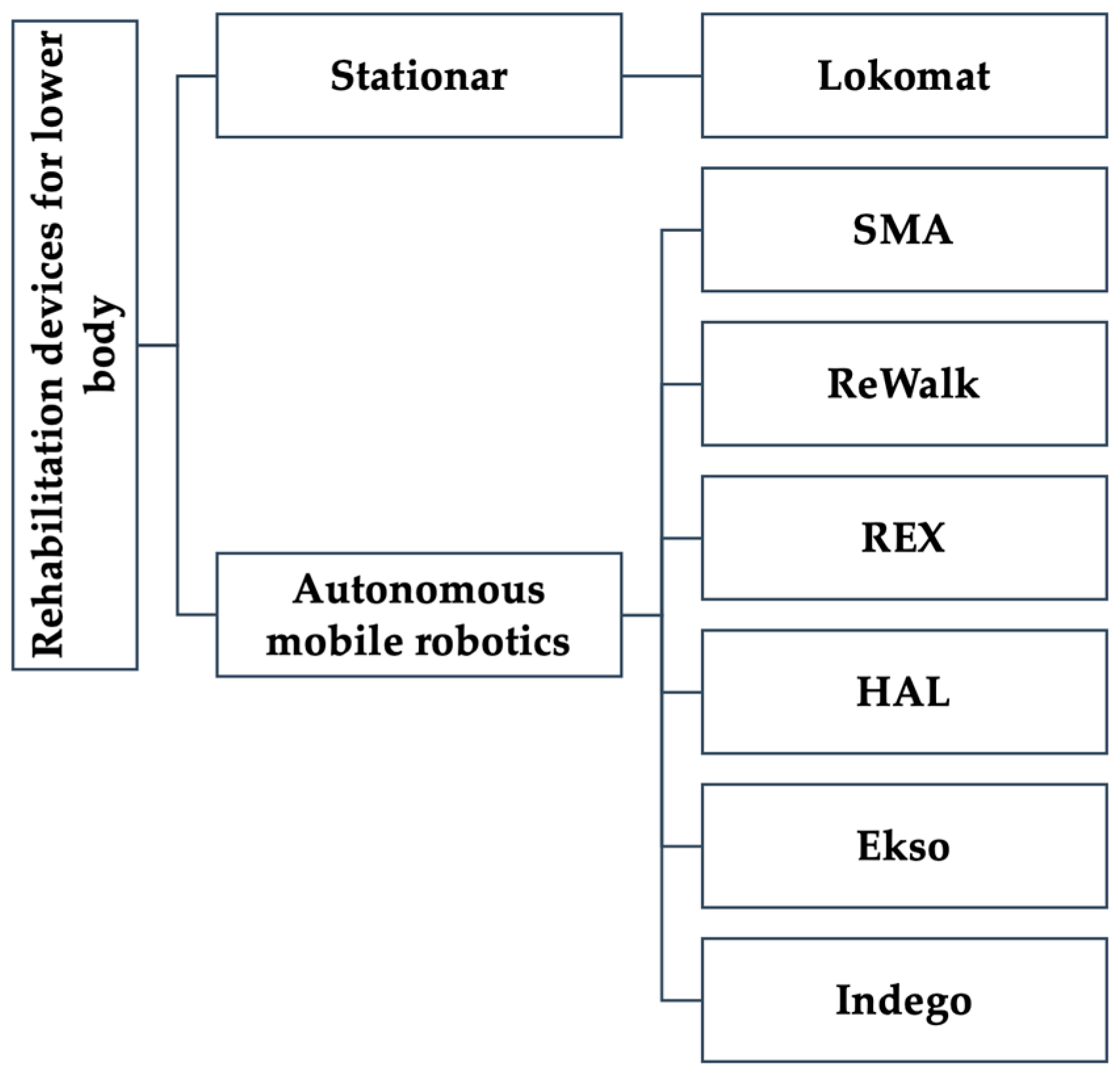
4.2. Rehabilitation Devices for Lower Body
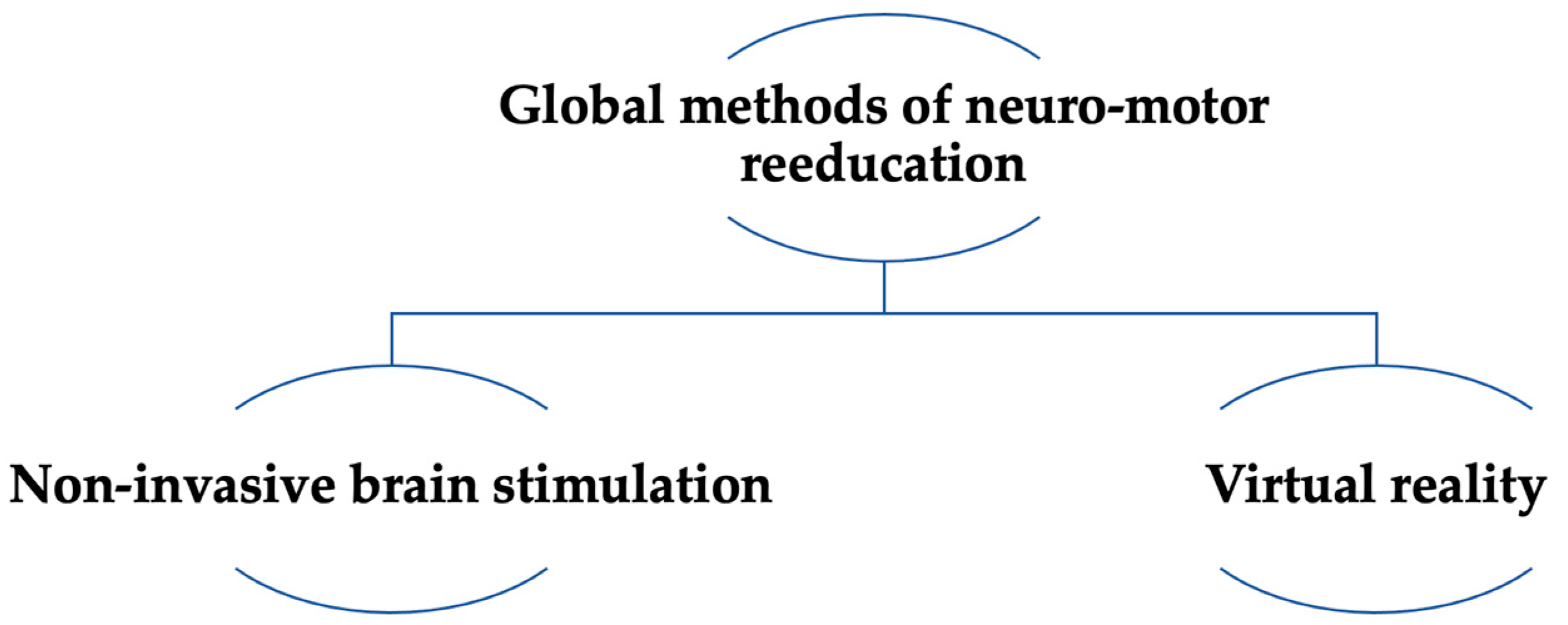
5. Extensive Methods of Neuro-Motor Rehabilitation
5.1. Virtual Reality
5.2. Non-Invasive Brain Stimulation
6. SCI-Associated Complications
6.1. Cardiovascular System
6.2. Autonomic Dysreflexia
6.3. Bone Mineral Density
6.4. Respiratory Recovery
6.5. Intestinal Function
6.6. Neurogenic Bladder
6.7. Erectile Dysfunction
6.8. Psychosocial Adjustment
6.9. Neuropathic Pain
7. Effects of Robotic Devices on SCI-Associated Complications
7.1. Effects on Gait
7.2. Effects on Elbow and RC Mobility
8. Discussion
8.1. Working Group on Artificial Intelligence and Robotics
8.2. Quality of Life of Patients with SCI
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nas, K.; Yazmalar, L.; Şah, V.; Aydın, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Tsai, J.L.; Li, G.S.; Lien, A.S.; Chang, Y.J. Effects of Robot-Assisted Gait Training in Individuals with Spinal Cord Injury: A Meta-analysis. Biomed. Res. Int. 2020, 2020, 2102785. [Google Scholar] [CrossRef]
- Tamburella, F.; Lorusso, M.; Tramontano, M.; Fadlun, S.; Masciullo, M.; Scivoletto, G. Overground robotic training effects on walking and secondary health conditions in individuals with spinal cord injury: Systematic review. J. Neuroeng. Rehabil. 2022, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef]
- Holanda, L.J.; Silva, P.M.M.; Amorim, T.C.; Lacerda, M.O.; Simão, C.R.; Morya, E. Robotic assisted gait as a tool for rehabilitation of individuals with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 126. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Devivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.L.; Nash, M.S. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004, 34, 727–751. [Google Scholar] [CrossRef]
- Uivarosan, D.; Tit, D.M.; Iovan, C.; Nistor-Cseppento, D.C.; Endres, L.; Lazar, L.; Sava, C.; Sabau, A.M.; Buhas, C.; Moleriu, L.C. Effects of combining modern recovery techniques with neurotrophic medication and standard treatment in stroke patients. Sci. Total Environ. 2019, 679, 80–87. [Google Scholar] [CrossRef]
- Uivarosan, D.; Abdel-Daim, M.M.; Endres, L.; Purza, L.; Iovan, C.; Bungau, S.; Furau, C.G.; Tit, D.M. Effects of a proteic swine extract associated to recovery treatment on functional independence and quality of life in patients post stroke. Farmacia 2018, 66, 826–830. [Google Scholar] [CrossRef]
- Esquenazi, A.; Talaty, M.; Jayaraman, A. Powered Exoskeletons for Walking Assistance in Persons with Central Nervous System Injuries: A Narrative Review. PM&R 2017, 9, 46–62. [Google Scholar] [CrossRef]
- Birch, N.; Graham, J.; Priestley, T.; Heywood, C.; Sakel, M.; Gall, A.; Nunn, A.; Signal, N. Results of the first interim analysis of the RAPPER II trial in patients with spinal cord injury: Ambulation and functional exercise programs in the REX powered walking aid. J. Neuroeng. Rehabil. 2017, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, M.A.; Öneş, K.; Gökşenoğlu, G. Early term effects of robotic assisted gait training on ambulation and functional capacity in patients with spinal cord injury. Turk. J. Med. Sci. 2019, 49, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Mekki, M.; Delgado, A.D.; Fry, A.; Putrino, D.; Huang, V. Robotic Rehabilitation and Spinal Cord Injury: A Narrative Review. Neurotherapeutics 2018, 15, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Villiger, M.; Grabher, P.; Hepp-Reymond, M.C.; Kiper, D.; Curt, A.; Bolliger, M.; Hotz-Boendermaker, S.; Kollias, S.; Eng, K.; Freund, P. Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: A longitudinal pilot study. Front. Hum. Neurosci. 2015, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Copaci, D.; Arias, J.; Moreno, L.; Blanco, D. Shape Memory Alloy (SMA)-Based Exoskeletons for Upper Limb Rehabilitation. In Rehabilitation of the Human Bone-Muscle System; Olaru, A.D., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Rodríguez-Fernández, A.; Lobo-Prat, J.; Font-Llagunes, J.M. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J. Neuroeng. Rehabil. 2021, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Meloni, F.; Bracalenti, M.; De Filippis, M.L. The effectiveness of powered, active lower limb exoskeletons in neurorehabilitation: A systematic review. NeuroRehabilitation 2015, 37, 321–340. [Google Scholar] [CrossRef]
- De Araújo, A.V.L.; Neiva, J.F.O.; Monteiro, C.B.M.; Magalhães, F.H. Efficacy of Virtual Reality Rehabilitation after Spinal Cord Injury: A Systematic Review. Biomed. Res. Int. 2019, 2019, 7106951. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aikat, R.; Labani, S.; Khanna, N. Efficacy of Virtual Reality in Upper Limb Rehabilitation in Patients with Spinal Cord Injury: A Pilot Randomized Controlled Trial. Asian Spine J. 2018, 12, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.; Gupta, A.; Khanna, M.; Rashmi Krishnan, U.K.; Chakrabarti, D. Role of Virtual Reality in Balance Training in Patients with Spinal Cord Injury: A Prospective Comparative Pre-Post Study. Asian Spine J. 2020, 14, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Polanía, R.; Nitsche, M.A.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Pascual-Leone, A. Technology Insight: Noninvasive brain stimulation in neurology—Perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Cardiovasc. Med. 2007, 3, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Santos, L.; Peterson, M.D.; Ehinger, M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015, 8, 76–87. [Google Scholar] [CrossRef]
- Yang, R.; Guo, L.; Wang, P.; Huang, L.; Tang, Y.; Wang, W.; Chen, K.; Ye, J.; Lu, C.; Wu, Y.; et al. Epidemiology of spinal cord injuries and risk factors for complete injuries in Guangdong, China: A retrospective study. PLoS ONE 2014, 9, e84733. [Google Scholar] [CrossRef]
- Warburton, D.E.; Eng, J.J.; Krassioukov, A.; Sproule, S. Cardiovascular Health and Exercise Rehabilitation in Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2007, 13, 98–122. [Google Scholar] [CrossRef]
- Harman, K.A.; DeVeau, K.M.; Squair, J.W.; West, C.R.; Krassioukov, A.V.; Magnuson, D.S.K. Effects of early exercise training on the severity of autonomic dysreflexia following incomplete spinal cord injury in rodents. Physiol. Rep. 2021, 9, e14969. [Google Scholar] [CrossRef]
- Rodriguez, B.; Santiago-Tovar, P.; Guerrero-Godinez, M.; Garcia Vences, E.E. Rehabilitation Therapies in Spinal Cord Injury Patients. In Paraplegia; IntechOpen: London, UK, 2020; pp. 1–18. [Google Scholar]
- Evans, N.; Hartigan, C.; Kandilakis, C.; Pharo, E.; Clesson, I. Acute Cardiorespiratory and Metabolic Responses During Exoskeleton-Assisted Walking Overground Among Persons with Chronic Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2015, 21, 122–132. [Google Scholar] [CrossRef]
- Ravensbergen, H.J.; de Groot, S.; Post, M.W.; Slootman, H.J.; van der Woude, L.H.; Claydon, V.E. Cardiovascular function after spinal cord injury: Prevalence and progression of dysfunction during inpatient rehabilitation and 5 years following discharge. Neurorehabil. Neural. Repair. 2014, 28, 219–229. [Google Scholar] [CrossRef]
- Lee, E.S.; Joo, M.C. Prevalence of Autonomic Dysreflexia in Patients with Spinal Cord Injury above T6. Biomed. Res. Int. 2017, 2017, 2027594. [Google Scholar] [CrossRef]
- Turiel, M.; Sitia, S.; Cicala, S.; Magagnin, V.; Bo, I.; Porta, A.; Caiani, E.; Ricci, C.; Licari, V.; De Gennaro Colonna, V.; et al. Robotic treadmill training improves cardiovascular function in spinal cord injury patients. Int. J. Cardiol. 2011, 149, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Escalona, M.J.; Brosseau, R.; Vermette, M.; Comtois, A.S.; Duclos, C.; Aubertin-Leheudre, M.; Gagnon, D.H. Cardiorespiratory demand and rate of perceived exertion during overground walking with a robotic exoskeleton in long-term manual wheelchair users with chronic spinal cord injury: A cross-sectional study. Ann. Phys. Rehabil. Med. 2018, 61, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Krassioukov, A. Malignant autonomic dysreflexia in spinal cord injured men. Spinal Cord 2006, 44, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Taweel, W.A.; Seyam, R. Neurogenic bladder in spinal cord injury patients. Res. Rep. Urol. 2015, 7, 85–99. [Google Scholar] [CrossRef]
- Gifre, L.; Vidal, J.; Carrasco, J.L.; Muxi, A.; Portell, E.; Monegal, A.; Guañabens, N.; Peris, P. Denosumab increases sublesional bone mass in osteoporotic individuals with recent spinal cord injury. Osteoporos. Int. 2016, 27, 405–410. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef]
- Ţiţ, D.M.; Pallag, A.; Iovan, C.; Furău, G.; Furău, C.; Bungău, S. Somatic-vegetative Symptoms Evolution in Postmenopausal Women Treated with Phytoestrogens and Hormone Replacement Therapy. Iran. J. Public Health 2017, 46, 1528–1534. [Google Scholar]
- Abdelrahman, S.; Ireland, A.; Winter, E.M.; Purcell, M.; Coupaud, S. Osteoporosis after spinal cord injury: Aetiology, effects and therapeutic approaches. J. Musculoskelet. Neuronal Interact. 2021, 21, 26–50. [Google Scholar]
- Frotzler, A.; Berger, M.; Knecht, H.; Eser, P. Bone steady-state is established at reduced bone strength after spinal cord injury: A longitudinal study using peripheral quantitative computed tomography (pQCT). Bone 2008, 43, 549–555. [Google Scholar] [CrossRef]
- Bauman, W.A.; Spungen, A.M.; Wang, J.; Pierson, R.N.; Schwartz, E. Continuous loss of bone during chronic immobilization: A monozygotic twin study. Osteoporos. Int. 1999, 10, 123–127. [Google Scholar] [CrossRef]
- Szollar, S.M.; Martin, E.M.; Parthemore, J.G.; Sartoris, D.J.; Deftos, L.J. Densitometric patterns of spinal cord injury associated bone loss. Spinal Cord 1997, 35, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Yilmaz, H.; Paker, N.; Onel, S. Osteoporosis after spinal cord injury. Spinal Cord 1998, 36, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Morse, L.R.; Sudhakar, S.; Danilack, V.; Tun, C.; Lazzari, A.; Gagnon, D.R.; Garshick, E.; Battaglino, R.A. Association between sclerostin and bone density in chronic spinal cord injury. J. Bone Miner. Res. 2012, 27, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Eser, P.; Frotzler, A.; Zehnder, Y.; Schiessl, H.; Denoth, J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos. Int. 2005, 16, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Berlowitz, D.J.; Wadsworth, B.; Ross, J. Respiratory problems and management in people with spinal cord injury. Breathe 2016, 12, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Randelman, M.; Zholudeva, L.V.; Vinit, S.; Lane, M.A. Respiratory Training and Plasticity After Cervical Spinal Cord Injury. Front. Cell. Neurosci. 2021, 15, 700821. [Google Scholar] [CrossRef]
- Zhou, H.L. Treatment of erectile dysfunction in patients with spinal cord injury. Zhonghua Nan Ke Xue 2017, 23, 99–102. [Google Scholar] [PubMed]
- Cadel, L.; DeLuca, C.; Hitzig, S.L.; Packer, T.L.; Lofters, A.K.; Patel, T.; Guilcher, S.J.T. Self-management of pain and depression in adults with spinal cord injury: A scoping review. J. Spinal Cord Med. 2020, 43, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Fundarò, C.; Giardini, A.; Maestri, R.; Traversoni, S.; Bartolo, M.; Casale, R. Motor and psychosocial impact of robot-assisted gait training in a real-world rehabilitation setting: A pilot study. PLoS ONE 2018, 13, e01918942018-13. [Google Scholar] [CrossRef] [PubMed]
- Stokes, S.; Drozda, M.; Lee, C. The past, present, and future of traumatic spinal cord injury therapies: A review. Bone Jt. Open 2022, 3, 348–358. [Google Scholar] [CrossRef]
- Pozeg, P.; Palluel, E.; Ronchi, R.; Solcà, M.; Al-Khodairy, A.W.; Jordan, X.; Kassouha, A.; Blanke, O. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology 2017, 89, 1894–1903. [Google Scholar] [CrossRef]
- Austin, P.D.; Siddall, P.J. Virtual reality for the treatment of neuropathic pain in people with spinal cord injuries: A scoping review. J. Spinal Cord Med. 2021, 44, 8–18. [Google Scholar] [CrossRef]
- Spiess, M.R.; Steenbrink, F.; Esquenazi, A. Getting the Best Out of Advanced Rehabilitation Technology for the Lower Limbs: Minding Motor Learning Principles. PM&R 2018, 10, S165–S173. [Google Scholar] [CrossRef]
- Wirz, M.; Mach, O.; Maier, D.; Benito-Penalva, J.; Taylor, J.; Esclarin, A.; Dietz, V. Effectiveness of Automated Locomotor Training in Patients with Acute Incomplete Spinal Cord Injury: A Randomized, Controlled, Multicenter Trial. J. Neurotrauma 2017, 34, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Alcobendas-Maestro, M.; Esclarín-Ruz, A.; Casado-López, R.M.; Muñoz-González, A.; Pérez-Mateos, G.; González-Valdizán, E.; Martín, J.L. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: Randomized controlled trial. Neurorehabilit. Neural Repair 2012, 26, 1058–1063. [Google Scholar] [CrossRef]
- Subbarao, J.V. Ambulation in spinal cord injured patients-options: Where do we stand? J. Spinal Cord Med. 1996, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.P.; Golding, D.G.; Rolle, W.A.; Graziani, V.; Ditunno, J.F. Recovery of ambulation in motor-incomplete tetraplegia. Arch. Phys. Med. Rehabil. 1997, 78, 1169–1172. [Google Scholar] [CrossRef]
- Van Hedel, H.J.; Dietz, V. Rehabilitation of locomotion after spinal cord injury. Restor. Neurol. Neurosci. 2010, 28, 123–134. [Google Scholar] [CrossRef]
- Burns, A.S.; Marino, R.J.; Flanders, A.E.; Flett, H. Clinical diagnosis and prognosis following spinal cord injury. Handb. Clin. Neurol. 2012, 109, 47–62. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.; Yousefifard, M.; Eskian, M.; Lu, Y.; Chalangari, M.; Harrop, J.S.; Jazayeri, S.B.; Seyedpour, S.; Khodaei, B.; Hosseini, M.; et al. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine 2019, 30, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Domingo, A.; Lam, T. Reliability and validity of using the Lokomat to assess lower limb joint position sense in people with incomplete spinal cord injury. J. Neuroeng. Rehabil. 2014, 11, 167. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, B.S.; Lee, H.J.; Kim, E.J.; Lee, J.A.; Yang, S.P.; Kim, T.Y.; Pak, H.R.; Kim, H.K.; Kim, H.Y.; et al. Energy Efficiency and Patient Satisfaction of Gait With Knee-Ankle-Foot Orthosis and Robot (ReWalk)-Assisted Gait in Patients With Spinal Cord Injury. Ann. Rehabil. Med. 2020, 44, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Zimmermann, A.K.; Herbert, W.G. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med. Devices 2016, 9, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, F.; Sun, L.; Chen, C. Comparison of Efficacy of Lokomat and Wearable Exoskeleton-Assisted Gait Training in People with Spinal Cord Injury: A Systematic Review and Network Meta-Analysis. Front. Neurol. 2022, 13, 772660. [Google Scholar] [CrossRef]
- Zariffa, J.; Kapadia, N.; Kramer, J.L.; Taylor, P.; Alizadeh-Meghrazi, M.; Zivanovic, V.; Willms, R.; Townson, A.; Curt, A.; Popovic, M.R.; et al. Feasibility and efficacy of upper limb robotic rehabilitation in a subacute cervical spinal cord injury population. Spinal Cord 2012, 50, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, H.J.; Cho, D.Y.; Lim, J.E.; Lee, B.S.; Kwon, S.H.; Kim, H.Y.; Lee, S.J. Effects of Combined Upper Limb Robotic Therapy in Patients with Tetraplegic Spinal Cord Injury. Ann. Rehabil. Med. 2019, 43, 445–457. [Google Scholar] [CrossRef]
- Kadivar, Z.; Sullivan, J.L.; Eng, D.P.; Pehlivan, A.U.; O’Malley, M.; Yozbatiran, N.; Francisco, G.E. Robotic Training and Kinematic Analysis of Arm and Hand after Incomplete Spinal Cord Injury: A Case Study, Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2011. [Google Scholar] [CrossRef]
- French, J.A.; Rose, C.G.; O’Malley, M.K. System Characterization of MAHI EXO-II: A Robotic Exoskeleton for Upper Extremity Rehabilitation. Proc. ASME Dyn. Syst. Control. Conf. 2014, 2014, V003T43A006. [Google Scholar] [CrossRef] [PubMed]
- Rudd, G.; Daly, L.; Jovanovic, V.; Cuckov, F. A Low-Cost Soft Robotic Hand Exoskeleton for Use in Therapy of Limited Hand–Motor. Appl. Sci. 2019, 9, 3751. [Google Scholar] [CrossRef]
- Takebayashi, T.; Takahashi, K.; Amano, S.; Uchiyama, Y.; Gosho, M.; Domen, K.; Hachisuka, K. Assessment of the Efficacy of ReoGo-J Robotic Training Against Other Rehabilitation Therapies for Upper-Limb Hemiplegia After Stroke: Protocol for a Randomized Controlled Trial. Front. Neurol. 2018, 9, 730. [Google Scholar] [CrossRef]
- Mulcahey, M.J.; Hutchinson, D.; Kozin, S. Assessment of upper limb in tetraplegia: Considerations in evaluation and outcomes research. J. Rehabil. Res. Dev. 2007, 44, 91–102. [Google Scholar] [CrossRef]
- Kim, C.M.; Eng, J.J.; Whittaker, M.W. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: Relationship with muscle strength. Spinal Cord 2004, 42, 156–162. [Google Scholar] [CrossRef]
- Cheung, E.Y.Y.; Ng, T.K.W.; Yu, K.K.K.; Kwan, R.L.C.; Cheing, G.L.Y. Robot-Assisted Training for People With Spinal Cord Injury: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2320–2331. [Google Scholar] [CrossRef]
- Alashram, A.R.; Annino, G.; Padua, E. Robot-assisted gait training in individuals with spinal cord injury: A systematic review for the clinical effectiveness of Lokomat. J. Clin. Neurosci. 2021, 91, 260–269. [Google Scholar] [CrossRef]
- Cseppento, C.D.N.; Iovanovici, I.; Andronie-Cioara, F.L.; Tarce, A.G.; Bochiș, C.F.; Bochiș, S.A.; Gabriela, D.B.G.D.B. The recovery management of patients with operated extramedullary spinal arteriovenous fistula, evolution and socio-professional reintegration: Case report and review of the literature. Balneo PRM Res. J. 2022, 13, 490. [Google Scholar] [CrossRef]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef]
- Sale, P.; Franceschini, M.; Waldner, A.; Hesse, S. Use of the robot assisted gait therapy in rehabilitation of patients with stroke and spinal cord injury. Eur. J. Phys. Rehabil. Med. 2012, 48, 111–121. [Google Scholar]
- Schwartz, I.; Sajina, A.; Neeb, M.; Fisher, I.; Katz-Luerer, M.; Meiner, Z. Locomotor training using a robotic device in patients with subacute spinal cord injury. Spinal Cord 2011, 49, 1062–1067. [Google Scholar] [CrossRef]
- Klamroth-Marganska, V.; Blanco, J.; Campen, K.; Curt, A.; Dietz, V.; Ettlin, T.; Felder, M.; Fellinghauer, B.; Guidali, M.; Kollmar, A.; et al. Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial. Lancet Neurol. 2014, 13, 159–166. [Google Scholar] [CrossRef]
- Sale, P.; Russo, E.F.; Russo, M.; Masiero, S.; Piccione, F.; Calabrò, R.S.; Filoni, S. Effects on mobility training and de-adaptations in subjects with Spinal Cord Injury due to a Wearable Robot: A preliminary report. BMC Neurol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Talaty, M.; Packel, A.; Saulino, M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 2012, 91, 911–921. [Google Scholar] [CrossRef]
- Vilimovsky, T.; Chen, P.; Hoidekrova, K.; Slavicek, O.; Harsa, P. Prism Adaptation Treatment Predicts Improved Rehabilitation Responses in Stroke Patients with Spatial Neglect. Healthcare 2022, 10, 2009. [Google Scholar] [CrossRef]
- Zeilig, G.; Weingarden, H.; Zwecker, M.; Dudkiewicz, I.; Bloch, A.; Esquenazi, A. Safety and tolerance of the ReWalk™ exoskeleton suit for ambulation by people with complete spinal cord injury: A pilot study. J. Spinal Cord Med. 2012, 35, 96–101. [Google Scholar] [CrossRef]
- Waters, R.L. Functional prognosis of spinal cord injuries. J. Spinal Cord Med. 1996, 19, 89–92. [Google Scholar]
- Oleson, C.V.; Burns, A.S.; Ditunno, J.F.; Geisler, F.H.; Coleman, W.P. Prognostic value of pinprick preservation in motor complete, sensory incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 2005, 86, 988–992. [Google Scholar] [CrossRef]
- Yozbatiran, N.; Keser, Z.; Davis, M.; Stampas, A.; O’Malley, M.K.; Cooper-Hay, C.; Frontera, J.; Fregni, F.; Francisco, G.E. Transcranial direct current stimulation (tDCS) of the primary motor cortex and robot-assisted arm training in chronic incomplete cervical spinal cord injury: A proof of concept sham-randomized clinical study. NeuroRehabilitation 2016, 39, 401–411. [Google Scholar] [CrossRef]
- Gordon, K.E.; Wald, M.J.; Schnitzer, T.J. Effect of parathyroid hormone combined with gait training on bone density and bone architecture in people with chronic spinal cord injury. PM&R 2013, 5, 663–671. [Google Scholar] [CrossRef]
- Lefeber, N.; Swinnen, E.; Kerckhofs, E. The immediate effects of robot-assistance on energy consumption and cardiorespiratory load during walking compared to walking without robot-assistance: A systematic review. Disabil. Rehabil. Assist. Technol. 2017, 12, 657–671. [Google Scholar] [CrossRef]
- Guest, R.S.; Klose, K.J.; Needham-Shropshire, B.M.; Jacobs, P.L. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: Part 4. Effect on physical self-concept and depression. Arch. Phys. Med. Rehabil. 1997, 78, 804–807. [Google Scholar] [CrossRef]
- Filipcic, T.; Sember, V.; Pajek, M.; Jerman, J. Quality of Life and Physical Activity of Persons with Spinal Cord Injury. Int. J. Environ. Res. Public Health 2021, 18, 9148. [Google Scholar] [CrossRef]
| Neurological Level of Injury | Functional Deficit of the Patient | Remaining Functional |
|---|---|---|
| C3 | The patient cannot breathe spontaneously and needs ventilator support. | - |
| C4 | The global functional deficit requires an electric chair controlled by breathing, tongue or jaw, and static wrist orthosis for forearm and hand posture. | Impairment of diaphragm function, necessary endotracheal intubation, and mechanical ventilation |
| C5 | Totally dependent on transfers and ADL | Breathe spontaneously Does elbow flexion, Joystick-controlled wheelchair |
| C6 | Assisted transfer, Needing an electric chair for longer distances Requirements of intermittent probing of the urinary bladder | Active wrist extension Independent in activities such as nutrition, grooming, hygiene, and dressing of the upper train |
| C6-C7 | Help for dressing the lower train Needing a manual wheelchair | Elbow extension (C7), finger flexion (C8) Quasi-independent for transfers |
| T11-T12 | Needing manual chair, Neurogenic bowel Neurogenic bladder | Independence to perform ADLs Walking with orthotics is initiated |
| L1-L2 | Traveling with a chair over long distances | Totally independent, capable to move short distances with walking orthotics, Can do knee flexion and partial plantar dorsiflexion Presenting bladder and intestinal sphincter control |
| Sub L5 | - | Total independence |
| Neurological Level of Injury | Transfers | Manual Wheelchair(W/C) Skills | Ambulation |
|---|---|---|---|
| C1-C4 | 100% assistance from another person | W/C propelled by another person | No functional ambulation |
| C5 | |||
| C6 | 80–100% assistance from another person | W/C propelled by another person outdoors, some are independent indoors | |
| C7-C8 | Independent in some cases | Independent indoors, sometimes outdoors, some need assistance with unlevel terrain | |
| T1-T9 | Independent | Independent indoors and outdoors on the level and unloved terrain | Functional ambulation is not typical |
| T10-L1 | Patients may be able to walk using KAFOs | ||
| L2-S5 | Patients may be able to walk using KAFOs, or AFOs and forearm crutches or cane(s) |
| Characteristics | Patients, Diagnostics | Outcomes |
|---|---|---|
| Complete vs. incomplete and paraplegia vs. tetraplegia | 246 patients with complete paraplegia, incomplete paraplegia and tetraplegia [63] | Complete paraplegia: 5% ambulators at one year Incomplete paraplegia: 76% ambulators at one year Incomplete tetraplegia: 46%ambulators at one year |
| ASIA classification | 80 with incomplete paraplegia, ASIA C and D [57] | About 75% of persons achieve walking ability |
| Initial ASIA classification and age | 105 patients with incomplete tetraplegia ASIA C and D [86] | Tetraplegia, ASIA C, <50 years old 91% ambulators by discharge from rehabilitation ward Tetraplegia, ASIA C, >50 years, 42% ambulators by discharge Tetraplegia, ASIA D, 100% ambulators by discharge, regardless of age. |
| Pinprick sensations spared in lower extremities | 97 patients with ASIA B paraplegia and tetraplegia as a result of trauma [59] | Pinprick sensation spared in >50% of L2–S1 dermatomes: 40% able to walk independently >150 feet 1 year after injury. |
| Preservation of iliopsoas muscle strength at 30 days after injury | 54 patients with incomplete paraplegia due to trauma [87] | All with >2/5 initial hip flexor or knee extensor strength were ambulators at one year. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor-Cseppento, C.D.; Gherle, A.; Negrut, N.; Bungau, S.G.; Sabau, A.M.; Radu, A.-F.; Bungau, A.F.; Tit, D.M.; Uivaraseanu, B.; Ghitea, T.C.; et al. The Outcomes of Robotic Rehabilitation Assisted Devices Following Spinal Cord Injury and the Prevention of Secondary Associated Complications. Medicina 2022, 58, 1447. https://doi.org/10.3390/medicina58101447
Nistor-Cseppento CD, Gherle A, Negrut N, Bungau SG, Sabau AM, Radu A-F, Bungau AF, Tit DM, Uivaraseanu B, Ghitea TC, et al. The Outcomes of Robotic Rehabilitation Assisted Devices Following Spinal Cord Injury and the Prevention of Secondary Associated Complications. Medicina. 2022; 58(10):1447. https://doi.org/10.3390/medicina58101447
Chicago/Turabian StyleNistor-Cseppento, Carmen Delia, Anamaria Gherle, Nicoleta Negrut, Simona Gabriela Bungau, Anca Maria Sabau, Andrei-Flavius Radu, Alexa Florina Bungau, Delia Mirela Tit, Bogdan Uivaraseanu, Timea Claudia Ghitea, and et al. 2022. "The Outcomes of Robotic Rehabilitation Assisted Devices Following Spinal Cord Injury and the Prevention of Secondary Associated Complications" Medicina 58, no. 10: 1447. https://doi.org/10.3390/medicina58101447
APA StyleNistor-Cseppento, C. D., Gherle, A., Negrut, N., Bungau, S. G., Sabau, A. M., Radu, A.-F., Bungau, A. F., Tit, D. M., Uivaraseanu, B., Ghitea, T. C., & Uivarosan, D. (2022). The Outcomes of Robotic Rehabilitation Assisted Devices Following Spinal Cord Injury and the Prevention of Secondary Associated Complications. Medicina, 58(10), 1447. https://doi.org/10.3390/medicina58101447










