Abstract
The number of patients with Alzheimer’s disease is increasing annually. Most of these patients are older adults with comorbid physical illnesses, which means that they are often treated with a combination of medications for the disease they have and those for Alzheimer’s disease. Thus, older adults with Alzheimer’s disease are potentially at risk for polypharmacy. In addition, the drug interactions between Alzheimer’s disease medications and those for the treatment of physical illnesses may reduce their efficacy and increase side effects. This article reviews polypharmacy and drug interactions in elderly patients with Alzheimer’s disease, with a focus on psychotropic drugs.
1. Introduction
It is widely known that the number of older adults with dementia is increasing worldwide. In the United States, the number of people aged 65 years and older was 52 million in 2018 and is predicted to increase to 95 million by 2060, 50 million of which will have dementia by 2050 [1,2]. Similarly, the number of elderly with dementia in Asia is increasing. According to a meta-analysis published in 2014, the prevalence of dementia among patients aged 65 years and older in South Korea is 9.2%, which is higher than that of patients of similar age in Europe, the United States, and other Asian countries [3,4]. Furthermore, the odds ratio for death from dementia and other neurodegenerative diseases is reportedly higher in Korea than that in other countries. In Japan, there were an estimated 3.5 million people with dementia in 2012 (approximately 8% of the global population), and the number of dementia patients is projected to reach 4.9 million by 2034, when the population over 65 years of age reaches its peak [5]. In addition, the cost of informal care for patients with dementia is estimated at approximately $54 billion.
Most patients with Alzheimer’s disease are elderly and have two or more chronic conditions [6,7,8]. For example, a review of Medicare claims data revealed that 67% of beneficiaries over the age of 65 years had two or more chronic diseases [9]. Patients with Alzheimer’s disease and related dementias often have comorbid physical diseases [10]. Considering the barriers to communication and cognitive decline associated with this disease, polypharmacy is more likely to occur in patients with dementia than in the general elderly population [11,12]. According to the currently accepted definition, polypharmacy is defined as taking five or more medications per day, with a prevalence of 30–60% among the elderly (65 years and older) [13,14,15,16,17]. Although in most cases polypharmacy results from prescribing necessary medications to treat diseases in the elderly, there are reports that polypharmacy is associated with adverse outcomes. Inappropriate polypharmacy leads to an increased incidence of falls, frailty, and decreased quality of life [18,19,20,21]. Such adverse events further increase the cost and burden of care for dementia patients.
Patients with dementia have a high incidence of potentially inappropriate medication (PIM), estimated to be 14–74% [22,23,24]. PIMs are medications whose benefits do not exceed the risks associated with taking them, such as adverse events, and are a common cause of adverse drug reactions in the elderly [25,26]. They have been associated with a decreased quality of life, poor nutrition, and depression in nursing home residents with dementia [27,28,29]. According to a survey of PIM use among older adults with dementia in seven European countries, patients with dementia had a high incidence of PIM intake, regardless of severity, even those with mild Alzheimer’s disease [30,31]. Inadequate communication between patients with dementia and healthcare providers triggers a prescription cascade in which healthcare providers misidentify the side effects of drugs as new symptoms and prescribe drugs to treat drug-related problems, thereby triggering polypharmacy [32,33,34]. In other words, prescribing additional drugs for adverse reactions to PIM may lead to polypharmacy, which is associated with many more adverse events, greater healthcare utilisation, and even mortality [35,36].
Increased prescriptions are associated with a higher incidence of drug-drug interactions and adverse effects. Particularly in the elderly, drug interactions and a high sensitivity to psychotropic drugs may lead to unanticipated increases in effects and adverse events. In this study, we also review the drug interactions associated with cholinesterase inhibitors or memantine, both of which are used in the treatment of Alzheimer’s disease.
This review aimed to understand polypharmacy and drug interactions in patients with dementia (Figure 1). The contents of this review are summarised in Figure 2.
Figure 1.
Aim of this review.
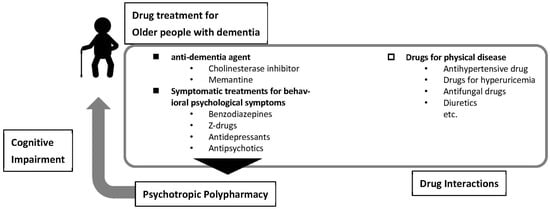
Figure 2.
Summary of contents in this review.
2. Polypharmacy in Alzheimer’s Disease Patients
The main symptoms of Alzheimer’s disease, namely memory impairment and behavioural and psychological symptoms, cause great distress to patients with dementia as well as caregivers [37,38]. Cognitive dysfunction in Alzheimer’s disease can be treated with cognitive-improving drugs, such as acetylcholinesterase inhibitors (donepezil) and NMDA-type glutamate receptor antagonists (memantine). Memantine and acetylcholinesterase inhibitors are sometimes used in combination to enhance efficacy; however, cognitive enhancers are generally used as a single medication [39]. Some countries do not have access to insurance coverage for cognitive-improving drugs because their effectiveness is limited [40].
The behavioural and psychological symptoms of dementia (BPSD) are customarily and widely treated symptomatically with psychotropic drugs [41,42]. Because of the wide variety of BPSD, including depression and delusions, the polypharmacy of psychotropic medication is increasing among the elderly, including patients without dementia [43]. The Beers Criteria, published by the American Geriatrics Society recommend avoiding use of more than three central nervous system (CNS)-active drugs, such as antidepressants, antipsychotics, benzodiazepines and “Z-drugs”, because they are associated with the risk of falls and fractures [44]. It is widely known that these drugs can produce falls due to sedation, daytime sleepiness, orthostatic hypotension, and motor disturbances.
Using Medicare claims data, a study examining psychotropic polypharmacy in patients with dementia revealed that 13.9% of older adults with dementia had “psychotropic polypharmacy” (three or more psychotropic medications for 30 or more consecutive days), and 29.4% of them were exposed to five or more psychotropic medications [1]. The drugs most frequently prescribed to patients with psychotropic polypharmacy were antidepressants (92.0% of days in polypharmacy during the study period), followed by antiepileptic drugs (62.1%), antipsychotics (47.1%), benzodiazepines (40.7%), opioids (32.3%), and benzodiazepine receptor agonists (6.0%). Although cognitive-improving drugs were not included in this study, considering that most dementia cases involve Alzheimer’s disease [45], it is assumed that the polypharmacy of CNS-acting drugs is even more serious with the added influence of cognitive-improving drugs.
A cross-sectional analysis using the National Ambulatory Medical Care Survey (NAMCS) showed that the number of medications was significantly higher in the elderly with dementia than in those without, although the number of outpatient visits did not differ based on dementia status [12]. In addition, the mean number of medications expected to be prescribed per visit was higher in patients with dementia when compared by age, sex, and number of comorbidities (standardised). Interestingly, elderly patients with dementia were more likely to be prescribed drugs that act on the CNS, as well as those that act on the gastrointestinal and urological systems and drugs for diabetes, indicating that polypharmacy with all drugs, not just CNS drugs for dementia symptoms, is likely to develop in patients with dementia.
Certain medications frequently included in polypharmacy, such as anticholinergics and sedatives, are associated with an increased risk of hospitalisation and death in patients with dementia [46]. Therefore, it is necessary to prescribe safer drugs and reduce the incidence of polypharmacy in this population [47,48]. The number of prescribed drugs should be reduced to a level consistent with the risk of hospitalisation and death in patients with dementia.
3. Cognitive Impairment Induced by Polypharmacy
Several reports have examined the association between polypharmacy and cognitive dysfunction, where polypharmacy is defined as the use of five or more drugs that are commonly used in clinical practice [9,17]. In a cross-sectional study on the association between polypharmacy and cognitive function and related comorbidities (depression, hypertension, and/or diabetes) in rural America, older adults afflicted with polypharmacy had 3.71 times higher odds of having cognitive impairment than older adults who were not [49]. In addition, even after adjusting for confounding factors using multivariate analysis, the odds of cognitive impairment were 2.86 times higher in patients with polypharmacy, whereas there was no significant association between comorbidities and cognitive impairment. Based on these results, it is considered that polypharmacy is independently associated with cognitive dysfunction. In general, patients with cognitive impairment have poor medication compliance. However, healthcare providers are unable to ascertain whether patients are taking their medications. Polypharmacy is thought to result from the addition of medications after determining that the prescribed medications are ineffective. Several reports have shown a negative correlation between the number of medications prescribed and adherence (i.e., the higher the number of medications, the worse the adherence) [50,51,52,53]. Future large cohort studies are needed to prospectively evaluate whether polypharmacy induces cognitive dysfunction or vice versa.
4. Drug Interactions in Alzheimer’s Disease
The risk of drug interaction is always present in patients undergoing polypharmacy. Since dementia patients with polypharmacy are prescribed not only psychotropic drugs but also multiple medications to treat physical illnesses, attention should also be paid to the status of physical illness medications. Since most patients with Alzheimer’s disease are elderly, the effects of aging must be considered in the pharmacokinetics of such patients. That is, muscle mass and total body water relatively decrease in the elderly, whereas body fat content increases [54,55,56]. These changes in body composition result in a reduction in the distribution volume of hydrophilic drugs and an increase in that of lipophilic drugs. In addition, drugs are more likely to reach the CNS in the elderly because of the reduced function of the blood-brain barrier [57]. The detoxification or disappearance of drugs absorbed into the body also declines in the elderly population. Hepatic blood flow decreases with age, and hepatic drug clearance can be reduced in the elderly [58,59]. Moreover, decreased renal blood flow is the most significant pharmacokinetic change associated with aging, leading to the decreased renal excretion of drugs.
4.1. Pharmacokinetic Drug Interactions
The major routes of disappearance and typical drug interactions of cognitive enhancers are listed in Table 1. Among the cognitive enhancers, donepezil and galantamine are metabolised in the liver via CYP2D6 and CYP3A4, and hepatic metabolism may be affected by specific substrates, inhibitors, or enhancers of the same enzymes [60]. The modes of metabolic inhibition by drug interactions fall into two major categories [61]: (1) direct enzyme inhibition (e.g., ketoconazole, a strong non-competitive inhibitor of CYP3A4), and (2) competitive inhibition of the catalytic site of the CYP3A4 enzyme. Ketoconazole significantly increased the plasma concentration of donepezil, presumably due to the inhibition of CYP3A4 [62]. In addition, according to the U.S. FDA, many drugs, such as ritonavir and other antivirals, clarithromycin and other macrolide antibiotics, and verapamil and fluvoxamine, have CYP3A4 inhibitory effects. The effect of CYP2D6 on donepezil metabolism is unclear, and donepezil is assumed to be less susceptible to renal or hepatic impairment in patients. Galantamine metabolism has been reported to be affected by both CYP2D6 and CYP3A4 inhibitors. The combination of galantamine with ketoconazole (a strong CYP3A4 inhibitor) or paroxetine (a strong CYP2D6 inhibitor) increased the area under the blood concentration-time curve (AUC) for galantamine by 30% and 40%, respectively, compared with the administration of galantamine alone [63]. Several antidepressants (paroxetine, fluoxetine, bupropion, duloxetine, escitalopram, fluvoxamine, and sertraline) have been alerted by the FDA to have CYP2D6 inhibitory activity to varying degrees. It was also noted that galantamine increases blood levels by approximately 30–60% in patients with impaired renal or hepatic function. While donepezil is not shown to exacerbate renal or hepatic impairment, blood levels of donepezil is increased in patients with renal or hepatic impairment. Therefore, greater caution should be exercised when galantamine is combined with other drugs in elderly patients with impaired renal or hepatic function, because both physiological changes and drug interactions can increase blood concentrations. Regarding drug classes, among psychotropic drugs, antidepressants should be used with caution because they may affect the pharmacokinetics of donepezil and galantamine.

Table 1.
Pharmacokinetic Drug Interactions in Alzheimer’s Disease.
Rivastigmine is rapidly metabolised by esterases in the blood, and is not metabolised by cytochrome P450. Thus, there was no risk of cytochrome P450-related drug interactions, and no significant differences in the ability to metabolise rivastigmine between patients with Alzheimer’s disease and healthy older adults were observed [64]. It was reported that rivastigmine does not increase adverse events in patients with Alzheimer’s disease when used in combination with 22 different drugs, including antidiabetic, cardiovascular, gastrointestinal, and nonsteroidal anti-inflammatory drugs [65].
Memantine is a weak base drug with a pKa of 10.27 and is excreted mainly from the kidneys as an unchanged drug [66,67]. Urinary pH has been shown to significantly affect the disappearance of drugs excreted renally in an unchanged form, such as memantine, and the acidification of urine increases excretion and decreases blood concentration [68]. Conversely, the marked alkalinisation of urine pH induces a decrease in memantine excretion, which may lead to the overexposure of tissues to memantine and toxic effects, especially in elderly patients with reduced renal function. Therefore, the use of drugs or foods that alkalize urine, such as acetazolamide, citric acid, and sodium bicarbonate, may decrease the excretion of memantine. In addition, renal cation transporters, particularly multidrug and toxin extrusion proteins (MATE1), may be involved in memantine excretion [69].
4.2. Pharmacodynamic Drug Interactions
Typical pharmacodynamic interactions of cognitive enhancers are summarized in Table 2. Because acetylcholine neurotransmission in the brain is reduced in patients with Alzheimer’s disease [70], a cognitive enhancer that increases acetylcholine in synaptic terminals by inhibiting cholinesterase in the brain has been developed. Anticholinergics have opposite mechanisms of action relative to cholinesterase inhibitors, thus their concomitant use may attenuate their mutual action [71].

Table 2.
Pharmacodynamic Drug Interaction in Alzheimer’s Disease.
Clinically used psychotropic drugs, including many tricyclic antidepressants (e.g., amitriptyline, amoxapine, and imipramine) and other antidepressants (nortriptyline and paroxetine), many first-generation antihistamines (e.g., diphenhydramine and hydroxy persons), anticholinergic antiparkinsonian agents (trihexyphenidyl and biperiden), and several antipsychotics (chlorpromazine, perphenazine, olanzapine and clozapine) [44]. As already noted, these drugs and cognitive enhancers are frequently administered to patients with Alzheimer’s disease. In fact, the rate of concomitant anticholinergic medications in patients using donepezil was found to be higher than that of elderly patients in the control group [44]. In addition, an increased prescription of anticholinergics at the start of cholinesterase inhibitor therapy, which is often inappropriately used, has also been reported [72]. One reason for this combination may be that atropine and other anticholinergics are used in combination with cholinesterase inhibitors to reduce their adverse effects [73]. Dose optimisation and minimisation of adverse events due to titration may be the most important factors in the use of these drugs for patients receiving cholinesterase inhibitor therapy [74].
Memantine is known to act on NMDA-type glutamate receptors. Therefore, the concomitant use of memantine with drugs that act on NMDA-type glutamate receptors (amantadine, ketamine, and dextromethorphan) may result in the competitive inhibition of its action [75]. Although reported at a nonclinical level, memantine’s NMDA receptor antagonism enhances dopamine release in the prefrontal cortex and striatum [76]. Decreased dopamine levels in the prefrontal cortex are known to be involved in cognitive-learning impairment and negative symptoms [77,78]. In addition, many antipsychotic drugs block dopamine receptors in these brain regions, as well as in the nucleus accumbens, resulting in negative symptoms and cognitive dysfunction. The efficacy of memantine in the treatment of learning and cognitive dysfunction and the negative symptoms of schizophrenia has become increasingly clear in recent years, although its effects on hallucinations via dopamine neurotransmission in the nucleus accumbens should be carefully monitored [79,80].
5. Conclusions
This study outlines the current state of the polypharmacy of drugs used in patients with Alzheimer’s disease and the drug interactions with cognitive enhancers. Patients with Alzheimer’s disease have increased sensitivity to psychotropic drugs owing to their decreased brain function and delayed drug elimination due to aging. These factors make them susceptible to polypharmacy and drug interactions. Adverse drug events can decrease the patients’ quality of life and worsen their prognosis. Considerable effort should be made to improve patients’ quality of life.
Author Contributions
Conceptualisation, S.E. and Y.Z.; writing—original draft preparation, S.E.; and writing—review and editing, S.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maust, D.T.; Strominger, J.; Kim, H.M.; Langa, K.M.; Bynum, J.P.W.; Chang, C.-H.; Kales, H.C.; Zivin, K.; Solway, E.; Marcus, S.C. Prevalence of Central Nervous System-Active Polypharmacy among Older Adults with Dementia in the US. JAMA 2021, 325, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778. [Google Scholar] [CrossRef]
- Kim, Y.J.; Han, J.W.; So, Y.S.; Seo, J.Y.; Kim, K.Y.; Kim, K.W. Prevalence and trends of dementia in Korea: A systematic review and meta-analysis. J. Korean Med. Sci. 2014, 29, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Park, B.; Kim, J.H.; Kim, J.H.; Kwon, M.J.; Kim, M. Causes of Mortality in Korean Patients with Neurodegenerative Dementia. Biomed. Res. Int. 2022, 2022, 3206594. [Google Scholar] [CrossRef] [PubMed]
- Kasajima, M.; Eggleston, K.; Kusaka, S.; Matsui, H.; Tanaka, T.; Son, B.K.; Iijima, K.; Goda, K.; Kitsuregawa, M.; Bhattacharya, J.; et al. Projecting prevalence of frailty and dementia and the economic cost of care in Japan from 2016 to 2043: A microsimulation modelling study. Lancet Public Health 2022, 7, e458–e468. [Google Scholar] [CrossRef]
- Salisbury, C. Multimorbidity: Redesigning health care for people who use it. Lancet 2012, 380, 7–9. [Google Scholar] [CrossRef]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Valderas, J.M.; Starfield, B.; Sibbald, B.; Salisbury, C.; Roland, M. Defining comorbidity: Implications for understanding health and health services. Ann. Fam. Med. 2009, 7, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Fried, T.R.; O’Leary, J.; Towle, V.; Goldstein, M.K.; Trentalange, M.; Martin, D.K. Health outcomes associated with polypharmacy in community-dwelling older adults: A systematic review. J. Am. Geriatr. Soc. 2014, 62, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Clague, F.; Mercer, S.W.; Mclean, G.; Reynish, E.; Guthrie, B. Comorbidity and polypharmacy in people with dementia: Insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing 2017, 46, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C. Polypharmacy and inappropriate medication use in patients with dementia: An underresearched problem. Ther. Adv. Drug Saf. 2017, 8, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Growdon, M.E.; Gan, S.; Yaffe, K.; Steinman, M.A. Polypharmacy among older adults with dementia compared with those without dementia in the United States. J. Am. Geriatr. Soc. 2021, 69, 2464–2475. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, C.J.; Smit, E.; Lee, D.S.H.; Alramadhan, F.; Odden, M.C. Polypharmacy among Adults Aged 65 Years and Older in the United States: 1988–2010. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Frazier, S.C. Health outcomes and polypharmacy in elderly individuals: An integrated literature review. J. Gerontol. Nurs. 2005, 31, 4–9. [Google Scholar] [CrossRef]
- Safran, D.G.; Neuman, P.; Schoen, C.; Kitchman, M.S.; Wilson, I.B.; Cooper, B.; Li, A.; Chang, H.; Rogers, W.H. Prescription drug coverage and seniors: Findings from a 2003 national survey. Health Aff. 2005, 24, W5–W152. [Google Scholar] [CrossRef]
- Mortazavi, S.S.; Shati, M.; Keshtkar, A.; Malakouti, S.K.; Bazargan, M.; Assari, S. Defining polypharmacy in the elderly: A systematic review protocol. BMJ Open 2016, 6. [Google Scholar] [CrossRef]
- Gnjidic, D.; Hilmer, S.N.; Blyth, F.M.; Naganathan, V.; Waite, L.; Seibel, M.J.; McLachlan, A.J.; Cumming, R.G.; Handelsman, D.J.; Le Couteur, D.G. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 2012, 65, 989–995. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Pilotto, A.; Vaona, A.; Demurtas, J.; Mueller, C.; Huntley, J.; Crepaldi, G.; et al. Polypharmacy Is Associated with Higher Frailty Risk in Older People: An 8-Year Longitudinal Cohort Study. J. Am. Med. Dir. Assoc. 2017, 18, 624–628. [Google Scholar] [CrossRef]
- Saum, K.U.; Schöttker, B.; Meid, A.D.; Holleczek, B.; Haefeli, W.E.; Hauer, K.; Brenner, H. Is Polypharmacy Associated with Frailty in Older People? Results from the ESTHER Cohort Study. J. Am. Geriatr. Soc. 2017, 65, e27–e32. [Google Scholar] [CrossRef]
- Morin, L.; Larrañaga, A.C.; Welmer, A.K.; Rizzuto, D.; Wastesson, J.W.; Johnell, K. Polypharmacy and injurious falls in older adults: A nationwide nested case-control study. Clin. Epidemiol. 2019, 11, 483–493. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Sarquis-Adamson, Y.; Song, H.Y.; Bray, N.W.; Pieruccini-Faria, F.; Speechley, M. Polypharmacy, Gait Performance, and Falls in Community-Dwelling Older Adults. Results from the Gait and Brain Study. J. Am. Geriatr. Soc. 2019, 67, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Disalvo, D.; Luckett, T.; Luscombe, G.; Bennett, A.; Davidson, P.; Chenoweth, L.; Mitchell, G.; Pond, D.; Phillips, J.; Beattie, E.; et al. Potentially Inappropriate Prescribing in Australian Nursing Home Residents with Advanced Dementia: A Substudy of the IDEAL Study. J. Palliat. Med. 2018, 21, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Hukins, D.; Macleod, U.; Boland, J.W. Identifying potentially inappropriate prescribing in older people with dementia: A systematic review. Eur. J. Clin. Pharm. 2019, 75, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Barry, H.E.; Cooper, J.A.; Ryan, C.; Passmore, A.P.; Robinson, A.L.; Molloy, G.J.; Darcy, C.M.; Buchanan, H.; Hughes, C.M. Potentially Inappropriate Prescribing among People with Dementia in Primary Care: A Retrospective Cross-Sectional Study Using the Enhanced Prescribing Database. J. Alzheimers Dis. 2016, 52, 1503–1513. [Google Scholar] [CrossRef]
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef]
- O’mahony, D.; O’sullivan, D.; Byrne, S.; O’connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef]
- Xing, X.X.; Zhu, C.; Liang, H.Y.; Wang, K.; Chu, Y.Q.; Zhao, L.B.; Jiang, D.C.; Wang, Y.Q.; Yan, S.Y. Associations between Potentially Inappropriate Medications and Adverse Health Outcomes in the Elderly: A Systematic Review and Meta-analysis. Ann. Pharmacother. 2019, 53, 1005–1019. [Google Scholar] [CrossRef]
- Porter, B.; Arthur, A.; Savva, G.M. How do potentially inappropriate medications and polypharmacy affect mortality in frail and non-frail cognitively impaired older adults? A cohort study. BMJ Open 2019, 9, e026171. [Google Scholar] [CrossRef]
- Harrison, S.L.; Kouladjian O’Donnell, L.; Bradley, C.E.; Milte, R.; Dyer, S.M.; Gnanamanickam, E.S.; Liu, E.; Hilmer, S.N.; Crotty, M. Associations between the Drug Burden Index, Potentially Inappropriate Medications and Quality of Life in Residential Aged Care. Drugs Aging 2018, 35, 83–91. [Google Scholar] [CrossRef]
- Renom-Guiteras, A.; Thürmann, P.A.; Miralles, R.; Klaaßen-Mielke, R.; Thiem, U.; Stephan, A.; Bleijlevens, M.H.C.; Jolley, D.; Leino-Kilpi, H.; Hallberg, I.R.; et al. Potentially inappropriate medication among people with dementia in eight European countries. Age Ageing 2018, 47, 68–74. [Google Scholar] [CrossRef]
- Murphy, C.; Dyer, A.H.; Lawlor, B.; Kennelly, S. Potentially inappropriate medication use in older adults with mild-moderate Alzheimer’s disease: Prevalence and associations with adverse events. Age Ageing 2020, 49, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Prescribing cascade: Calcium-channel blockers and diuretics. Drug Ther. Bull 2021, 59, 19. [CrossRef] [PubMed]
- Kverno, K. First Do No Harm: Psychotropic Prescribing Principles and Guidelines for Older Adults. J. Psychosoc. Nurs. Ment. Health Serv. 2020, 58, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Cocoros, N.M.; Haynes, K.; Nair, V.P.; Harkins, T.P.; Rochon, P.A.; Platt, R.; Dashevsky, I.; Reynolds, J.; Mazor, K.M.; et al. Antidopaminergic-Antiparkinsonian Medication Prescribing Cascade in Persons with Alzheimer’s Disease. J. Am. Geriatr. Soc. 2021, 69, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Storms, H.; Marquet, K.; Aertgeerts, B.; Claes, N. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: A systematic review. Eur. J. Gen. Pract. 2017, 23, 69–77. [Google Scholar] [CrossRef]
- Sköldunger, A.; Fastbom, J.; Wimo, A.; Fratiglioni, L.; Johnell, K. Impact of Inappropriate Drug Use on Hospitalizations, Mortality, and Costs in Older Persons and Persons with Dementia: Findings from the SNAC Study. Drugs Aging 2015, 32, 671–678. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; Dekosky, S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Christine, D.; Bray, T.; Castellon, S.; Masterman, D.; MacMillan, A.; Ketchel, P.; DeKosky, S.T. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: The Neuropsychiatric Inventory Caregiver Distress Scale. J. Am. Geriatr. Soc. 1998, 46, 210–215. [Google Scholar] [CrossRef]
- Gauthier, S.; Molinuevo, J.L. Benefits of combined cholinesterase inhibitor and memantine treatment in moderate-severe Alzheimer’s disease. Alzheimers Dement 2013, 9, 326–331. [Google Scholar] [CrossRef]
- Krolak-Salmon, P.; Dubois, B.; Sellal, F.; Delabrousse-Mayoux, J.P.; Vandel, P.; Amieva, H.; Jeandel, C.; Andrieu, S.; Perret-Liaudet, A. France Will No More Reimburse Available Symptomatic Drugs against Alzheimer’s Disease. J. Alzheimers Dis. 2018, 66, 425–427. [Google Scholar] [CrossRef]
- Moore, T.J.; Mattison, D.R. Adult Utilization of Psychiatric Drugs and Differences by Sex, Age, and Race. JAMA Intern. Med. 2017, 177, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Maust, D.T.; Strominger, J.; Bynum, J.P.W.; Langa, K.M.; Gerlach, L.B.; Zivin, K.; Marcus, S.C. Prevalence of Psychotropic and Opioid Prescription Fills among Community-Dwelling Older Adults with Dementia in the US. JAMA 2020, 324, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Maust, D.T.; Gerlach, L.B.; Gibson, A.; Kales, H.C.; Blow, F.C.; Olfson, M. Trends in Central Nervous System-Active Polypharmacy among Older Adults Seen in Outpatient Care in the United States. JAMA Intern. Med. 2017, 177, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Fick, D.M.; Semla, T.P.; Steinman, M.; Beizer, J.; Brandt, N.; Dombrowski, R.; DuBeau, C.E.; Pezzullo, L.; Epplin, J.J.; Flanagan, N.; et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Girotra, P.; Behl, T.; Sehgal, A.; Singh, S.; Bungau, S. Investigation of the Molecular Role of Brain-Derived Neurotrophic Factor in Alzheimer’s Disease. J. Mol. Neurosci. 2022, 72, 173–186. [Google Scholar] [CrossRef]
- Gnjidic, D.; Hilmer, S.N.; Hartikainen, S.; Tolppanen, A.M.; Taipale, H.; Koponen, M.; Bell, J.S. Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer’s disease: A national population cohort study. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Thorpe, J.M.; Thorpe, C.T.; Gellad, W.F.; Good, C.B.; Hanlon, J.T.; Mor, M.K.; Pleis, J.R.; Schleiden, L.J.; Van Houtven, C.H. Dual Health Care System Use and High-Risk Prescribing in Patients with Dementia: A National Cohort Study. Ann. Intern. Med. 2017, 166, 157–163. [Google Scholar] [CrossRef]
- Bayliss, E.A.; Shetterly, S.M.; Drace, M.L.; Norton, J.; Green, A.R.; Reeve, E.; Weffald, L.A.; Wright, L.; Maciejewski, M.L.; Sheehan, O.C.; et al. The OPTIMIZE patient- and family-centered, primary care-based deprescribing intervention for older adults with dementia or mild cognitive impairment and multiple chronic conditions: Study protocol for a pragmatic cluster randomized controlled trial. Trials 2020, 21, 542. [Google Scholar] [CrossRef]
- Rasu, R.S.; Shrestha, N.; Karpes Matusevich, A.R.; Zalmai, R.; Large, S.; Johnson, L.; O’Bryant, S.E. Polypharmacy and Cognition Function among Rural Adults. J. Alzheimers Dis. 2021, 82, 607–619. [Google Scholar] [CrossRef]
- Stoehr, G.P.; Lu, S.Y.; Lavery, L.; Bilt J vander Saxton, J.A.; Chang, C.C.H.; Ganguli, M. Factors associated with adherence to medication regimens in older primary care patients: The Steel Valley Seniors Survey. Am. J. Geriatr. Pharm. 2008, 6, 255–263. [Google Scholar] [CrossRef]
- Turner, B.J.; Hollenbeak, C.; Weiner, M.G.; ten Have, T.; Roberts, C. Barriers to adherence and hypertension control in a racially diverse representative sample of elderly primary care patients. Pharm. Drug Saf. 2009, 18, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Mahoney, J.E.; Blough, D.K. Medication adherence in elderly patients receiving home health services following hospital discharge. Ann. Pharm. 2001, 35, 539–545. [Google Scholar] [CrossRef]
- Chapman, R.H.; Petrilla, A.A.; Benner, J.S.; Schwartz, J.S.; Tang, S.S.K. Predictors of adherence to concomitant antihypertensive and lipid-lowering medications in older adults: A retrospective, cohort study. Drugs Aging 2008, 25, 885–892. [Google Scholar] [CrossRef]
- Ginsberg, G.; Hattis, D.; Russ, A.; Sonawane, B. Pharmacokinetic and pharmacodynamic factors that can affect sensitivity to neurotoxic sequelae in elderly individuals. Environ. Health Perspect. 2005, 113, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.J.; le Couteur, D.G. Aging biology and geriatric clinical pharmacology. Pharm. Rev. 2004, 56, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Wórum, I.; Csongor, J.; Fóris, G.; Leövey, A. Body composition in elderly people. I. Determination of body composition by multiisotope method and the elimination kinetics of these isotopes in healthy elderly subjects. Gerontology 1985, 31, 6–14. [Google Scholar] [CrossRef]
- Bentué-Ferrer, D.; Tribut, O.; Polard, E.; Allain, H. Clinically significant drug interactions with cholinesterase inhibitors: A guide for neurologists. CNS Drugs 2003, 17, 947–963. [Google Scholar] [CrossRef]
- Wynne, H.A.; Cope, L.H.; Mutch, E.; Rawlins, M.D.; Woodhouse, K.W.; James, O.F.W. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 1989, 9, 297–301. [Google Scholar] [CrossRef]
- Zoli, M.; Magalotti, D.; Bianchi, G.; Gueli, C.; Orlandini, C.; Grimaldi, M.; Marchesini, G. Total and functional hepatic blood flow decrease in parallel with ageing. Age Ageing 1999, 28, 29–34. [Google Scholar] [CrossRef]
- Spina, E.; Scordo, M.G.; D’Arrigo, C. Metabolic drug interactions with new psychotropic agents. Fundam. Clin. Pharm. 2003, 17, 517–538. [Google Scholar] [CrossRef]
- Sweeney, B.P.; Bromilow, J. Liver enzyme induction and inhibition: Implications for anaesthesia. Anaesthesia 2006, 61, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, P.J.; Perdomo, C.A.; Friedhoff, L.T. Concurrent administration of donepezil HCl and ketoconazole: Assessment of pharmacokinetic changes following single and multiple doses. Br. J. Clin. Pharmacol. 1998, 46 (Suppl. S1), 30–34. [Google Scholar] [CrossRef]
- Huang, F.; Fu, Y. A review of clinical pharmacokinetics and pharmacodynamics of galantamine, a reversible acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease, in healthy subjects and patients. Curr. Clin. Pharm. 2010, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Polinsky, R.J. Clinical pharmacology of rivastigmine: A new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin. Ther. 1998, 20, 634–647. [Google Scholar] [CrossRef]
- Grossberg, G.T.; Stahelin, H.B.; Messina, J.C.; Anand, R.; Veach, J. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int. J. Geriatr. Psychiatry 2000, 15, 242–247. [Google Scholar] [CrossRef]
- Noetzli, M.; Eap, C.B. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin. Pharm. 2013, 52, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Hasegawa, S.; Ishizuka, T.; Shiosakai, K.; Ishizuka, H. Pharmacokinetics and Bioequivalence of Memantine Tablet and a New Dry Syrup Formulation in Healthy Japanese Males: Two Single-Dose Crossover Studies. Adv. Ther. 2019, 36, 2930. [Google Scholar] [CrossRef]
- Freudenthaler, S.; Meineke, I.; Schreeb, K.H.; Boakye, E.; Gundert-Remy, U.; Gleiter, C.H. Influence of urine pH and urinary flow on the renal excretion of memantine. Br. J. Clin. Pharm. 1998, 46, 541–546. [Google Scholar] [CrossRef]
- Müller, F.; Weitz, D.; Derdau, V.; Sandvoss, M.; Mertsch, K.; König, J.; Fromm, M.F. Contribution of MATE1 to Renal Secretion of the NMDA Receptor Antagonist Memantine. Mol. Pharm. 2017, 14, 2991–2998. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.M.; Anderson, M.J.; Spivack, B. Use of anticholinergic medications by older adults with dementia. J. Am. Geriatr. Soc. 2002, 50, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, R.M.; Lund, B.C.; Perry, P.J.; Chrischilles, E.A. The concurrent use of anticholinergics and cholinesterase inhibitors: Rare event or common practice? J. Am. Geriatr. Soc. 2004, 52, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, G.; Klein-Schwartz, W.; Edwards, R. Donepezil overdose: A tenfold dosing error. Ann. Pharm. 1999, 33, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Han, H.J.; Youn, Y.C.; Park, K.W.; Yang, D.W.; Kim, S.; Kim, H.J.; Kim, J.E.; Lee, J.H. Safety and tolerability of donepezil 23 mg with or without intermediate dose titration in patients with Alzheimer’s disease taking donepezil 10 mg: A multicenter, randomized, open-label, parallel-design, three-arm, prospective trial. Alzheimers Res. Ther. 2019, 11, 37. [Google Scholar] [CrossRef]
- Herrmann, N.; Li, A.; Lanctôt, K. Memantine in dementia: A review of the current evidence. Expert Opin. Pharm. 2011, 12, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Spanagel, R.; Eilbacher, B.; Wilke, R. Memantine-induced dopamine release in the prefrontal cortex and striatum of the rat—A pharmacokinetic microdialysis study. Eur. J. Pharm. 1994, 262, 21–26. [Google Scholar] [CrossRef]
- Miller, E.K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000, 1, 59–65. [Google Scholar] [CrossRef]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar] [CrossRef]
- Hassanpour, F.; Zarghami, M.; Mouodi, S.; Moosazadeh, M.; Barzegar, F.; Bagheri, M.; Hendouei, N. Adjunctive Memantine Treatment of Schizophrenia: A Double-Blind, Randomized Placebo-Controlled Study. J. Clin. Psychopharmacol. 2019, 39, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Sandström, K.O.; Baltzersen, O.B.; Marsman, A.; Lemvigh, C.K.; Boer, V.O.; Bojesen, K.B.; Nielsen, M.Ø.; Lundell, H.; Sulaiman, D.K.; Sørensen, M.E.; et al. Add-on MEmaNtine to Dopamine Antagonism to Improve Negative Symptoms at First Psychosis—the AMEND Trial Protocol. Front. Psychiatry 2022, 13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).