HLA-B*27 Heavy Chain Homo-Oligomers Promote the Cytotoxicity of NK Cells via Activation of PI3K/AKT Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Stable Over-Expression of HLA-B*2704 C67S in HMy2.C1R Cells
2.2. Biotinylation of Cell Surface Proteins and Pulldown by Avidin Beads
2.3. Analysis of the Perforin Production of NK-92MI Co-Cultured with HMy2.C1R or C1R-B*2704 or C1R-B*2704 C67S Cells
2.4. Analysis of NK Cell-Mediated Cytotoxicity
2.5. Analysis of the Signaling Pathway of NK Cells Induced by Co-Culturing with C1R, C1R-B*2704, or C1R-B*2704 C67S Cells
2.6. Targeted Delivery of HLA-B27-Binding Peptide by THU Vehicle into the ER of C1R-B*2704 Cells and Analysis of the Perforin Production of NK-92MI Cells Co-Cultured with Peptide-Targeted C1R-B*2704 Cells
2.7. Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
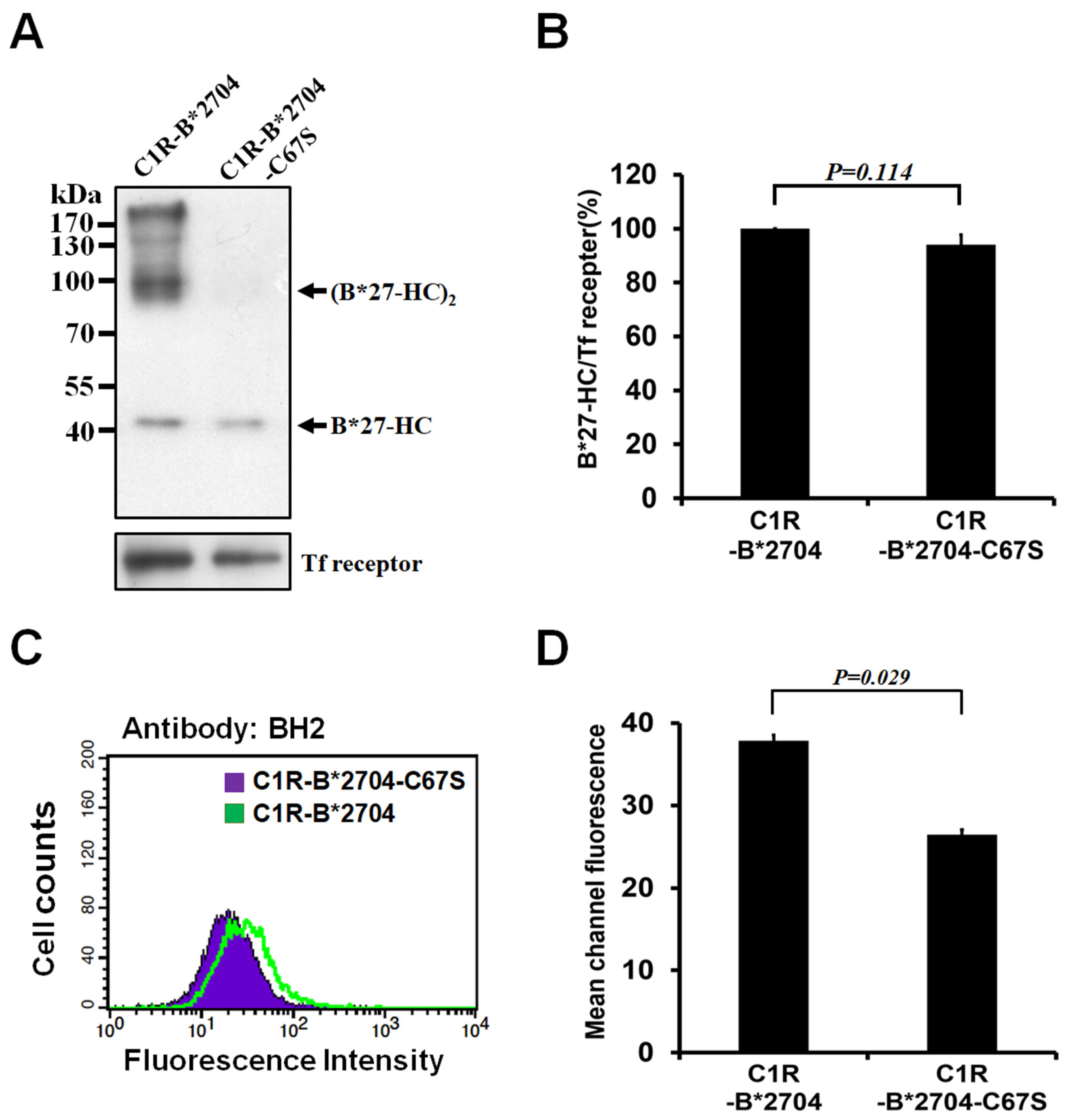
3.1. Replacement of Cys-67 with Ser Avoids the Oligomeric Formation of HLA-B*2704
3.2. The Perforin Production and Cytotoxicity of NK-92MI Cells were Promoted by Co-Culturing with C1R-B*2704 Cells
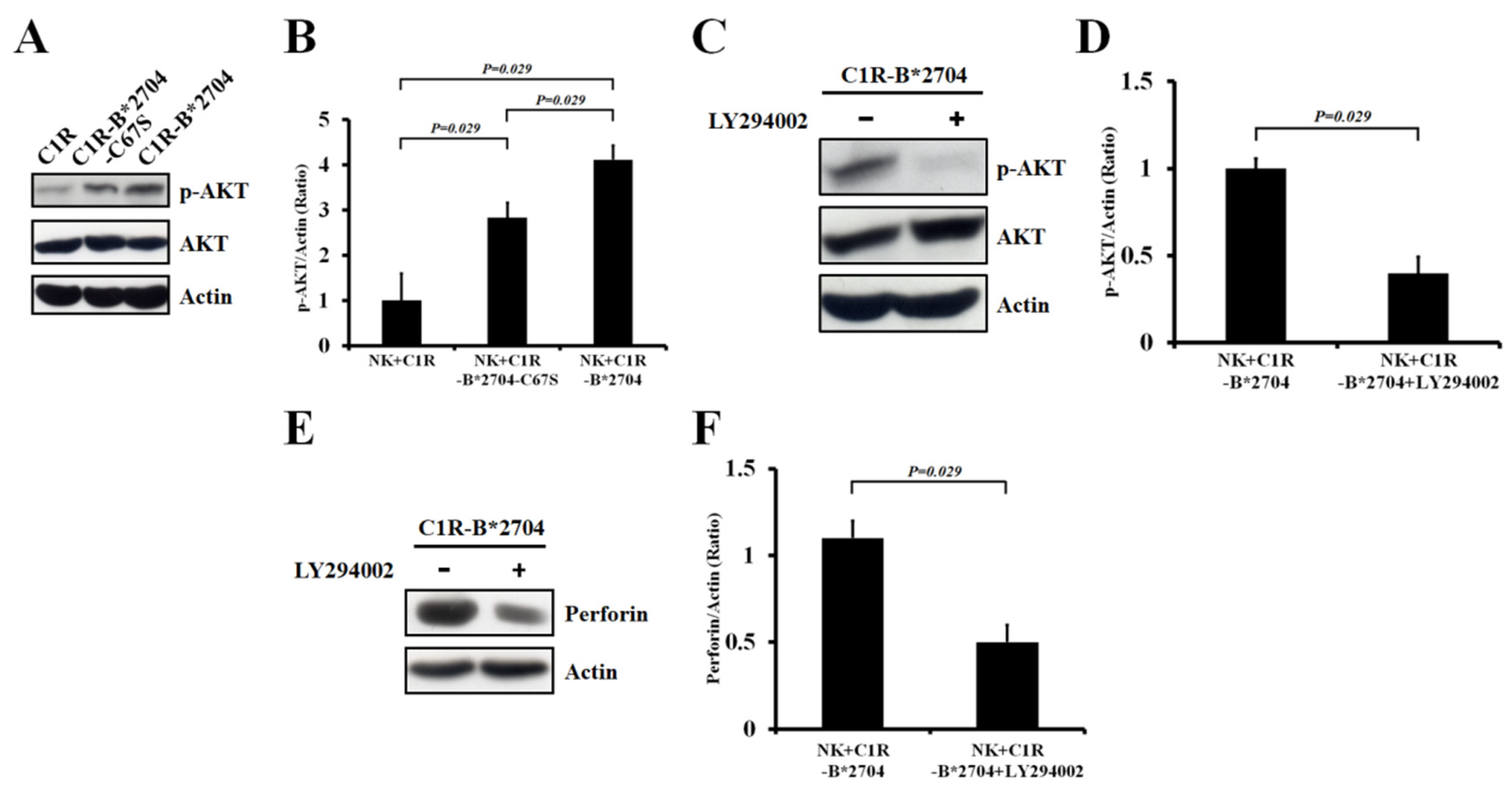
3.3. The PI3K/AKT Signaling of NK-92MI Cells were Activated by Co-Culturing with C1R-B*2704 Cells
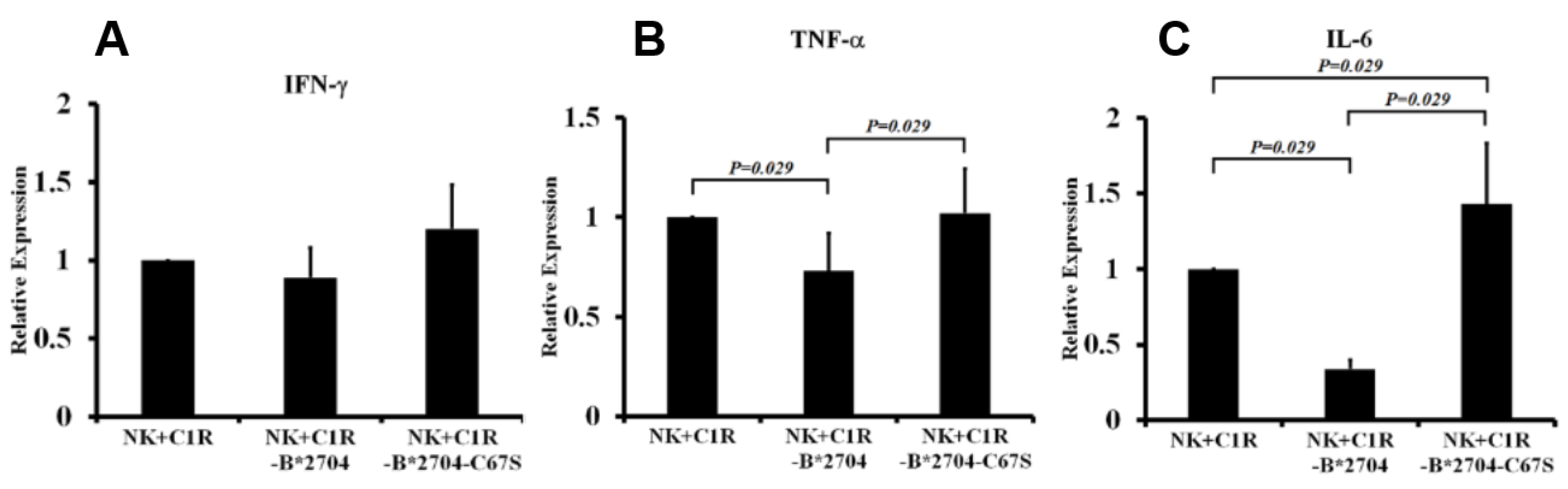
3.4. The IL6 and TNF-α Expressions of NK-92MI Cells were Suppressed by Co-Culturing with C1R-B*2704 Cells
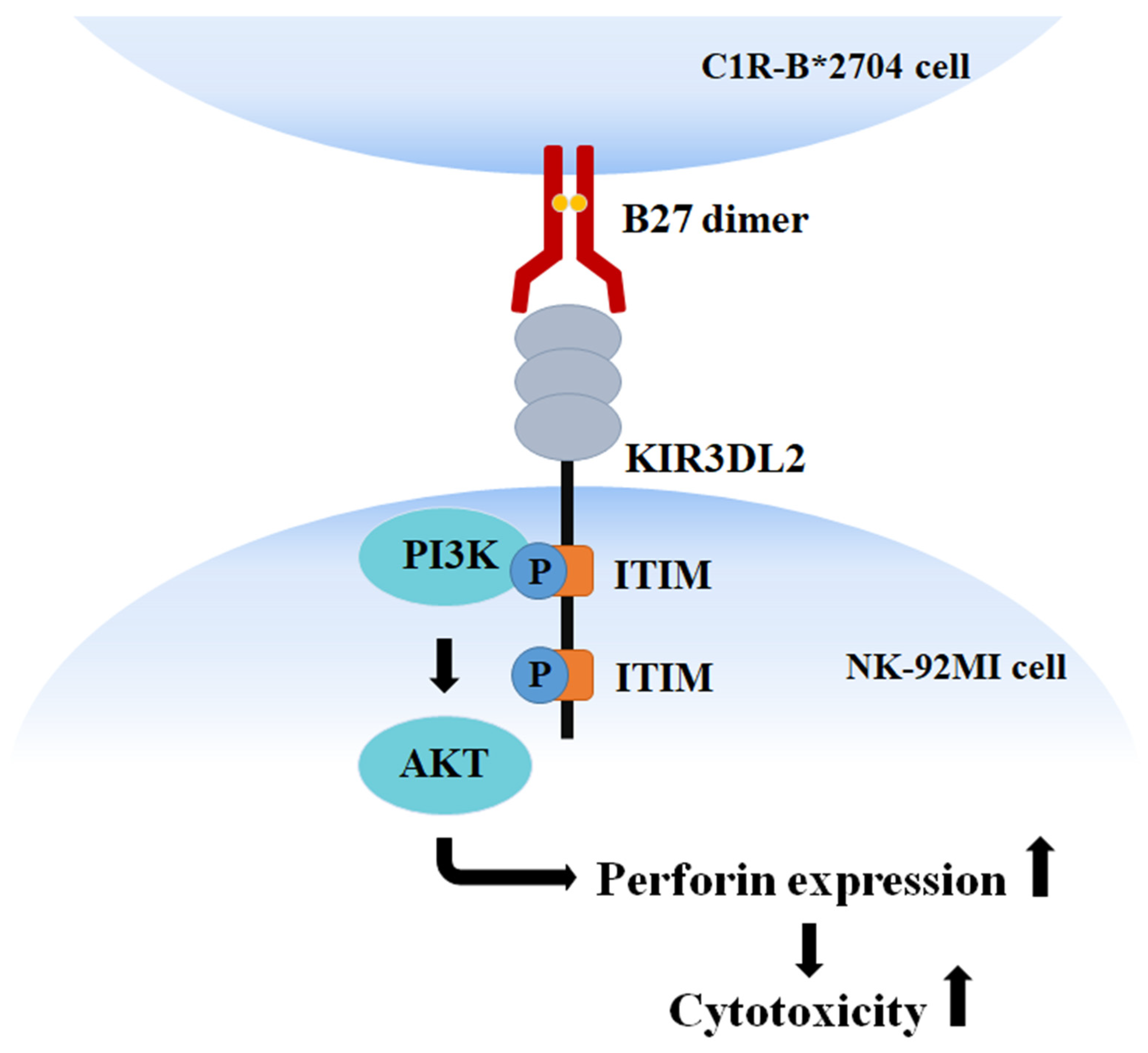
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Colbert, R.A.; DeLay, L.; Klenk, E.I.; Layh-Schmitt, G. From HLA-B27 to spondyloarthritis: A journey through the ER. Immunol. Rev. 2010, 233, 181–202. [Google Scholar] [CrossRef]
- Melis, L.; Elewaut, D. Immunopathogenesis of spondykoarthritis: Which cells drive disease? Arthritis Res. Ther. 2009, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Marker-Hermann, E.; Colbert, R.A. Pathogenesis of ankylosing spondylitis: Current concepts. Best Pract. Res. Clin. Rheumatol. 2006, 20, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Reveille, J.D.; Arnett, F.C. Spondyloarthitis: Update on pathogenesis and management. Am. J. Med. 2005, 118, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M.F.; James, D.C. Human lymphocyte antigen association in ankylosing spondylitis. Nature 1973, 5393, 121. [Google Scholar] [CrossRef] [PubMed]
- Brewerton, D.A.; Hart, F.D.; Nicholls, A.; Caffrey, M.; James, D.C.; Sturrock, R.D. Ankylosing spondylitis and HL-A 27. Lancet 1973, 1, 904–907. [Google Scholar] [CrossRef]
- Madden, D.R. The three-dimensional structure of peptide-MHC complexes. Ann. Rev. Immunol. 1995, 13, 587–622. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Larrea, C.; González, S.; Borra, J.M. The role of HLA-B27 polymorphism and molecular mimicry in spondyloarthropathy. Mol. Med. Today 1998, 4, 540–549. [Google Scholar] [CrossRef]
- Taurog, J.D.; Maika, S.D.; Satumtira, N.; Dorris, M.L.; McLean, I.L.; Yanagisawa, H.; Sayad, A.; Stagg, A.J.; Fox, G.M.; Le O’Brein, A.; et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999, 169, 209–223. [Google Scholar]
- May, E.; Dorris, M.L.; Satumtira, N.; Iqbal, I.; Rehman, M.I.; Lightfoot, E.; Taurog, J.D. CD8αβ T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J. Immunol. 2003, 170, 1099–1105. [Google Scholar] [CrossRef]
- Taurog, J.D.; Dorris, M.L.; Satumtira, N.; Tran, T.M.; Sharma, R.; Dressel, R.; van den Brandt, J.; Reichardt, H.M. Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 2009, 60, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.L.; O’Callaghan, C.A.; McMichael, A.J.; Bowness, P. HLA-B27 can form a novel β2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999, 162, 5045–5048. [Google Scholar] [PubMed]
- Kollnberger, S.; Bird, L.; Sun, M.Y.; Retiere, C.; Braud, V.M.; McMichael, A.; Bowness, P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002, 46, 2972–2982. [Google Scholar] [CrossRef] [PubMed]
- Dangoria, N.; DeLay, M.L.; Kingsbury, D.J.; Mear, J.P.; Uchanska-Ziegler, B.; Ziegler, A.; Colbert, R.A. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J. Biol. Chem. 2002, 277, 23459–23468. [Google Scholar] [CrossRef] [PubMed]
- Kollnberger, S.; Bird, L.A.; Roddis, M.; Hacquard-Bouder, C.; Kubagawa, H.; Bodmer, H.C.; Breban, M.; McMichael, A.J.; Bowness, P. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J. Immunol. 2004, 173, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Kollnberger, S.D.; Wedderburn, L.R.; Bowness, P. Expansion and enchanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 inspondylarthritis. Arthritis Rheum. 2005, 52, 3586–3596. [Google Scholar] [CrossRef] [PubMed]
- Bowness, P.; Ridley, A.; Shaw, J.; Chan, A.T.; Wong-Baeza, I.; Fleming, M.; Cummings, F.; McMichael, A.; Kollnberger, S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. 2011, 186, 2672–2680. [Google Scholar] [CrossRef]
- Wong-Baeza, I.; Ridley, A.; Shaw, J.; Hatano, H.; Rysnik, O.; McHugh, K.; Piper, C.; Brackenbridge, S.; Fernandes, R.; Chan, A.; et al. KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA Class I and promotes the expansion of T Cells in ankylosing spondylitis. J. Immunol. 2013, 190, 3216–3224. [Google Scholar] [CrossRef]
- Payeli, S.K.; Kollnberger, S.; Belaunzaran, O.M.; Thiel, M.; McHugh, K.; Giles, J.; Shaw, J.; Kleber, S.; Ridley, A.; Wong-Baeza, I.; et al. Inhibiting HLA–B27 homodimer–driven immune cell inflammation in spondylarthritis. Arthritis Rheum. 2012, 64, 3139–3149. [Google Scholar] [CrossRef]
- Vivier, E.; Anfossi, N. Inhibitory NK-cell receptors on T cells: Witness of the past, actors of the future. Nat. Rev. Immunol. 2004, 4, 191–198. [Google Scholar] [CrossRef]
- Marti, F.; Xu, W.; Selvakumar, A.; Brent, R.; DuPont, B.; King, P.D. LCK-phosphorylated human killer cell inhibitory receptors recruit and activate phosphatidylinositol 3-kinase. Proc. Nat. Acad. Sci. USA 1998, 95, 11810–11815. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Regulation of immune responses through inhibitory receptors. Ann. Rev. Immunol. 1999, 17, 875–904. [Google Scholar] [CrossRef] [PubMed]
- Storkus, W.J.; Alexander, J.; Payne, J.A.; Dawson, J.R.; Cresswell, P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc. Nat. Acad. Sci. USA 1989, 86, 2361–2364. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Li, M.C.; Huang, H.L.; Huang, K.Y.; Liu, S.Q.; Lai, N.S.; Huang, H.B. Targeted delivery of an antigenic peptide to the endoplasmic reticulum: Application for development of a peptide therapy for ankylosing spondylitis. PLoS ONE 2013, 8, e77451. [Google Scholar] [CrossRef]
- Yu, H.C.; Huang, K.Y.; Lu, M.C.; Huang, H.L.; Liu, S.Q.; Lai, N.S.; Huang, H.B. Targeted delivery of the HLA-B*27-binding peptide into the endoplasmic reticulum suppresses the IL-23/IL-17 axis of immune cells in spondylarthritis. Mediat. Inflamm. 2017, 2017, 4016802. [Google Scholar] [CrossRef]
- Zielinska, H.A.; Daly, C.S.; Alghamdi, A.; Alghamdi, A.; Babl, A.; Sohail, M.; White, P.; Dean, S.R.; Holly, J.M.P.; Perks, C.M. Interaction between GRP78 and IGFBP-3 affects 2 tumorigenesis and prognosis in breast cancer patients. Cancers 2020, 12, 3821. [Google Scholar] [CrossRef]
- Yu, H.C.; Huang, K.Y.; Lu, M.C.; Huang, H.L.; Liu, W.T.; Lee, W.C.; Liu, S.Q.; Huang, H.B.; Lai, N.S. Characterization of the recognition specificity of BH2, a monoclonal antibody Prepared against the HLA-B27 Heavy Chain. Int. J. Mol. Sci. 2015, 16, 8142–8150. [Google Scholar] [CrossRef]
- Yu, H.C.; Huang, K.Y.; Lu, M.C.; Huang Tseng, H.Y.; Liu, S.Q.; Lai, N.S.; Huang, H.B. Down-regulation of LOC645166 in T cells of ankylosing spondylitis patients promotes the NF-κB signaling via decreasingly blocking recruitment of the IKK complex to K63-linked polyubiquitin chains. Front. Immunol. 2021, 12, 5917056. [Google Scholar] [CrossRef]
- Shaw, J.; Kollnberger, S. New perspectives on the ligands and function of the killer cell immunoglobulin-like receptor KIR3DL2 in health and disease. Front. Immunol. 2012, 3, 339. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 12, 1124. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA class-I molecules in human natural killer cells. Ann. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Storkus, W.J.; Salter, R.D.; Cresswell, P.; Dawson, J.R. Peptide-induced modulation of target cell sensitivity to natural killing. J. Immunol. 1992, 149, 1185–1190. [Google Scholar] [PubMed]
- Moretta, L.; Ciccone, E.; Mingari, M.C.; Biassoni, R.; Moretta, A. Human NK cells: Origin, clonality, specificity and receptors. Adv. Immunol. 1994, 55, 341–380. [Google Scholar] [PubMed]
- Cifaldi, L.; Prencipe, G.; Caiello, I.; Bracaglia, C.; Locatelli, F.; de Benedetti, F.; Strippoli, R. Inhibition of natural killer cell cytotoxicity by interleukin-6 implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 2015, 67, 3037–3046. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-C.; Huang, K.-Y.; Lu, M.-C.; Huang Tseng, H.-Y.; Liu, S.-Q.; Lai, N.-S.; Huang, H.-B. HLA-B*27 Heavy Chain Homo-Oligomers Promote the Cytotoxicity of NK Cells via Activation of PI3K/AKT Signaling. Medicina 2022, 58, 1411. https://doi.org/10.3390/medicina58101411
Yu H-C, Huang K-Y, Lu M-C, Huang Tseng H-Y, Liu S-Q, Lai N-S, Huang H-B. HLA-B*27 Heavy Chain Homo-Oligomers Promote the Cytotoxicity of NK Cells via Activation of PI3K/AKT Signaling. Medicina. 2022; 58(10):1411. https://doi.org/10.3390/medicina58101411
Chicago/Turabian StyleYu, Hui-Chun, Kuang-Yung Huang, Ming-Chi Lu, Hsien-Yu Huang Tseng, Su-Qin Liu, Ning-Sheng Lai, and Hsien-Bin Huang. 2022. "HLA-B*27 Heavy Chain Homo-Oligomers Promote the Cytotoxicity of NK Cells via Activation of PI3K/AKT Signaling" Medicina 58, no. 10: 1411. https://doi.org/10.3390/medicina58101411
APA StyleYu, H.-C., Huang, K.-Y., Lu, M.-C., Huang Tseng, H.-Y., Liu, S.-Q., Lai, N.-S., & Huang, H.-B. (2022). HLA-B*27 Heavy Chain Homo-Oligomers Promote the Cytotoxicity of NK Cells via Activation of PI3K/AKT Signaling. Medicina, 58(10), 1411. https://doi.org/10.3390/medicina58101411






