TENS Improves Cisplatin-Induced Neuropathy in Lung Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
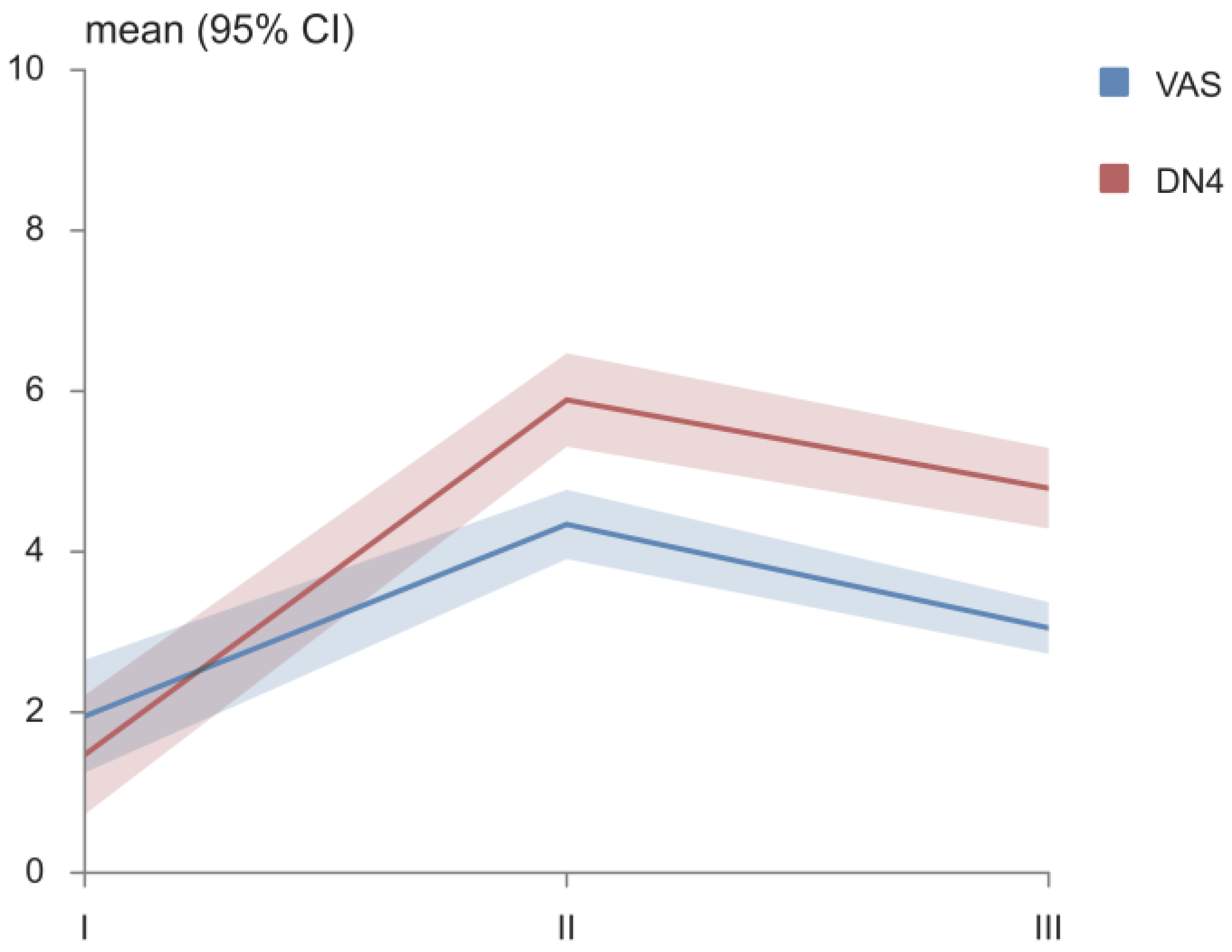
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Stefens-Stawna, P.; Piorunek, T.; Gabryel-Batura, H.; Kozubski, W.; Michalak, S. Neurological Paraneoplastic Syndromes in Lung Cancer Patients; Springer: Berlin/Heidelberg, Germany, 2012; Volume 756, pp. 333–339. [Google Scholar] [CrossRef]
- Hausheer, F.H.; Schilsky, R.L.; Bain, S.; Berghorn, E.J.; Lieberman, F. Diagnosis, Management, and Evaluation of Chemotherapy-Induced Peripheral Neuropathy. Semin. Oncol. 2006, 33, 15–49. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.-Y.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Drug-Induced Neurological Disorders; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Gregg, R.W.; Molepo, J.M.; Monpetit, V.J.; Mikael, N.Z.; Redmond, D.; Gadia, M.; Stewart, D.J. Cisplatin neurotoxicity: The relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J. Clin. Oncol. 1992, 10, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Mollman, J.; Glover, D.; Hogan, M.; Furman, R. Cisplatin neuropathy Risk Factors, Prognosis, and Protection by WR-2721 JOAN. Cancer 1988, 61, 2192–2195. [Google Scholar] [CrossRef]
- Andres, A.L.; Gong, X.; Di, K.; Bota, D.A. Low-doses of cisplatin injure hippocampal synapses: A mechanism for ‘chemo’ brain? Exp. Neurol. 2014, 255, 137–144. [Google Scholar] [CrossRef]
- Brydøy, M.; Oldenburg, J.; Klepp, O.; Bremnes, R.M.; Wist, E.A.; Wentzel-Larsen, T.; Hauge, E.R.; Dahl, O.; Fosså, S.D. Observational Study of Prevalence of Long-term Raynaud-Like Phenomena and Neurological Side Effects in Testicular Cancer Survivors. JNCI J. Natl. Cancer Inst. 2009, 101, 1682–1695. [Google Scholar] [CrossRef]
- Von Schlippe, M.; Fowler, C.J.; Harland, S.J. Cisplatin neurotoxicity in the treatment of metastatic germ cell tumour: Time course and prognosis. Br. J. Cancer 2001, 85, 823–826. [Google Scholar] [CrossRef][Green Version]
- Nalley, C. Risk Factors for Chemotherapy-Induced Peripheral Neuropathy. Oncol. Times 2021, 43, 33. [Google Scholar] [CrossRef]
- Molassiotis, A.; Cheng, H.L.; Leung, K.T.; Li, Y.C.; Wong, K.H.; Au, J.S.K.; Sundar, R.; Chan, A.; De Ng, T.R.; Suen, L.K.P.; et al. Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav. 2019, 9, e01312. [Google Scholar] [CrossRef] [PubMed]
- Hertz, D.L.; Tofthagen, C.; Faithfull, S. Predisposing Factors for the Development of Chemotherapy-Induced Peripheral Neuropathy (CIPN). In Diagnosis, Management and Emerging Strategies for Chemotherapy-Induced Neuropathy; Springer: Cham, Switzerland, 2021; pp. 19–51. [Google Scholar] [CrossRef]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Huh, B.; Kim, H.K.; Kim, K.-H.; Abdi, S. Treatment of Chemotherapy-Induced Peripheral Neuropathy: Systematic Review and Recommendations. Pain Physician 2018, 21, 571–592. [Google Scholar] [PubMed]
- Attal, N.; Cruccu, G.; Baron, R.A.; Haanpää, M.; Hansson, P.; Jensen, T.S.; Nurmikko, T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol. 2010, 17, 1113–1188. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, M.C.; Twilling, L.; Davies, P.S.; Reisner, L.; Taylor, K.; Mohr, D. Oral Opioid Therapy for Chronic Peripheral and Central Neuropathic Pain. New Engl. J. Med. 2003, 348, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aziz, T.Z.; Garcia-Larrea, L.; Hansson, P.; Jensen, T.S.; Lefaucheur, J.-P.; Simpson, B.A.; Taylor, R.S. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur. J. Neurol. 2007, 14, 952–970. [Google Scholar] [CrossRef]
- Kilinç, M.; Livanelioglu, A.; Yildirim, S.A.; Tan, E. Effects of transcutaneous electrical nerve stimulation in patients with peripheral and central neuropathic pain. J. Rehabil. Med. 2014, 46, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Püsküllüoğlu, M.; Tomaszewski, K.A.; Grela-Wojewoda, A.; Pacholczak-Madej, R.; Ebner, F. Effects of Transcutaneous Electrical Nerve Stimulation on Pain and Chemotherapy-Induced Peripheral Neuropathy in Cancer Patients: A Systematic Review. Medicina 2022, 58, 284. [Google Scholar] [CrossRef]
- Medical Reasrch Council. Aids to the Examination of the Peripheral Nervous System; Her Majesty’s Station Off: London, UK, 1976; pp. 1–61.
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.H.S.; Patterson, D.G. Experimental development of the graphic rating method. Psychol. Bull. 1921, 18, 98–99. [Google Scholar]
- Bergman, B.; Aaronson, N.; Ahmedzai, S.; Kaasa, S.; Sullivan, M. The EORTC QLQ-LC13: A modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur. J. Cancer 1994, 30, 635–642. [Google Scholar] [CrossRef]
- Cella, D.F.; Bonomi, A.E.; Lloyd, S.R.; Tulsky, D.S.; Kaplan, E.; Bonomi, P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995, 12, 199–220. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Savic, M.; Winham, S.J.; Stanisavljevic, D.; Garovic, V.D.; Milic, N.M. Data visualization, bar naked: A free tool for creating interactive graphics. J. Biol. Chem. 2017, 292, 20592–20598. [Google Scholar] [CrossRef] [PubMed]
- SPSS for Windows, release 25.0; SPSS: Chicago, IL, USA, 2017.
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Barton, D.; Kottschade, L.; Grothey, A.; Loprinzi, C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur. J. Cancer 2008, 44, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Coraci, D.; Brau, F.; Galluzzo, V.; Loreti, C.; Caliandro, P.; Padua, L.; Maccauro, G.; Biscotti, L.; Bernabei, R. Neuropathic Pain in the Elderly. Diagnostics 2021, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Mc Leod, J. Paraneoplastic neuropathies. In Peripheral Neuropathy. II; Dyck, P.J., Thomas, P.K., Eds.; Saunders: Philadelphia, PA, USA, 1993; pp. 1583–1590. [Google Scholar]
- Antoine, J.-C.; Mosnier, J.-F.; Absi, L.; Convers, P.; Honnorat, J.; Michel, D. Carcinoma associated paraneoplastic peripheral neuropathies in patients with and without anti-onconeural antibodies. J. Neurol. Neurosurg. Psychiatry 1999, 67, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, N.; Watanabe, N.; Kita, N.; Bandoh, S.; Tadokoro, A.; Ishii, T.; Dobashi, H.; Matsunaga, T. Paraneoplastic syndromes associated with lung cancer Nobuhiro Kanaji, Naoki Watanabe, Nobuyuki Kita, Shuji Bandoh, Akira Tadokoro, Tomoya Ishii, Hiroaki Dobashi, Takuya Matsunaga. World J. Clin. Oncol. 2014, 5, 197–223. [Google Scholar] [CrossRef]
- Miltenburg, N.C.; Boogerd, W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat. Rev. 2014, 40, 872–882. [Google Scholar] [CrossRef]
- Tofthagen, C. Patient Perceptions Associated With Chemotherapy-Induced Peripheral Neuropathy. Clin. J. Oncol. Nurs. 2010, 14, E22–E28. [Google Scholar] [CrossRef]
- Ruelle, L.; Bentea, G.; Sideris, S.; El Koulali, M.; Holbrechts, S.; Lafitte, J.-J.; Grigoriu, B.; Sculier, C.; Meert, A.-P.; Durieux, V.; et al. Autoimmune paraneoplastic syndromes associated to lung cancer: A systematic review of the literature Part 4: Neurological paraneoplastic syndromes, involving the peripheral nervous system and the neuromuscular junction and muscles. Lung Cancer 2017, 111, 150–163. [Google Scholar] [CrossRef]
- Krarup-Hansen, A.; Helweg-Larsen, S.; Schmalbruch, H.; Rorth, M.; Krarup, C. Neuronal involvement in cisplatin neuropathy: Prospective clinical and neurophysiological studies. Brain 2006, 130, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- McQuay, H.J.; Moore, R.A.; Eccleston, C.; Morley, S.; Williams, A.C. Systematic review of outpatient services for chronic pain control. Health Technol. Assess. 1997, 1, i–iv. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, L.; Labrenz, S.; Ziegler, D.; Martin, S. Effective treatment of symptomatic diabetic polyneuropathy by high-frequency external muscle stimulation. Diabetologia 2005, 48, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Marshall, H.J. Diabetic Peripheral Neuropathy: Amelioration of Pain With Transcutaneous Electrostimulation. Diabetes Care 1997, 20, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.A.; White, P.F.; Craig, W.F.; Ghoname, E.S.; Ahmed, H.E.; Proctor, T.J.; Noe, C.E.; Vakharia, A.S.; Gajraj, N. Percutaneous electrical nerve stimulation: A novel analgesic therapy for diabetic neuropathic pain. Diabetes Care 2000, 23, 365–370. [Google Scholar] [CrossRef]
- Forst, T.; Nguyen, M.; Forst, S.; Disselhoff, B.; Pohlmann, T.; Pfützner, A. Impact of low fre- quency transcutaneous electrical nerve stimulation on symptomatic diabetic neuropathy using the new salutaris device. Diabetes 2004, 17, 163–168. [Google Scholar]
- Cheing, G.L.Y.; Luk, M.L.M. Transcutaneous electrical nerve stimulation for neuropathic pain. J. Hand Surg. 2005, 30, 50–55. [Google Scholar] [CrossRef]
- Thorsteinsson, G.; Stonnington, H.H.; Stillwell, G.K.; Elveback, L.R. Transcutaneous electrical stimulation: A double-blind trial of its efficacy for pain. Arch. Phys. Med. Rehabil. 1977, 58, 8–13. [Google Scholar] [CrossRef]
- Rutgers, M.; Van Romunde, L.; Osman, P. A small randomized comparative trial of acupuncture versus transcutaneous electrical neurostimulation in postherpetic neuralgia. Pain Clin. 1988, 2, 87–89. [Google Scholar]
- Bloodworth, D.M.; Nguyen, B.N.; Garver, W.; Moss, F.; Pedroza, C.; Tran, T.; Chiou-Tan, F.Y. Comparison of stochastic vs. conventional transcutaneous electrical stimulation for pain modulation in patients with electromyographically documented radiculopathy. Am. J. Phys. Med. Rehabil. 2004, 83, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, R.M.; Miyasaki, J. Assessment: Efficacy of transcutaneous electric nerve stimulation in the treatment of pain in neurologic disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2009, 74, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Siemens, W.; Boehlke, C.; Bennett, M.I.; Offner, K.; Becker, G.; Gaertner, J. Transcutaneous electrical nerve stimulation for advanced cancer pain inpatients in specialist palliative care—A blinded, randomized, sham-controlled pilot cross-over trial. Support Care Cancer 2020, 28, 5323–5333. [Google Scholar] [CrossRef]
- Laurent, H.; Galvaing, G.; Thivat, E.; Coudeyre, E.; Aubreton, S.; Richard, R.; Kwiatkowski, F.; Costes, F.; Filaire, M. Effect of an intensive 3-week preoperative home rehabilitation programme in patients with chronic obstructive pulmonary disease eligible for lung cancer surgery: A multicentre randomised controlled trial. BMJ Open 2017, 7, e017307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gagnon, G.J.; Nasr, N.M.; Liao, J.J.; Molzahn, I.; Marsh, D.; McRae, D.; Henderson, F.C., Sr. Treatment of spinal tumors using cyberKnife fractionated stereotactic radiosurgery: Pain and quality-of-life assessment after treatment in 200 patients. Neurosurgery 2009, 64, 297–306. [Google Scholar] [CrossRef]
- Fiorelli, A.; Morgillo, F.; Milione, R.; Pace, M.C.; Passavanti, M.B.; Laperuta, P.; Aurilio, C.; Santini, M. Control of post-thoracotomy pain by transcutaneous electrical nerve stimulation: Effect on serum cytokine levels, visual analogue scale, pulmonary function and medication. Eur. J. Cardio-Thorac. Surg. 2011, 41, 861–868. [Google Scholar] [CrossRef]
- Ferreira, F.C.; Issy, A.M.; Sakata, R.K. Avaliação do efeito da estimulação nervosa elétrica transcutânea (TENS) para analgesia após toracotomia. Rev. Bras. Anestesiol. 2011, 61, 564–567. [Google Scholar] [CrossRef]
- Solak, O.; Turna, A.; Pekcolaklar, A.; Metin, M.; Sayar, A.; Gürses, A. Transcutaneous Electric Nerve Stimulation for the Treatment of Postthoracotomy Pain: A Randomized Prospective Study. Thorac. Cardiovasc. Surg. 2007, 55, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Banavaliker, J.N.; Das, P.K.; Hasti, S. Use of transcutaneous electrical nerve stimulation as an adjunctive to epidural analgesia in the management of acute thoracotomy pain. Indian J. Anaesth. 2010, 54, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.; Erdogan, A.; Erbil, N.; Karakaya, H.; Demircan, A. Prospective, Randomized, Placebo-controlled Study of the Effect of TENS on Postthoracotomy Pain and Pulmonary Function. World J. Surg. 2005, 29, 1563–1570. [Google Scholar] [CrossRef]
- DeSantana, J.M.; Sluka, K.A.; Lauretti, G.R. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: A randomized controlled trial. Clin. J. Pain 2009, 25, 12–19. [Google Scholar] [CrossRef] [PubMed]

| All Lung Cancer Patients n = 106 | without Neuropathy n = 68 | with Neuropathy n = 38 | Patients without Neuropathy before Cisplatin Therapy n = 60 | Patients without Neuropathy after Cisplatin Therapy n = 20 | Cisplatin-Induced Neuropathy n = 40 | |
|---|---|---|---|---|---|---|
| Gender, n (%) | ||||||
| Male | 74 (70%) | 45 (66%) | 29 (76%) | 42 (70%) | 15 (75%) | 27 (68%) |
| Female | 32 (30%) | 23 (34%) | 9 (24%) | 18 (30%) | 5 (25%) | 13 (32%) |
| Age, mean (range) | 64 (47–83) | 62 (47–83) | 65 (51–77) | 63 (47–82) | 63 (49–78) | 63 (47–82) |
| Smoking habits, n (%) | ||||||
| Never | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ex | 33 (31%) | 24 (35%) | 9 (24%) | 23 (38%) | 11 (55%) | 12 (30%) |
| Smoker | 72 (68%) | 44 (65%) | 28 (74%) | 37 (62%) | 9 (45%) | 28 (70%) |
| Stage of disease, n (%) | ||||||
| I | 2 (2%) | 1 (1%) | 1 (3%) | 1 (2%) | 0 (0%) | 1 (3%) |
| II | 5 (5%) | 2 (3%) | 3 (8%) | 1 (2%) | 0 (0%) | 1 (3%) |
| III | 55 (53%) | 35 (52%) | 20 (54%) | 31 (53%) | 13 (65%) | 18 (46%) |
| IV | 42 (40%) | 29 (43%) | 13 (35%) | 26 (44%) | 7 (35%) | 19 (49%) |
| Type of lung cancer, n (%) | ||||||
| Small-cell | 33 (31%) | 23 (34%) | 10 (26%) | 22 (37%) | 11 (55%) | 11 (28%) |
| Adenocarcinoma | 33 (31%) | 22 (33%) | 11 (29%) | 18 (31%) | 3 (15%) | 15 (38%) |
| Squamous | 39 (37%) | 22 (33%) | 17 (45%) | 19 (32%) | 6 (30%) | 13 (33%) |
| Small-cell/non-small-cell, n (%) | ||||||
| Small-cell | 32 (30%) | 22 (32%) | 10 (26%) | 21 (35%) | 10 (50%) | 11 (28%) |
| Non-small-cell | 74 (70%) | 46 (68%) | 28 (74%) | 39 (65%) | 10 (50%) | 29 (72%) |
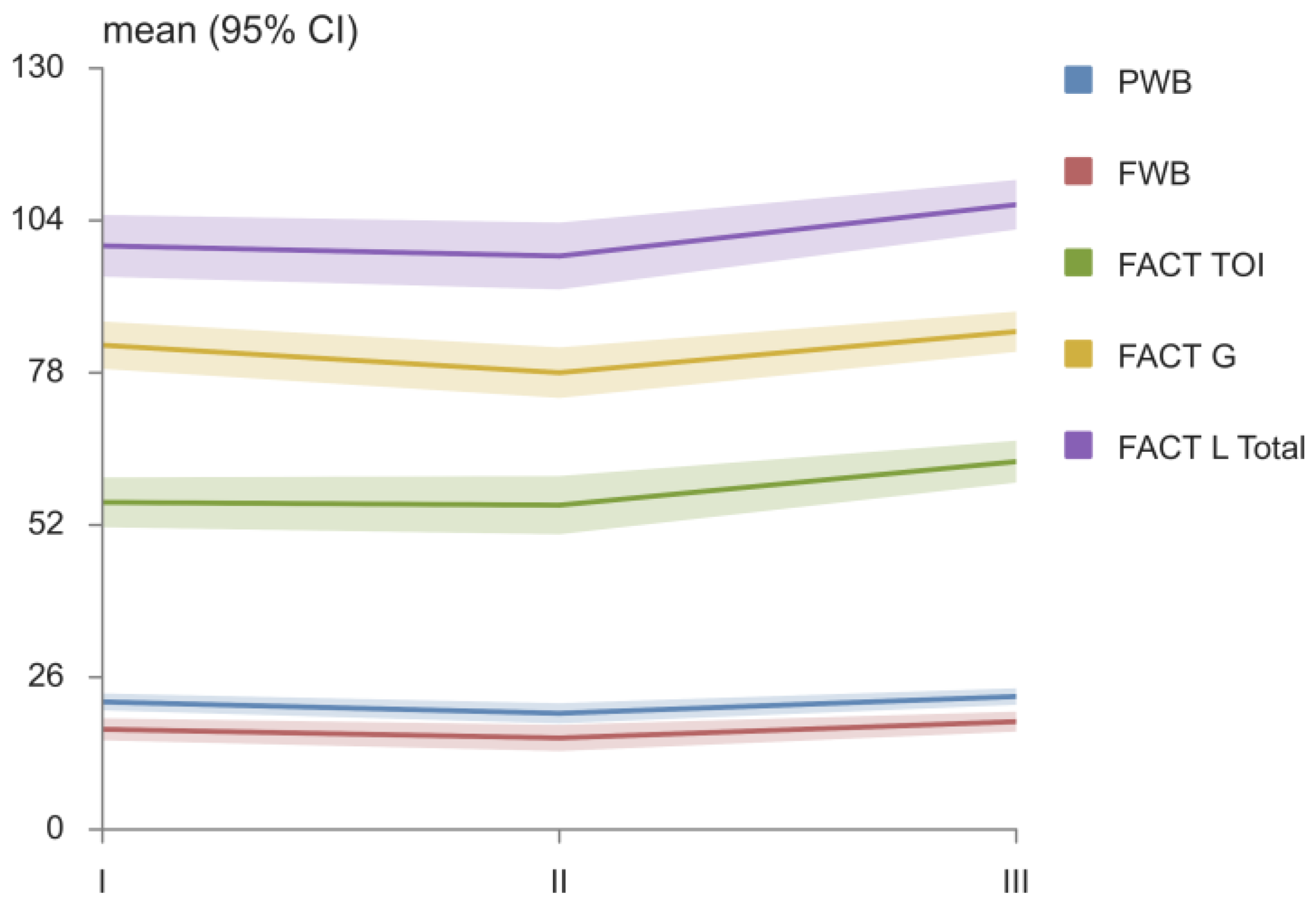
| FACT Domains | I | II | III | p |
|---|---|---|---|---|
| PWB, mean (95% CI) | 21.8 (20.4–23.2) | 19.8 (18.1–21.6) | 22.7 (21.3–24.1) | II vs. III * |
| SWB, mean (95% CI) | 25.3 (24.3–26.3) | 23.4 (22.7–24.1) | 23.4 (22.7–24.1) | I vs. II*, I vs. III * |
| EWB, mean (95% CI) | 18.5 (17.2–19.9) | 19.1 (17.9–20.4) | 20.5 (19.3–21.7) | I vs. III * |
| FWB, mean (95% CI) | 17.1 (15.2–19.0) | 15.6 (13.4–17.9) | 18.4 (16.7–20.1) | II vs. III * |
| LCS, mean (95% CI) | 17.0 (15.2–18.8) | 19.9 (18.1–21.7) | 21.7 (20.6–22.8) | I vs. II *, I vs. III *, II vs. III * |
| FACT L TOI, mean (95% CI) | 55.9 (51.6–60.1) | 55.4 (50.4–60.4) | 62.8 (59.3–66.4) | I vs. III *, II vs. III * |
| FACT G, mean (95% CI) | 82.7 (78.7–86.7) | 78.0 (73.7–82.3) | 85.0 (81.6–88.4) | II vs. III* |
| FACT L TOTAL, mean (95% CI) | 99.7 (94.4–104.9) | 97.9 (92.2–103.6) | 106.7 (102.5–110.9) | I vs. III *, II vs. III * |
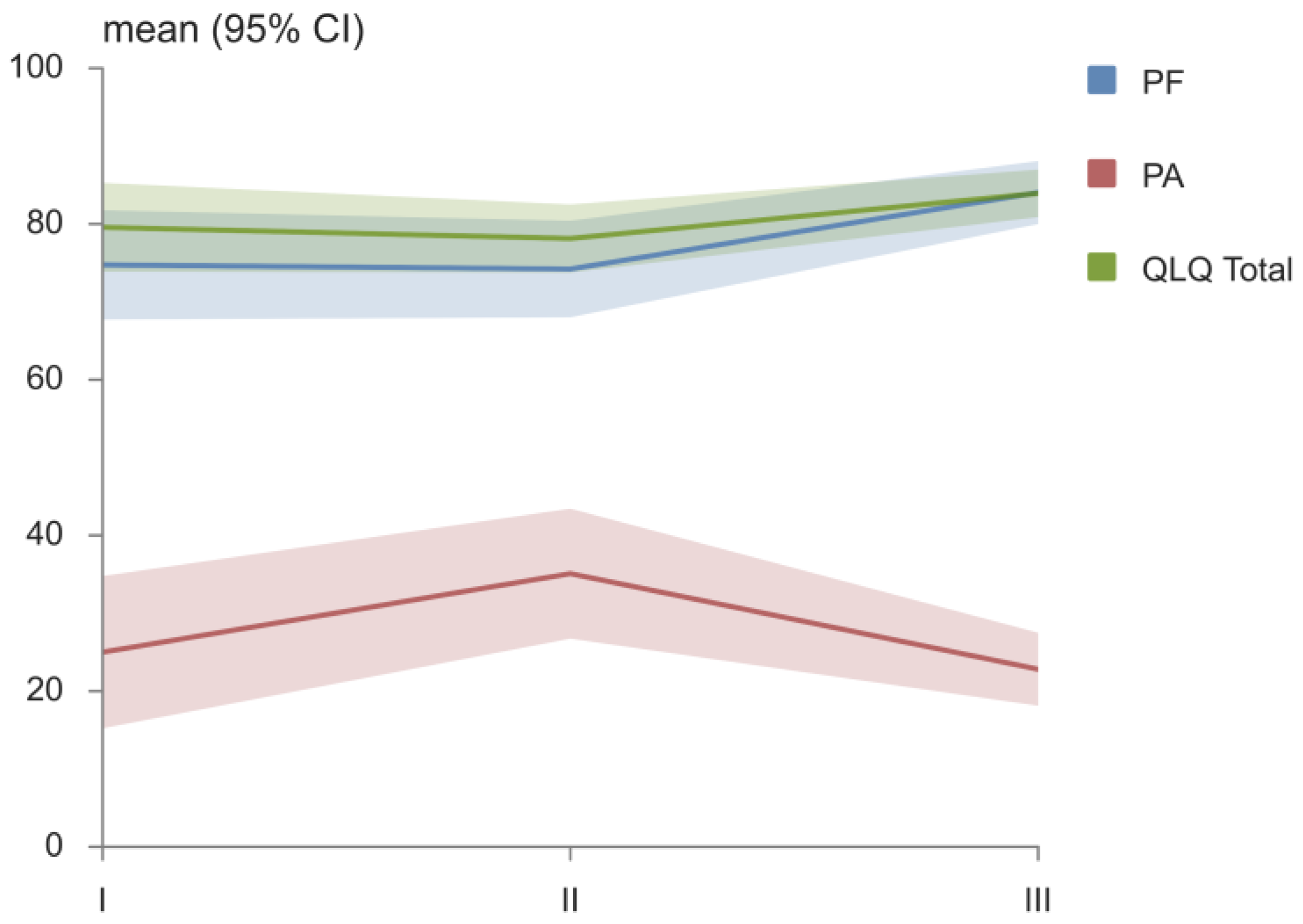
| EORTC QLQ-C30 Domains | I | II | III | p |
|---|---|---|---|---|
| PF, mean (95% CI) | 74.7 (67.7–81.7) | 74.2 (68.0–80.4) | 84.0 (80.0–88.1) | I vs. III *, II vs. III * |
| RF, mean (95% CI) | 67.6 (58.5–76.5) | 64.9 (55.2–74.6) | 71.1 (62.7–79.4) | II vs. III * |
| EF, mean (95% CI) | 73.9 (65.4–82.4) | 76.3 (69.4–83.2) | 79.8 (72.8–86.9) | |
| CF, mean (95% CI) | 93.4 (88.6–98.3) | 92.1 (86.0–98.2) | 94.7 (89.5–100.0) | |
| SF, mean (95% CI) | 82.9 (75.2–90.6) | 72.4 (64.2–80.6) | 76.8 (70.2–83.3) | |
| FA, mean (95% CI) | 36.5 (26.7–46.4) | 33.0 (26.3–39.8) | 28.9 (23.3–34.6) | |
| NV, mean (95% CI) | 7.0 (1.1–12.9) | 9.2 (2.5–15.9) | 6.6 (1.4–11.8) | |
| PA, mean (95% CI) | 25.0 (15.2–34.8) | 35.1 (26.8–43.4) | 22.8 (18.1–27.5) | II vs. III * |
| DY, mean (95% CI) | 22.8 (11.4–34.2) | 12.3 (4.9–19.7) | 14.0 (8.6–19.5) | |
| SL, mean (95% CI) | 25.4 (14.5–36.4) | 27.2 (17.7–36.7) | 15.8 (9.7–21.9) | II vs. III * |
| AP, mean (95% CI) | 24.6 (13.3–35.9) | 26.3 (15.5–37.2) | 14.0 (7.5–20.6) | II vs. III * |
| CO, mean (95% CI) | 12.3 (4.1–20.5) | 9.6 (4.0–15.3) | 8.8 (3.9–13.7) | |
| DI, mean (95% CI) | 4.4 (0.6–8.1) | 11.4 (5.0–17.8) | 4.4 (0.6–8.1) | I vs. II *, II vs. III * |
| FI, mean (95% CI) | 22.8 (12.6–33.0) | 35.1 (24.6–45.6) | 42.1 (30.8–53.4) | I vs. III * |
| QL, mean (95% CI) | 59.4 (52.8–66.0) | 57.0 (53.0–61.1) | 62.5 (59.3–65.7) | II vs. III * |
| QLQ Total, mean (95% CI) | 79.6 (73.9–85.3) | 78.1 (73.8–82.5) | 83.9 (80.9–87.0) | II vs. III * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomanovic Vujadinovic, S.; Ilic, N.; Selakovic, I.; Nedeljkovic, U.; Krstic, N.; Mujovic, N.; Dubljanin Raspopovic, E.; Jovanovic, D. TENS Improves Cisplatin-Induced Neuropathy in Lung Cancer Patients. Medicina 2022, 58, 1405. https://doi.org/10.3390/medicina58101405
Tomanovic Vujadinovic S, Ilic N, Selakovic I, Nedeljkovic U, Krstic N, Mujovic N, Dubljanin Raspopovic E, Jovanovic D. TENS Improves Cisplatin-Induced Neuropathy in Lung Cancer Patients. Medicina. 2022; 58(10):1405. https://doi.org/10.3390/medicina58101405
Chicago/Turabian StyleTomanovic Vujadinovic, Sanja, Nela Ilic, Ivan Selakovic, Una Nedeljkovic, Nevena Krstic, Natasa Mujovic, Emilija Dubljanin Raspopovic, and Dragana Jovanovic. 2022. "TENS Improves Cisplatin-Induced Neuropathy in Lung Cancer Patients" Medicina 58, no. 10: 1405. https://doi.org/10.3390/medicina58101405
APA StyleTomanovic Vujadinovic, S., Ilic, N., Selakovic, I., Nedeljkovic, U., Krstic, N., Mujovic, N., Dubljanin Raspopovic, E., & Jovanovic, D. (2022). TENS Improves Cisplatin-Induced Neuropathy in Lung Cancer Patients. Medicina, 58(10), 1405. https://doi.org/10.3390/medicina58101405





