The Association between Admission Procalcitonin Level and The Severity of COVID-19 Pneumonia: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
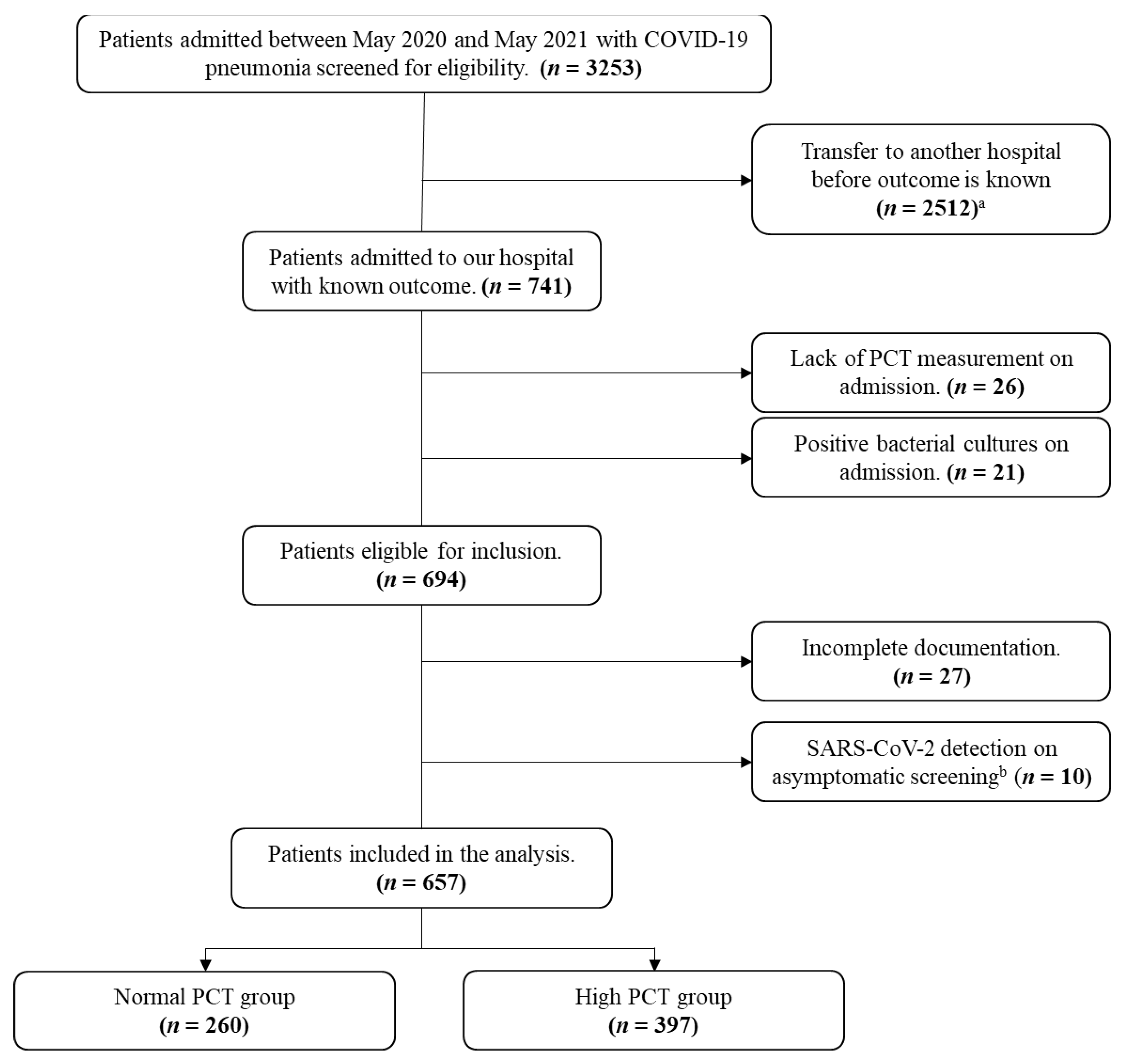
2.2. Inclusion and Exclusion Criteria
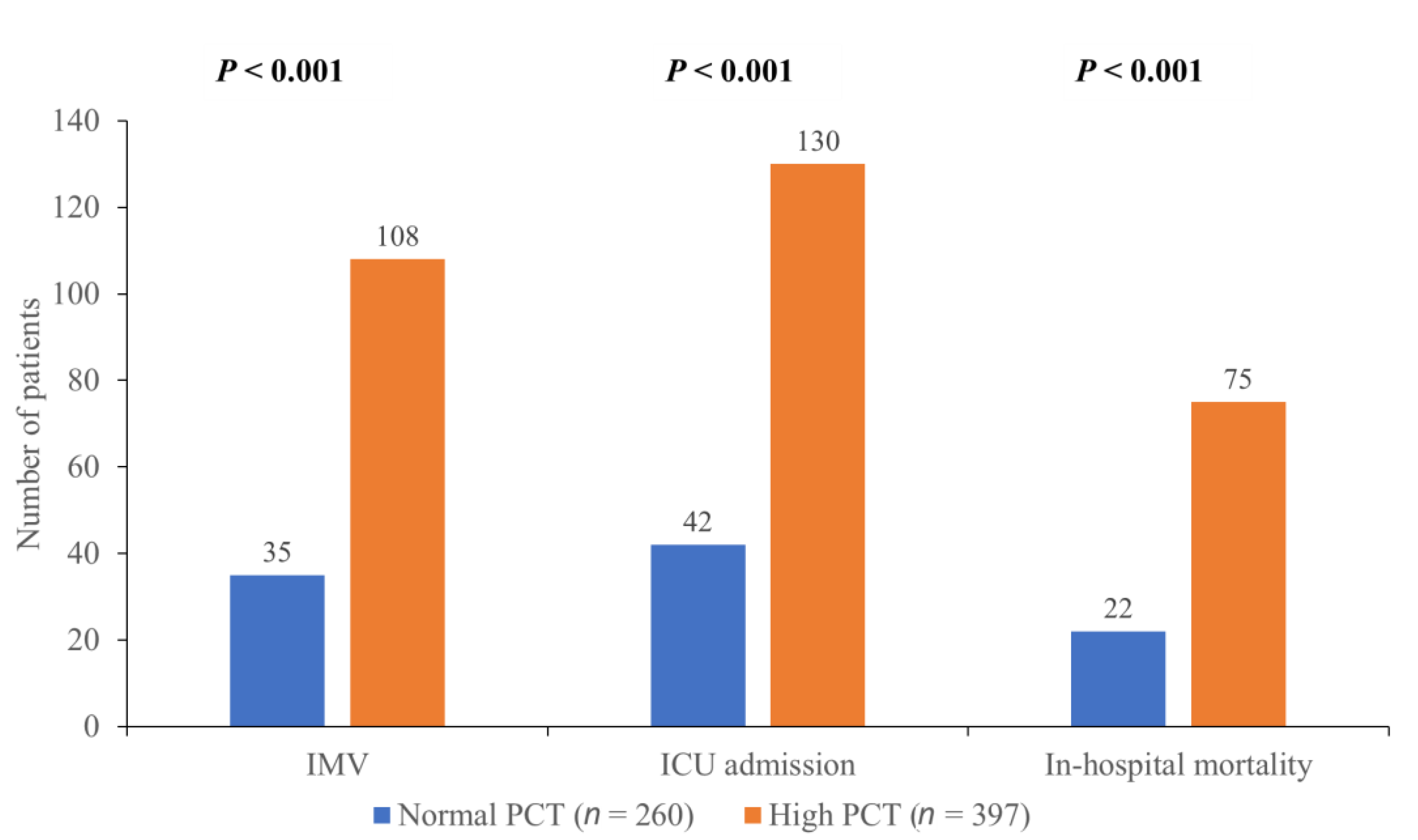
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
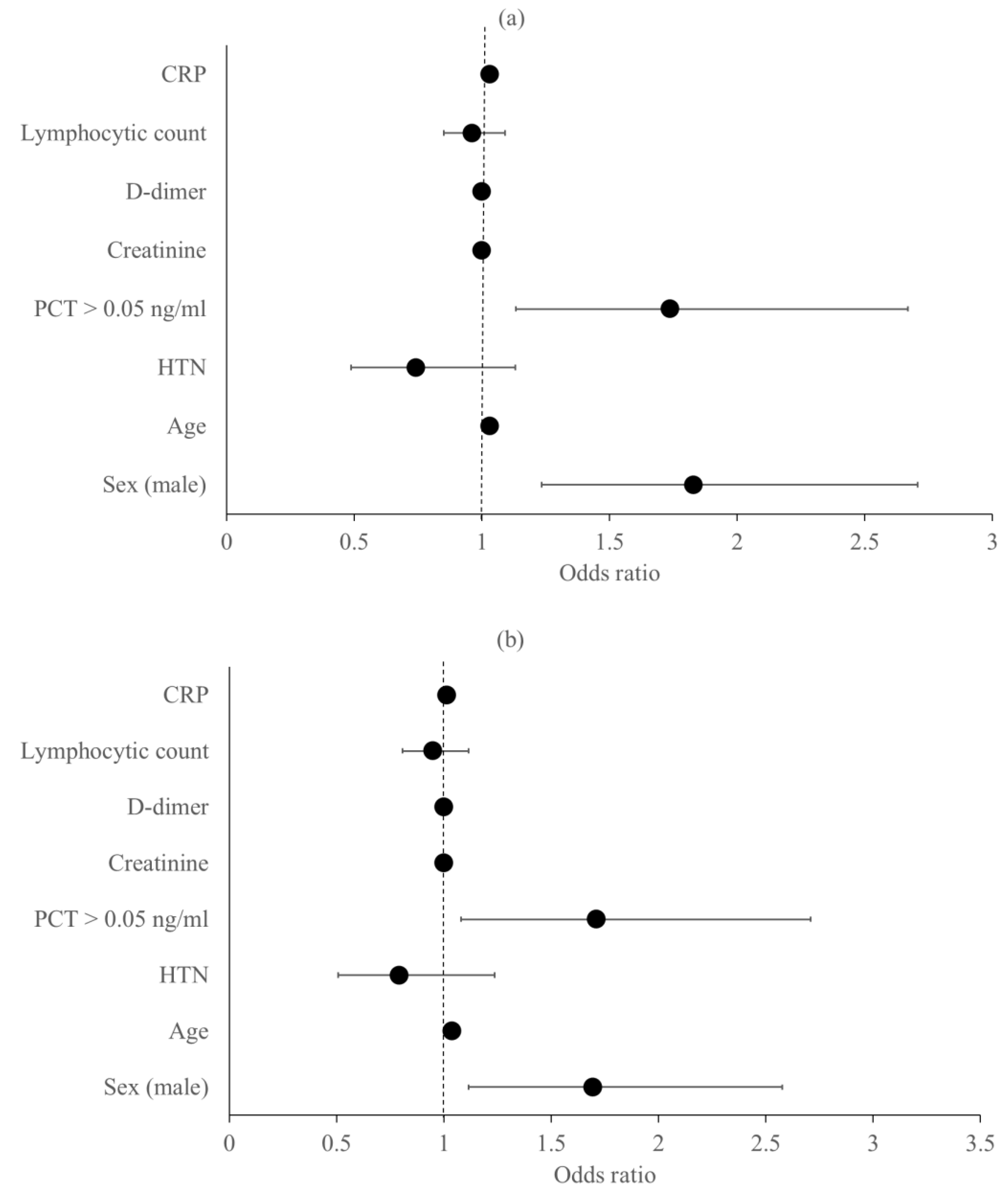
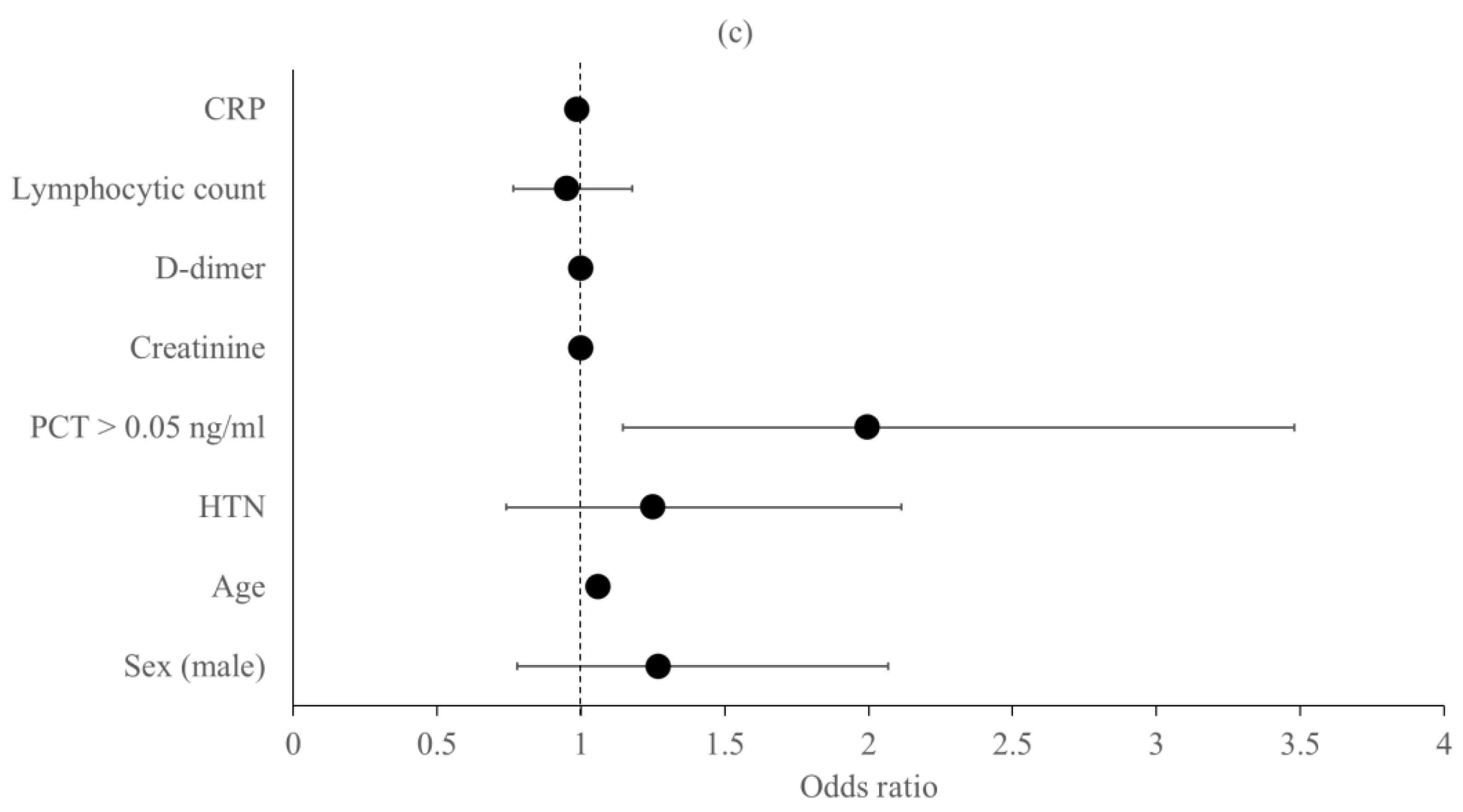
Predictors of the Primary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int (accessed on 1 February 2022).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Y.; Xu, B.; Wang, S.; Li, S.; Li, H.; Huang, Z.; Luo, Y.; Hu, M.; Wu, W.; et al. Early identification of patients with severe COVID-19 at increased risk of in-hospital death: A multicenter case-control study in Wuhan. J. Thorac. Dis. 2021, 13, 1380–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Shi, B.; Xu, S.; Chen, W.; et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Simon, T.; Sinnathamby, M.A.; Shirin, A.; Shaun, R.; Ross, H.J.; Russell, H.; Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2021, 22, S1473309921004758. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- Cecconi, M.; Piovani, D.; Brunetta, E.; Aghemo, A.; Greco, M.; Ciccarelli, M.; Angelini, C.; Voza, A.; Omodei, P.; Vespa, E.; et al. Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy. J. Clin. Med. 2020, 9, 1548. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Meisner, M. Update on Procalcitonin Measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chim. Acta 2020, 505, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.B.; Xu, C.; Zhang, R.; Bing, O.; Wu, M.; Pan, C.-K.; Li, X.-J.; Wang, Q.; Zeng, F.-F.; Zhu, S. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci. Rep. 2020, 10, 15058. [Google Scholar] [CrossRef] [PubMed]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.-P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef]
- Powell, N.; Howard, P.; Llewelyn, M.J.; Szakmany, T.; Albur, M.; Bond, S.E.; Euden, J.; Brookes-Howell, L.; Dark, P.; Hellyer, T.P.; et al. Use of Procalcitonin during the First Wave of COVID-19 in the Acute NHS Hospitals: A Retrospective Observational Study. Antibiotics 2021, 10, 516. [Google Scholar] [CrossRef]
- May, M.; Chang, M.; Dietz, D.; Shoucri, S.; Laracy, J.; Sobieszczyk, M.; Uhlemann, A.-C.; Zucker, J.; Kubin, C.J. Limited Utility of Procalcitonin in Identifying Community-Associated Bacterial Infections in Patients Presenting with Coronavirus Disease 2019. Antimicrob. Agents Chemother. 2021, 65, e02167–e02187. [Google Scholar] [CrossRef]
- Heer, R.S.; Mandal, A.K.; Kho, J.; Pinelli, V.; Novelli, F.; Carletti, A.M.; Chiesa, F.; Celi, A.; Sivori, M.; Giannoni, U. Elevated procalcitonin concentrations in severe Covid-19 may not reflect bacterial co-infection. Ann. Clin. Biochem. Int. J. Lab. Med. 2021, 58, 520–527. [Google Scholar] [CrossRef]
- NICE. Recommendations|COVID-19 Rapid Guideline: Managing COVID-19|Guidance|NICE. 2021. Available online: https://www.nice.org.uk/guidance/ng191/chapter/Recommendations (accessed on 10 November 2021).
- WHO. Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. 2020. Available online: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis (accessed on 2 January 2022).
- Liu, Z.M.; Li, J.P.; Wang, S.P.; Chen, D.-Y.; Zeng, W.; Chen, S.-C.; Huang, Y.-H.; Huang, J.-L.; Long, W.; Li, M.; et al. Association of procalcitonin levels with the progression and prognosis of hospitalized patients with COVID-19. Int. J. Med. Sci. 2020, 17, 2468–2476. [Google Scholar] [CrossRef]
- Abate, B.B.; Kassie, A.M.; Kassaw, M.W.; Aragie, T.G.; Masresha, S.A. Sex difference in coronavirus disease (COVID-19): A systematic review and meta-analysis. BMJ Open 2020, 10, e040129. [Google Scholar] [CrossRef]
- Saurabh, A.; Dey, B.; Raphael, V.; Prahbu, S.; Umakanth, S.; Bhat, V.; Sampathila, N. Evaluation of Hematological Parameters in Predicting Intensive Care Unit Admission in COVID-19 Patients. SN Compr. Clin. Med. 2022, 4, 39. [Google Scholar] [CrossRef]
- Hughes, S.; Mughal, N.; Moore, L.S.P. Procalcitonin to Guide Antibacterial Prescribing in Patients Hospitalised with COVID-19. Antibiotics 2021, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; He, M.; Kang, Y. A risk score based on procalcitonin for predicting acute kidney injury in COVID-19 patients. J. Clin. Lab. Anal. 2021, 35, 23805. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.; Cueto, P.; Rovira, C.; Garcia, E.; Parra, A.; Enriquez, R.; Pinos, A.; Sosa, M.; Aguilera, A.; Vallversu, I. Clinical value of procalcitonin in critically ill patients infected by SARS-CoV-2. Am. J. Emerg. Med. 2021, 46, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Asoğlu, R.; Tibilli, H.; Afşin, A.; Türkmen, S.; Barman, H.A.; Asoğlu, E. Procalcitonin is a predictor of disseminated intravascular coagulation in patients with fatal COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11953–11959. [Google Scholar] [CrossRef]
- Krause, M.; Douin, D.J.; Tran, T.T.; Fernandez-Bustamante, A.; Aftab, M.; Bartels, K. Association between procalcitonin levels and duration of mechanical ventilation in COVID-19 patients. PLoS ONE 2020, 15, e0239174. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef]
- Vazzana, N.; Dipaola, F.; Ognibene, S. Procalcitonin and secondary bacterial infections in COVID-19: Association with disease severity and outcomes. Acta Clin. 2020, 77, 1–5. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Gautam, S.; Cohen, A.J.; Stahl, Y.; Toro, P.V.; Young, G.M.; Datta, R.; Yan, X.; Ristic, N.T.; Bermejo, S.D.; Sharma, L.; et al. Severe respiratory viral infection induces procalcitonin in the absence of bacterial pneumonia. Thorax 2020, 75, 974–981. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, C.; Zheng, X.; Jin, Y.; Duan, G.; Chen, M.; Chen, S. Elevated Procalcitonin Is Positively Associated with the Severity of COVID-19: A Meta-Analysis Based on 10 Cohort Studies. Medicina 2021, 57, 594. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Fabre, V.; Karaba, S.; Amoah, J.; Robinson, M.; Jones, G.; Dzintars, K.; Katz, M.; Landrum, B.M.; Qasba, S.; Gupta, P.; et al. The role of procalcitonin results in antibiotic decision-making in coronavirus disease 2019 (COVID-19). Infect. Control Hosp. Epidemiol. 2021, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983. [Google Scholar] [CrossRef]
- Calderon, M.; Li, A.; Bazo-Alvarez, J.C.; Dennis, J.; Baker, K.F.; van der Loeff, I.S.; Hanrath, A.T.; Capstick, R.; Payne, A.I.; Weiand, D. Evaluation of procalcitonin-guided antimicrobial stewardship in patients admitted to hospital with COVID-19 pneumonia. JAC-Antimicrob. Resist. 2021, 3, dlab133. [Google Scholar] [CrossRef]
- Peters, C.; Williams, K.; Un, E.A.; Little, L.; Saad, A.; Lendrum, K.; Thompson, N.; Weatherley, N.D.; Pegden, A. Use of procalcitonin for antibiotic stewardship in patients with COVID-19: A quality improvement project in a district general hospital. Clin. Med. 2021, 21, e71–e76. [Google Scholar] [CrossRef]
- Dolci, A.; Robbiano, C.; Aloisio, E.; Chibireva, M.; Serafini, L.; Falvella, F.S.; Pasqualetti, S.; Panteghini, M. Searching for a role of procalcitonin determination in COVID-19: A study on a selected cohort of hospitalized patients. Clin. Chem. Lab. Med. 2021, 59, 433–440. [Google Scholar] [CrossRef]
- Nazerian, P.; Gagliano, M.; Suardi, L.R.; Fanelli, A.; Rossolini, G.M.; Grifoni, S. Procalcitonin for the differential diagnosis of COVID-19 in the emergency department. Prospective monocentric study. Intern Emerg. Med. 2021, 16, 1733–1735. [Google Scholar] [CrossRef]
- Sayah, W.; Berkane, I.; Guermache, I.; Sabri, M.; Lakhal, F.Z.; Rahali, S.Y.; Djideli, A.; Mahammed, L.L.; Merah, F.; Belaid, B.; et al. Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: Potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19. Cytokine 2021, 141, 155428. [Google Scholar] [CrossRef]
- Tang, J.; Lin, J.; Zhang, E.; Zhong, M.; Luo, Y.; Fu, Y.; Yang, Y. Serum IL-6 and procalcitonin are two promising novel biomarkers for evaluating the severity of COVID-19 patients. Medicine 2021, 100, e26131. [Google Scholar] [CrossRef] [PubMed]
- Girija, A.S.S.; Shankar, E.M.; Larsson, M. Could SARS-CoV-2-Induced Hyperinflammation Magnify the Severity of Coronavirus Disease (CoViD-19) Leading to Acute Respiratory Distress Syndrome? Front. Immunol. 2020, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 657) | Normal PCT Group (n = 260) | High PCT Group (n = 397) | p-Value | NR | |
|---|---|---|---|---|---|
| Age (y), mean ± SD | 55.12 ± 15.1 | 52.55 ± 14.78 | 56.8 ± 15.08 | <0.001 | |
| Sex Male, n (%) Female, n (%) | 335 (51) 322 (49) | 107 (41.2) 153 (58.8) | 228 (57.4) 169 (42.6) | <0.001 | |
| Diabetes, n (%) Hypertension, n (%) Renal disease, n (%) ASCVD a, n (%) Lung disease, n (%) | 393 (59.8) 278 (42.3) 78 (11.9) 210 (32) 58 (8.8) | 145 (55.8) 92 (35.4) 18 (6.9) 73 (28.1) 24 (9.2) | 248 (62.5) 186 (46.9) 60 (15.1) 137 (34.5) 34 (8.6) | 0.088 0.004 0.001 0.088 0.78 | |
| White blood cells | 6.59 (4.73–9.66) | 5.6 (4.29–7.77) | 7.43 (5.39–10.3) | <0.001 | 4–10 × 109/L |
| Neutrophils | 4.89 (3.3–7.67) | 3.91 (2.7–6.14) | 5.9 (3.79–8.37) | <0.001 | 2–7 × 109/L |
| Lymphocytes | 1.09 (0.7–1.54) | 1.18 (0.8–1.7) | 1 (0.65–1.48) | 0.003 | 1–3 × 109/L |
| Hemoglobin | 128 (114–141) | 128 (115–140) | 128 (114–141.5) | 0.807 | 130–170 g/L |
| Platelets | 214 (164–265) | 209.5 (159.5–263) | 219 (169–265.5) | 0.227 | 150–410 × 109/L |
| Creatinine | 83.91 (66–111.65) | 75.86 (60.05–92.81) | 93.24 (70.75–127) | <0.001 | 57–113 umol/L |
| ALT | 30 (20–46.5) | 24.5 (18–37.75) | 35 (22–54.35) | <0.001 | 8–41 IU/L |
| AST | 38 (28–58) | 32 (25–43) | 47 (30–69) | <0.001 | 10–40 IU/L |
| LDH | 313 (244–388) | 272 (217–320) | 322 (263.5–439) | <0.001 | 95–200 IU/L |
| D-dimer | 315 (205–620.8) | 251.5 (168.5–433.75) | 363 (240–859) | <0.001 | <232 ng/ml |
| CRP | 9.5 (6.935–13.2) | 8.1 (4.93–9.65) | 10.2 (7.82–15.6) | <0.001 | 0–0.8 mg/dl |
| Admission PCT | 0.1 (0.05–0.265) | 0.05 (0.05–0.05) | 0.21 (0.11–0.56) | <0.001 | ≤0.05 ng/ml |
| Total (n = 657) | Normal PCT Group (n = 260) | High PCT Group (n = 397) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Admission scale a 3 4 5 6 | 111 539 6 1 | 16.9 82 0.9 0.2 | 49 210 1 0 | 18.8 80.8 0.4 0 | 62 329 5 1 | 15.6 82.9 1.3 0.2 | |
| Patients who received empiric antibiotics on admission b | 587 | 89.3 | 219 | 84.2 | 368 | 92.7 | |
| Clinical deterioration c | 146 | 22.2 | 36 | 13.8 | 110 | 27.7 | <0.001 |
| High flow O2 therapy d | 226 | 34.4 | 59 | 22.7 | 167 | 42.1 | <0.001 |
| Hospital LOS (days) e | 8 (5–13) | 7 (5–10) | 9 (6–15) | <0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aon, M.; Alsaeedi, A.; Alzafiri, A.; Ibrahim, M.M.; Al-Shammari, A.; Al-Shammari, O.; Tawakul, M.; Taha, S.; Alherz, N.; Alshammari, J.; et al. The Association between Admission Procalcitonin Level and The Severity of COVID-19 Pneumonia: A Retrospective Cohort Study. Medicina 2022, 58, 1389. https://doi.org/10.3390/medicina58101389
Aon M, Alsaeedi A, Alzafiri A, Ibrahim MM, Al-Shammari A, Al-Shammari O, Tawakul M, Taha S, Alherz N, Alshammari J, et al. The Association between Admission Procalcitonin Level and The Severity of COVID-19 Pneumonia: A Retrospective Cohort Study. Medicina. 2022; 58(10):1389. https://doi.org/10.3390/medicina58101389
Chicago/Turabian StyleAon, Mohamed, Abdullah Alsaeedi, Azeez Alzafiri, Mohamed M. Ibrahim, Abdelrahman Al-Shammari, Omar Al-Shammari, Mahmoud Tawakul, Sherif Taha, Naser Alherz, Jarrah Alshammari, and et al. 2022. "The Association between Admission Procalcitonin Level and The Severity of COVID-19 Pneumonia: A Retrospective Cohort Study" Medicina 58, no. 10: 1389. https://doi.org/10.3390/medicina58101389
APA StyleAon, M., Alsaeedi, A., Alzafiri, A., Ibrahim, M. M., Al-Shammari, A., Al-Shammari, O., Tawakul, M., Taha, S., Alherz, N., Alshammari, J., Albazee, E., Alharbi, T., Alshammari, D., Alenezi, Z., Alenezi, M., Aldouseri, S., Eyadah, M., Aldhafeeri, M., & Aoun, A. H. (2022). The Association between Admission Procalcitonin Level and The Severity of COVID-19 Pneumonia: A Retrospective Cohort Study. Medicina, 58(10), 1389. https://doi.org/10.3390/medicina58101389






