Magnesium Potentiates the Vortioxetine’s Effects on Physical Performances and Biological Changes in Exercise-Induced Stress in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Substances
2.3. Experimental Protocol
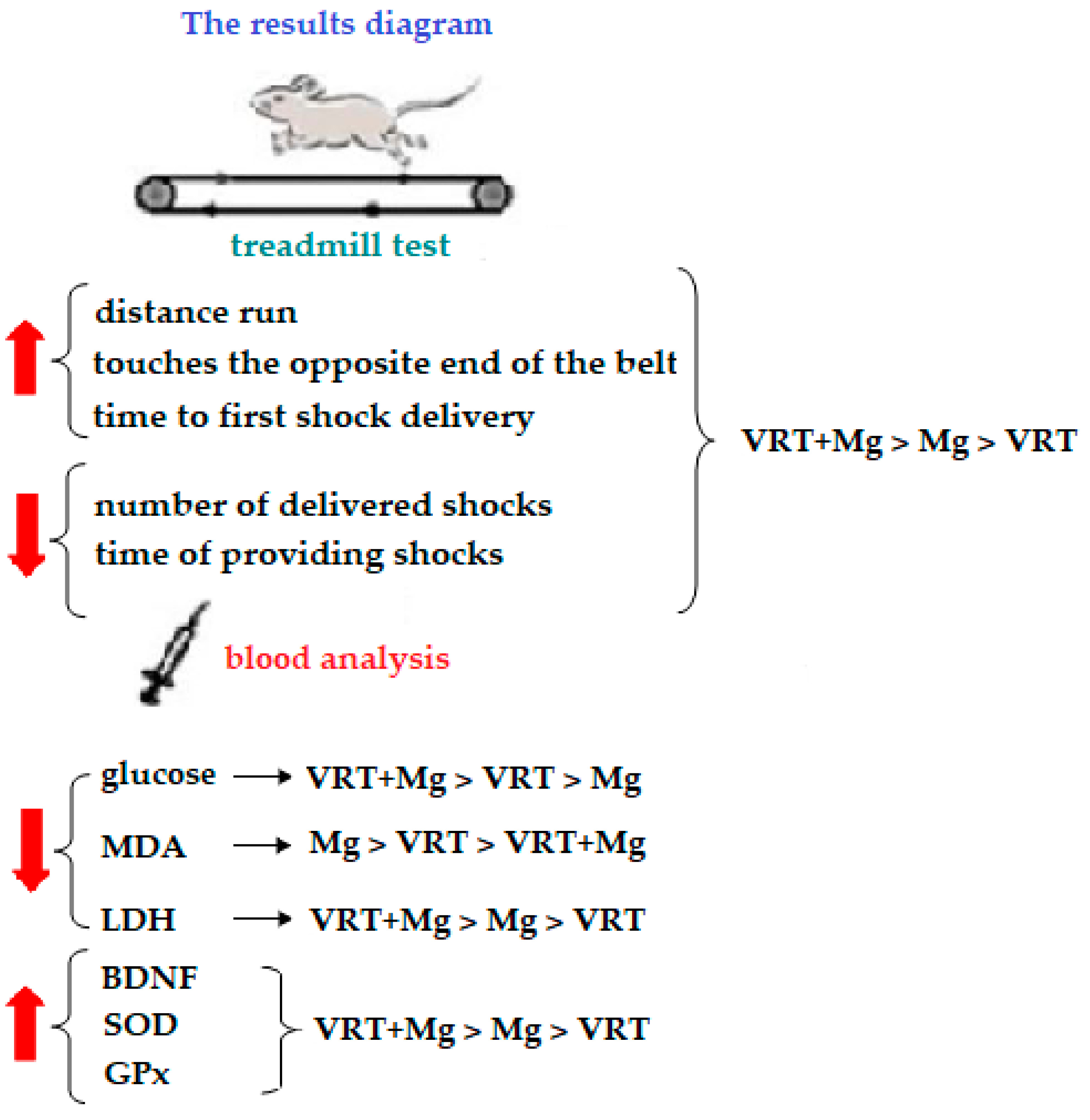
2.3.1. The Treadmill Test
- Group 1 (Positive control): distilled water 0.3 mL/100g body;
- Group 3 (VRT): VRT 20 mg/kg body [31];
- Group 4 (VRT+Mg): VRT 20 mg/kg body + magnesium chloride 200 mg/kg body.
2.3.2. The Laboratory Investigations
2.4. Statistical Data Processing
3. Results
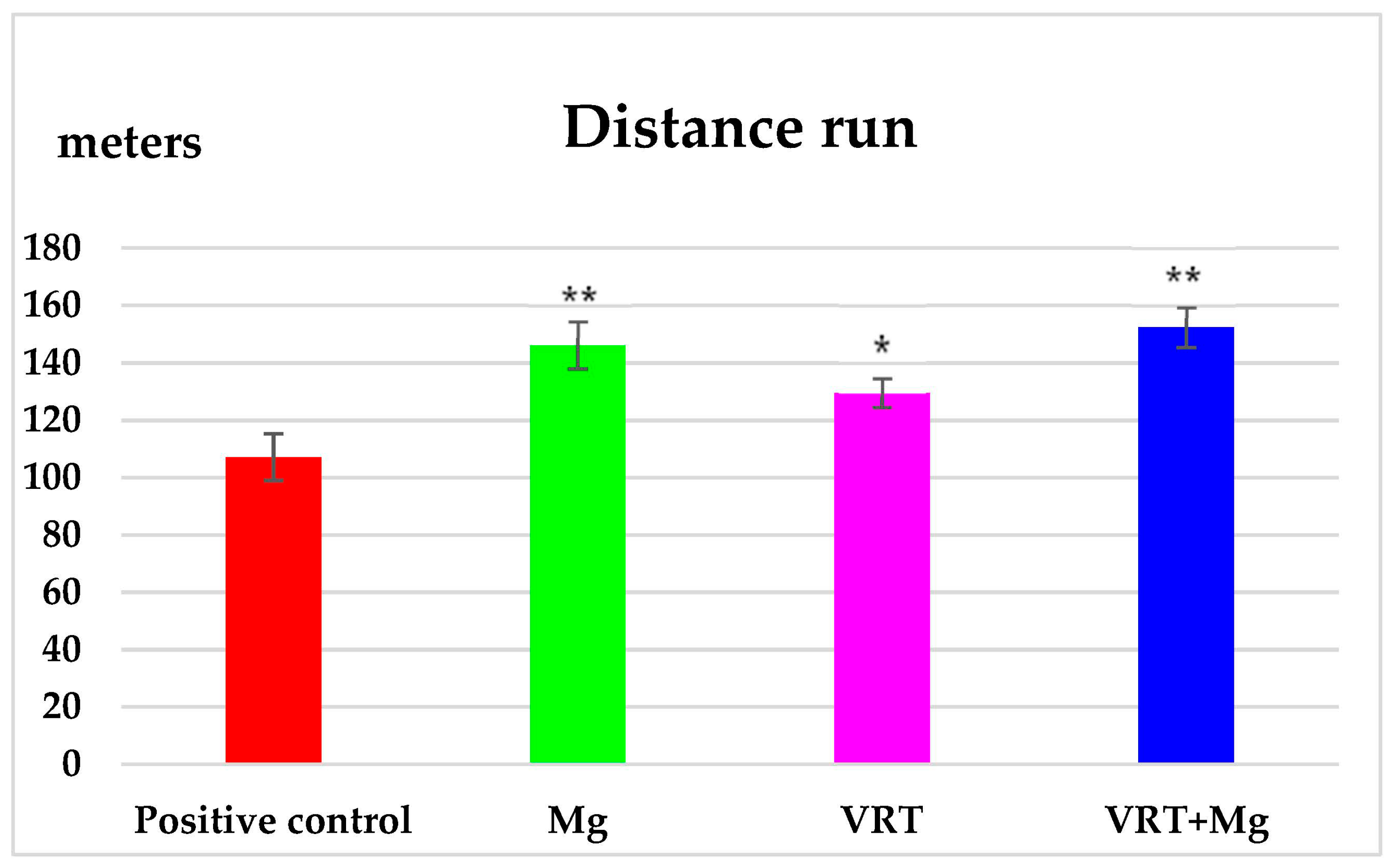
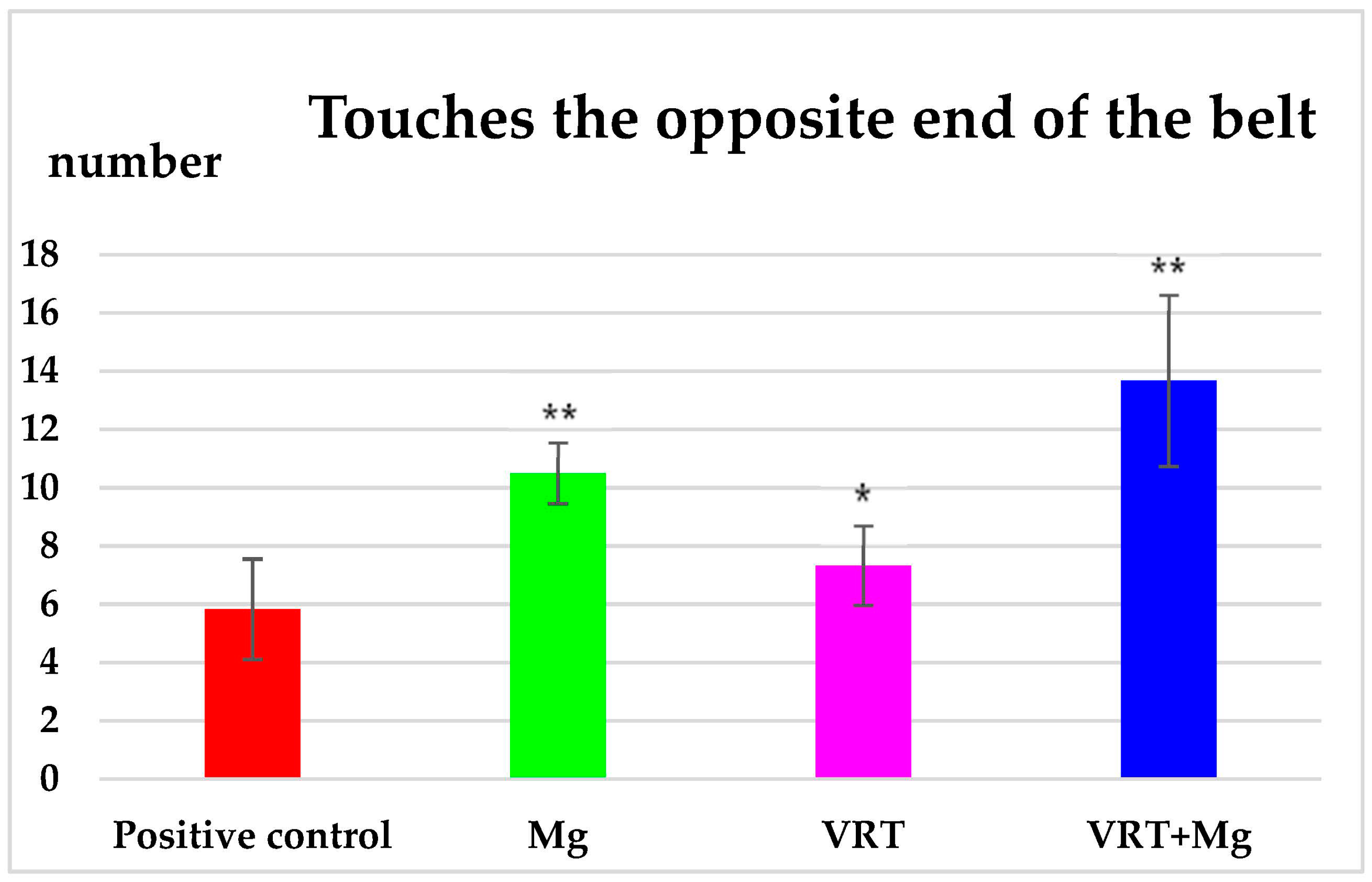
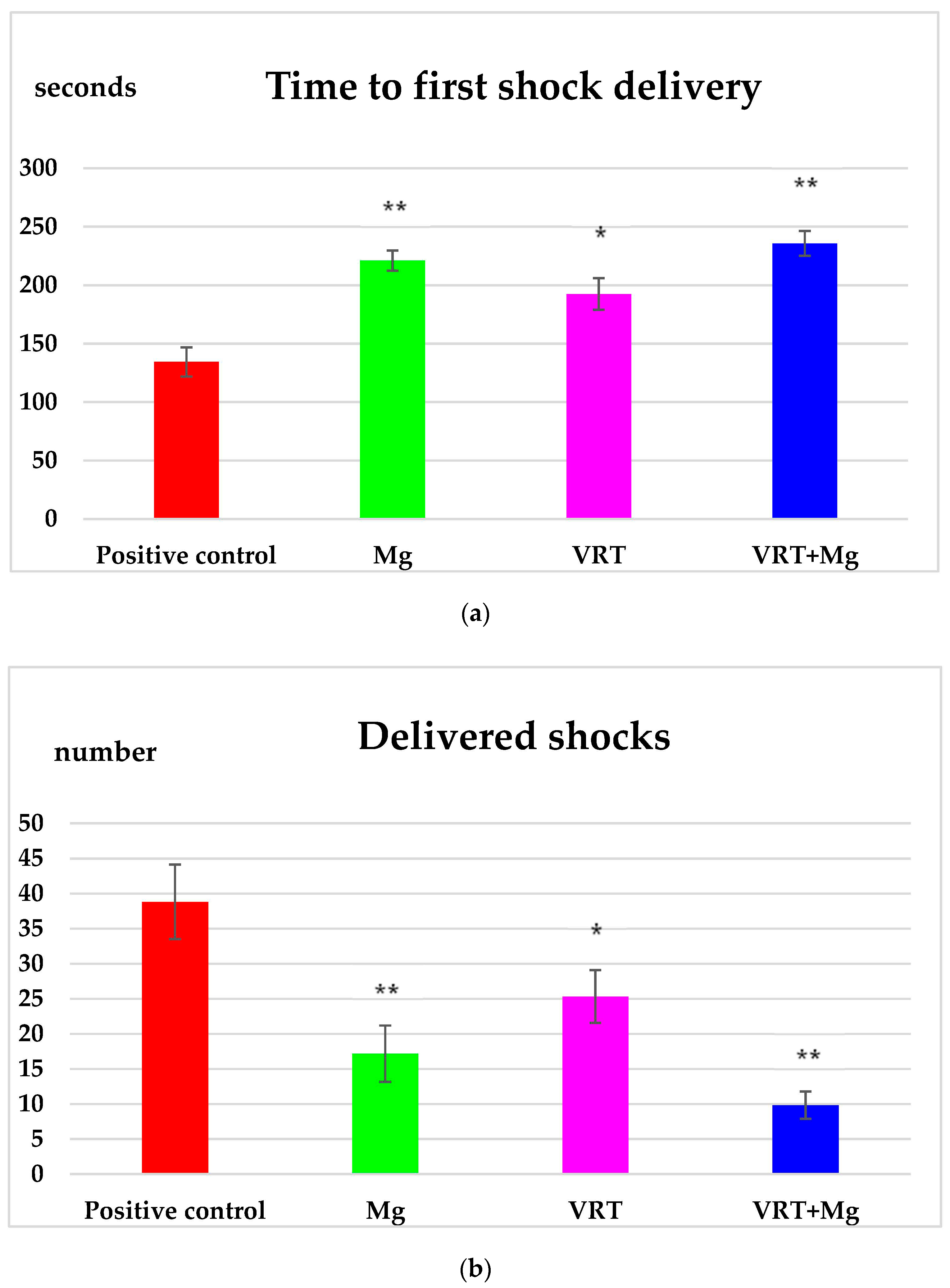
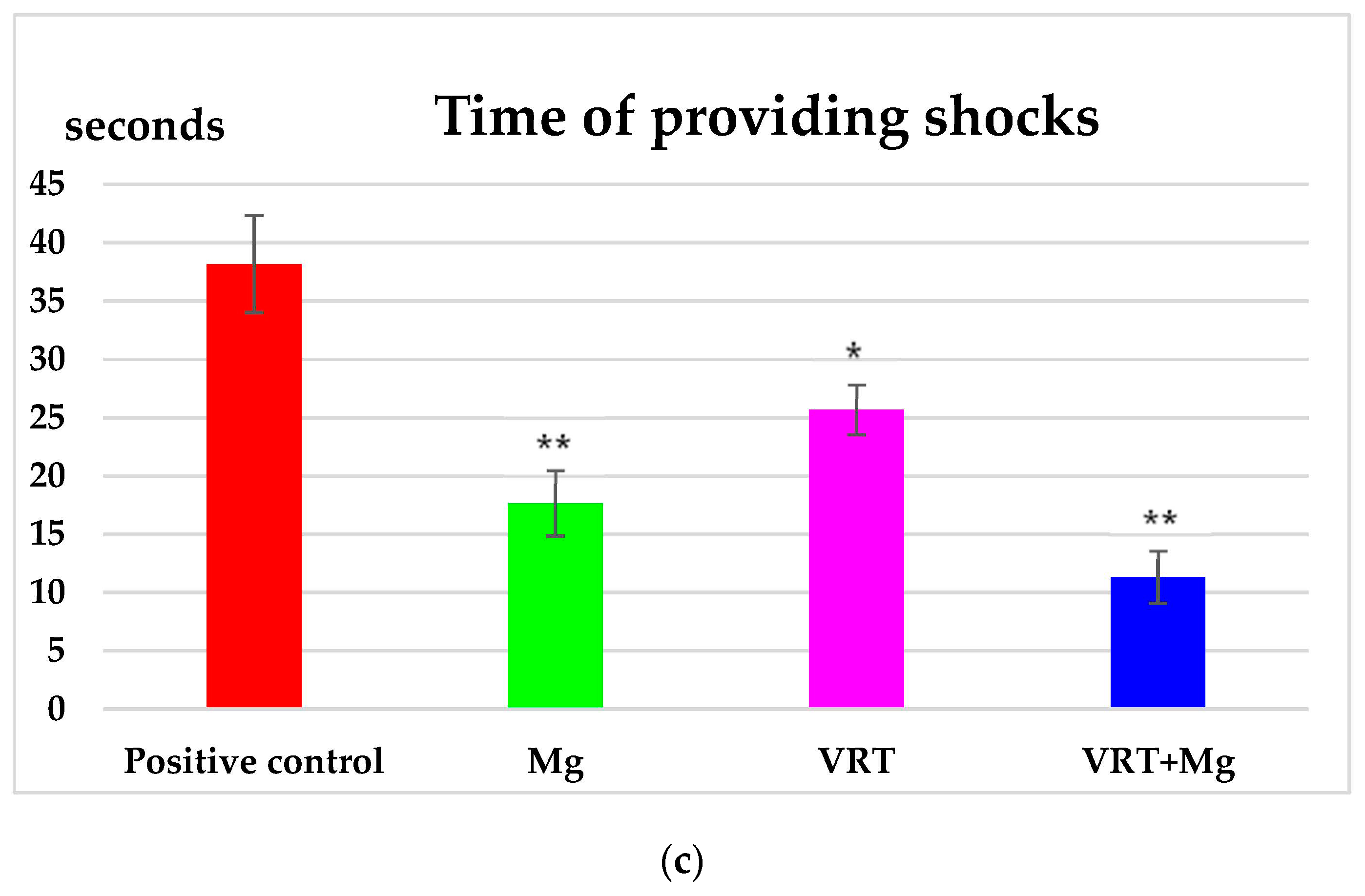
3.1. The Treadmill Test
3.2. The Laboratory Investigations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, C.P.; Schmidt, D.; Stein, C.M.; Morrow, J.D.; Salomon, R.M. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res. 2013, 206, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Fagan, M.; Subramaniapillai, M.; Lee, Y.; Park, C.; Mansur, R.B.; McIntyre, R.S.; Faulkner, G.E.J. Are early increases in physical activity a behavioral marker for successful antidepressant treatment? J. Affect. Disord. 2020, 260, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, G.; Bortoli, M.; Pavan, C.; Zagotto, G.; Orian, L. Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design. Antioxidants 2020, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Ayers, D. Physiology, Stress Reaction. [Updated 18 September 2021]; In StatPearls; [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541120/ (accessed on 4 July 2022).
- Terenina, E.E.; Cavigelli, S.; Mormede, P.; Zhao, W.; Parks, C.; Lu, L.; Jones, B.C.; Mulligan, M.K. Genetic factors mediate the impact of chronic stress and subsequent response to novel acute stress. Front. Neurosci. 2019, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Postovit, L.; Cattaneo, A.; Binder, E.B.; Aitchison, K.J. Epigenetic modifications in stress response genes associated with childhood trauma. Front. Psychiatry 2019, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- McCue, A.D.; Nuthikattu, S.; Reeder, S.H.; Slotkin, R.K. Gene expression and stress response mediated by the epigenetic regulation of a transposable element small RNA. PLoS Genet. 2012, 8, e1002474. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2017, 8, 383–395. [Google Scholar] [CrossRef]
- Hing, B.; Sathyaputri, L.; Potash, J.B. A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 143–167. [Google Scholar] [CrossRef]
- Pochwat, B.; Sowa-Kucma, M.; Kotarska, K.; Misztak, P.; Nowak, G.; Szewczyk, B. Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacology 2015, 232, 355–367. [Google Scholar] [CrossRef]
- Haider, S.; Sadir, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Khaliq, S.; Anis, L.; Sajid, I.; Haleem, D.J. Magnesium treatment palliates noise-induced behavioral deficits by normalizing DAergic and 5-HTergic metabolism in adult male rats. Metab. Brain Dis. 2016, 31, 815–825. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, Y.K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef]
- Salagre, E.; Grande, I.; Solé, B.; Sanchez-Moreno, J.; Vieta, E. Vortioxetine: A new alternative for the treatment of major depressive disorder. Rev. Psiquiatr. Salud. Ment. 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Nielsen, R.Z.; Dragheim, M.; Tonnoir, B. Efficacy and tolerability of vortioxetine versus agomelatine, categorized by previous treatment, in patients with major depressive disorder switched after an inadequate response. J. Psychiatr. Res. 2018, 101, 72–79. [Google Scholar] [CrossRef]
- Fagiolini, A.; Florea, I.; Loft, H.; Christensen, M.C. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J. Affect. Disord. 2021, 283, 472–479. [Google Scholar] [CrossRef]
- Findling, R.L.; Robb, A.S.; DelBello, M.; Huss, M.; McNamara, N.; Sarkis, E.; Scheffer, R.; Poulsen, L.H.; Chen, G.; Lemming, O.M.; et al. Pharmacokinetics and Safety of Vortioxetine in Pediatric Patients. J. Child Adolesc. Psychopharmacol. 2017, 27, 526–534. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Gastaldon, C.; Barbato, A.; D’Avanzo, B.; Tettamanti, M.; Monti, I.; Aguglia, A.; Aguglia, E.; Alessi, M.C.; Amore, M.; et al. Tolerability and efficacy of vortioxetine versus SSRIs in elderly with major depression. Study protocol of the VESPA study: A pragmatic, multicentre, open-label, parallel-group, superiority, randomized trial. Trials 2020, 21, 695. [Google Scholar] [CrossRef]
- Gibb, A.; Deeks, E.D. Vortioxetine: First global approval. Drugs 2014, 74, 135–145. [Google Scholar] [CrossRef]
- Alvarez, E.; Perez, V.; Artigas, F. Pharmacology and clinical potential of vortioxetine in the treatment of major depressive disorder. Neuropsychiatr. Dis. Treat. 2014, 15, 1297–1307. [Google Scholar] [CrossRef]
- Areberg, J.; Søgaard, B.; Højer, A.M. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin. Pharmacol. Toxicol. 2012, 111, 198–205. [Google Scholar] [CrossRef]
- Schatzberg, A.F.; Blier, P.; Culpepper, L.; Jain, R.; Papakostas, G.I.; Thase, M.E. An overview of vortioxetine. J. Clin. Psychiatry 2014, 75, 1411–1418. [Google Scholar] [CrossRef]
- Adamo, D.; Calabria, E.; Coppola, N.; Pecoraro, G.; Mignogna, M.D. Vortioxetine as a new frontier in the treatment of chronic neuropathic pain: A review and update. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211034320. [Google Scholar] [CrossRef]
- Okada, M.; Okubo, R.; Fukuyama, K. Vortioxetine subchronically activates serotonergic transmission via desensitization of serotonin 5-HT1A receptor with 5-HT3 receptor inhibition in rats. Int. J. Mol. Sci. 2019, 20, 46235. [Google Scholar] [CrossRef]
- Sanchez, C.; Asin, K.E.; Artigas, F. Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol. Ther. 2015, 145, 43–57. [Google Scholar] [CrossRef]
- D’Agostino, A.; English, C.D.; Rey, J.A. Vortioxetine (brintellix): A new serotonergic antidepressant. P T A Peer-Rev. J. Formul. Manag. 2015, 40, 36–40. [Google Scholar]
- Al-Sukhni, M.; Maruschak, N.A.; McIntyre, R.S. Vortioxetine: A review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin. Drug Saf. 2015, 14, 1291–1304. [Google Scholar] [CrossRef]
- Chen, G.; Højer, A.M.; Areberg, J.; Nomikos, G. Vortioxetine: Clinical pharmacokinetics and drug interactions. Clin. Pharmacokinet. 2018, 57, 673–686. [Google Scholar] [CrossRef]
- Dalla, C.; Pitychoutis, P.M.; Kokras, N.; Papadopoulou-Daifoti, Z. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 2009, 106, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Dhibi, S.; Bouzenna, H.; Elfeki, A.; Hfaiedh, N. Effects of MgCl2 supplementation on blood parameters and kidney injury of rats exposed to CCl4. Open Life Sci. 2016, 11, 250–258. [Google Scholar] [CrossRef][Green Version]
- Tartau, L.; Valeanu, A.S.; Lupusoru, C.E. Effects of magnesium and zinc on the memory processes performance in mice. Eur. Neuropsychopharmacol. 2012, 22 (Suppl. S2), S191. [Google Scholar] [CrossRef]
- Sun, B.; Lv, Y.; Xu, H.; Qi, C.; Li, C.; Liu, P. Effects of vortioxetine on depression model rats and expression of BDNF and Trk B in hippocampus. Exp. Ther. Med. 2020, 20, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.B.; Cho, H.S.; Shin, M.S.; Kim, C.J.; Ji, E.S.; Baek, S.S. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J. Exerc. Rehabil. 2013, 9, 220–229. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, H.Y.; Wang, M.F.; Hsu, M.H.; Liang, W.M.; Cheng, F.C. Effects of magnesium on exercise performance and plasma glucose and lactate concentrations in rats using a novel blood-sampling technique. Appl. Physiol. Nutr. Metab. 2009, 34, 1040–1047. [Google Scholar] [CrossRef]
- Marcaletti, S.; Thomas, C.; Feige, J.N. Exercise performance tests in mice. Curr. Protoc. Mouse Biol. 2011, 1, 141–154. [Google Scholar] [CrossRef]
- Cechetti, F.; Worm, P.V.; Elsner, V.R.; Bertoldi, K.; Sanches, E.; Ben, J.; Siqueira, I.R.; Netto, C.A. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol. Learn. Mem. 2012, 97, 90–96. [Google Scholar] [CrossRef]
- Lee, G.; Goosens, K.A. Sampling blood from the lateral tail vein of the rat. J. Vis. Exp. 2015, 99, e52766. [Google Scholar] [CrossRef]
- Zou, W.; Yang, Y.; Gu, Y.; Zhu, P.; Zhang, M.; Cheng, Z.; Liu, X.; Yu, Y.; Peng, X. Repeated blood collection from tail vein of non-anesthetized rats with a vacuum blood collection system. J. Vis. Exp. 2017, 130, 55852. [Google Scholar] [CrossRef]
- Béjot, Y.; Mossiat, C.; Giroud, M.; Prigent-Tessier, A.; Marie, C. Circulating and brain BDNF levels in stroke rats. Relevance to clinical studies. PLoS ONE 2011, 7, e29405. [Google Scholar] [CrossRef]
- Elfving, B.; Plougmann, P.H.; Wegener, G. Detection of brain-derived neurotrophic factor (BDNF) in rat blood and brain preparations using ELISA: Pitfalls and solutions. J. Neurosci. Methods 2010, 187, 73–77. [Google Scholar] [CrossRef]
- Bahrami, S.; Shahriari, A.; Tavalla, M.; Azadmanesh, S.; Hamidinejat, H. Blood levels of oxidant/antioxidant parameters in rats infected with Toxoplasma gondii. In Oxidative Stress in Disease and Aging: Mechanisms and Therapies; Cabello-Verrugio, C., Ed.; Hindawi Publishing House: London, UK, 2016; p. 8045969. [Google Scholar]
- Ebner, K.; Singewald, N. Individual differences in stress susceptibility and stress inhibitory mechanisms. Curr. Opin. Behav. Sci. 2017, 14, 54–64. [Google Scholar] [CrossRef]
- Gorban, A.N.; Tyukina, T.A.; Smirnova, E.V.; Pokidysheva, L.I. Evolution of adaptation mechanisms: Adaptation energy, stress, and oscillating death. J. Theor Biol. 2016, 405, 127–139. [Google Scholar] [CrossRef]
- Miyanishi, H.; Nitta, A. A role of BDNF in the depression pathogenesis and a potential target as antidepressant: The modulator of stress sensitivity “Shati/Nat8l-BDNF System” in the dorsal striatum. Pharmaceuticals 2021, 14, 889. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Nishii, A.; Amemiya, S.; Kubota, N.; Nishijima, T.; Kita, I. Effects of acute treadmill running at different intensities on activities of serotonin and corticotropin-releasing factor neurons, and anxiety-and depressive-like behaviors in rats. Behav. Brain Res. 2016, 298 (Pt B), 44–51. [Google Scholar] [CrossRef] [PubMed]
- Strohe, A. Physical activity, exercise, depression and anxiety disorders. J. Neural. Transm 2009, 116, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Ohiwa, N.; Saito, T.; Chang, H.; Nakamura, T.; Soya, H. Differential responsiveness of c-Fos expression in the rats medulla oblongata to different treadmill running speeds. Neurosci. Res. 2006, 54, 124–132. [Google Scholar] [CrossRef]
- Soya, H.; Mukai, A.; Deocaris, C.C.; Ohiwa, N.; Chang, H.; Nishijima, T.; Fujikawa, T.; Tagashi, K.; Saito, T. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running> establishment of a minimum running stress (MRS) rat model. Neurosci. Res. 2007, 58, 342–348. [Google Scholar] [CrossRef]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium status and stress: The vicious circle concept revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef]
- Seo, M.K.; Ly, N.N.; Lee, C.H.; Cho, H.Y.; Choi, C.M.; Nhu, L.H.; Lee, J.G.; Lee, B.J.; Kim, G.-M.; Yoon, B.J.; et al. Early life stress increases stress vulnerability through BDNF gene epigenetic changes in the rat hippocampus. Neuropharmacology 2016, 105, 388–397. [Google Scholar] [CrossRef]
- Jiang, N.; Lv, J.-W.; Wang, H.-X.; Lu, C.; Wang, Q.; Xia, T.-J.; Bao, Y.; Li, S.-S.; Liu, X.-M. Dammarane sapogenins alleviates depression-like behaviours induced by chronic social defeat stress in mice through the promotion of the BDNF signalling pathway and neurogenesis in the hippocampus. Brain Res. Bull. 2019, 153, 239–249. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2015, 102, 72–79. [Google Scholar] [CrossRef]
- Youssef, M.M.; Underwood, M.D.; Huang, Y.Y.; Hsiung, S.C.; Liu, Y.; Simpson, N.R.; Bakalian, M.J.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int. J. Neuropsychopharmacol. 2018, 21, 528–538. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Kao, C.-F.; Liu, Y.-L.; Yu, Y.W.-Y. Gene-based analysis of genes related to neurotrophic pathway suggests association of BDNF and VEGFA with antidepressant treatment-response in depressed patients. Sci. Rep. 2018, 8, 6983. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, S.; Li, C.; Lu, N.; Yue, Y.; Yin, Y.; Zhang, Y.; Zhi, X.; Zhang, D.; Yuan, Y. The serum protein levels of the tPA–BDNF pathway are implicated in depression and antidepressant treatment. Transl. Psychiatry 2017, 7, e1079. [Google Scholar] [CrossRef]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-derived neurotrophic factor and major depressive disorder: Evidence from meta-analyses. Front. Psychiatry 2018, 8, 308. [Google Scholar] [CrossRef]
- Duman, R.S. Neurobiology of stress, depression, and rapid acting antidepressants: Remodeling synaptic connections. Depress. Anxiety 2014, 31, 291–296. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Ferrarelli, L.K. Antidepressants suppress DNA methylation. Science 2015, 350, 1051–1053. [Google Scholar] [CrossRef]
- Tadić, A.; Müller-Engling, L.; Schlicht, K.F.; Kotsiari, A.; Dreimüller, N.; Kleimann, A.; Bleich, S.; Lieb, K.; Frieling, H. Methylation of the promoter of brain-derived neurotrophic factor exon IV and antidepressant response in major depression. Mol. Psychiatry 2013, 19, 281–283. [Google Scholar] [CrossRef]
- Marshall, J.; Zhou, X.-Z.; Chen, G. Antidepression action of BDNF requires and is mimicked by Gαi1/3 expression in the hippocampus. Proc. Natl. Acad. Sci. USA 2018, 115, E3549–E3558. [Google Scholar] [CrossRef]
| Group | Glucose (mg/dL) | Cortisol (pg/mL) | BDNF (pg/mL) |
|---|---|---|---|
| Negative control | 88.50 ± 6.24 | 0.14 ± 0.01 | 575.67 ± 47.25 |
| Positive control | 116.33 ± 1.53 ♦♦ | 0.19 ± 0.02 ♦ | 477.50 ± 33.23 ♦♦ |
| Mg | 93.33 ± 8.02 * | 0.16 ± 0.03 | 569.50 ± 31.82 ** |
| VRT | 92.00 ± 1.41 * | 0.16 ± 0.05 | 563.00 ± 41.01 ** |
| VRT+Mg | 89.17 ± 3.25 ** | 0.15 ± 0.02 * | 573.33 ± 45.78 ** |
| Group | SOD (μg/mL) | MDA (nmol/L) | GPx (pg/mL) | LDH (U/L) |
|---|---|---|---|---|
| Negative control | 24.33 ± 2.46 | 27.83 ± 3.16 | 321.17 ± 33.27 | 2069.67 ± 21.92 |
| Positive control | 15.00 ± 2.83 ♦♦ | 38.5 ± 2.40 * | 194.00 ± 35.36 ♦♦ | 2407.50 ± 21.92 ♦♦ |
| Mg | 23.00 ± 1.41 ** | 26.65 ± 3.46 ** | 311.50 ± 113.84 ** | 2094.00 ± 156.98 ** |
| VRT | 22.33 ± 2.12 * | 26.93 ± 1.12 * | 309.00 ± 165.46 ** | 2100.50 ± 144.96 * |
| VRT+Mg | 24.17 ± 2.33 ** | 27.65 ± 2.59 ** | 319.50 ± 101.41 ** | 2075.33 ± 132.83 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotache, P.A.; Mititelu-Tartau, L.; Bogdan, M.; Buca, B.R.; Pavel, L.L.; Pelin, A.-M.; Meca, A.-D.; Tartau, C.-G.; Popa, G.E. Magnesium Potentiates the Vortioxetine’s Effects on Physical Performances and Biological Changes in Exercise-Induced Stress in Rats. Medicina 2022, 58, 1363. https://doi.org/10.3390/medicina58101363
Fotache PA, Mititelu-Tartau L, Bogdan M, Buca BR, Pavel LL, Pelin A-M, Meca A-D, Tartau C-G, Popa GE. Magnesium Potentiates the Vortioxetine’s Effects on Physical Performances and Biological Changes in Exercise-Induced Stress in Rats. Medicina. 2022; 58(10):1363. https://doi.org/10.3390/medicina58101363
Chicago/Turabian StyleFotache, Paula Alina, Liliana Mititelu-Tartau, Maria Bogdan, Beatrice Rozalina Buca, Liliana Lacramioara Pavel, Ana-Maria Pelin, Andreea-Daniela Meca, Cosmin-Gabriel Tartau, and Gratiela Eliza Popa. 2022. "Magnesium Potentiates the Vortioxetine’s Effects on Physical Performances and Biological Changes in Exercise-Induced Stress in Rats" Medicina 58, no. 10: 1363. https://doi.org/10.3390/medicina58101363
APA StyleFotache, P. A., Mititelu-Tartau, L., Bogdan, M., Buca, B. R., Pavel, L. L., Pelin, A.-M., Meca, A.-D., Tartau, C.-G., & Popa, G. E. (2022). Magnesium Potentiates the Vortioxetine’s Effects on Physical Performances and Biological Changes in Exercise-Induced Stress in Rats. Medicina, 58(10), 1363. https://doi.org/10.3390/medicina58101363








