Predictors of Recurrence after Catheter Ablation of Paroxysmal Atrial Fibrillation in Different Follow-Up Periods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Cardiac Biomarkers
2.3. Echocardiographic Evaluations
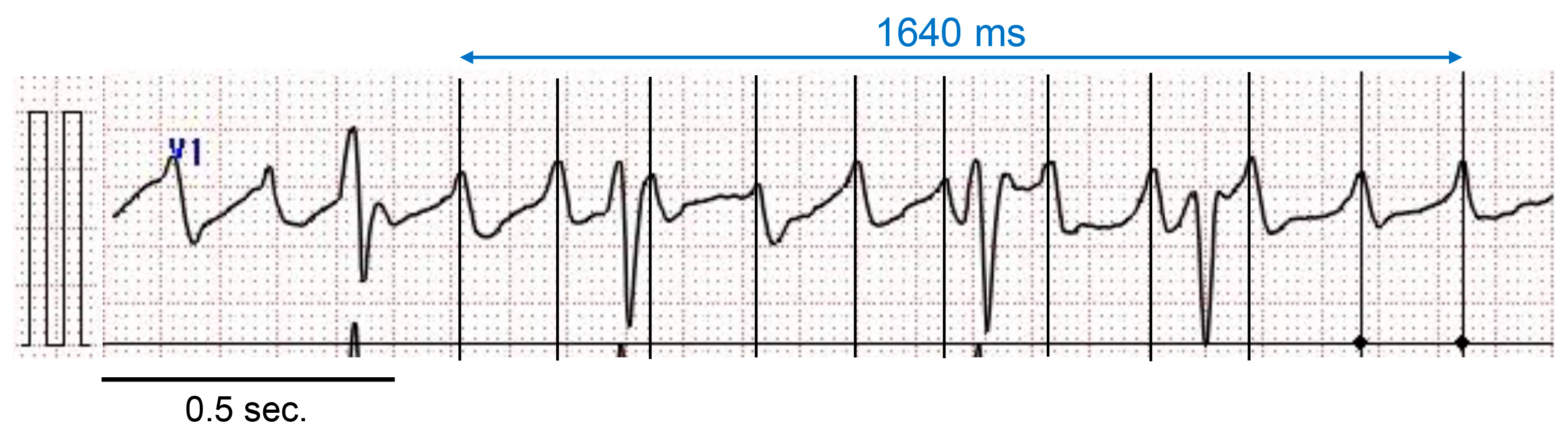
2.4. Measurement of AF Cycle Length in Lead V1
2.5. Catheter Ablation
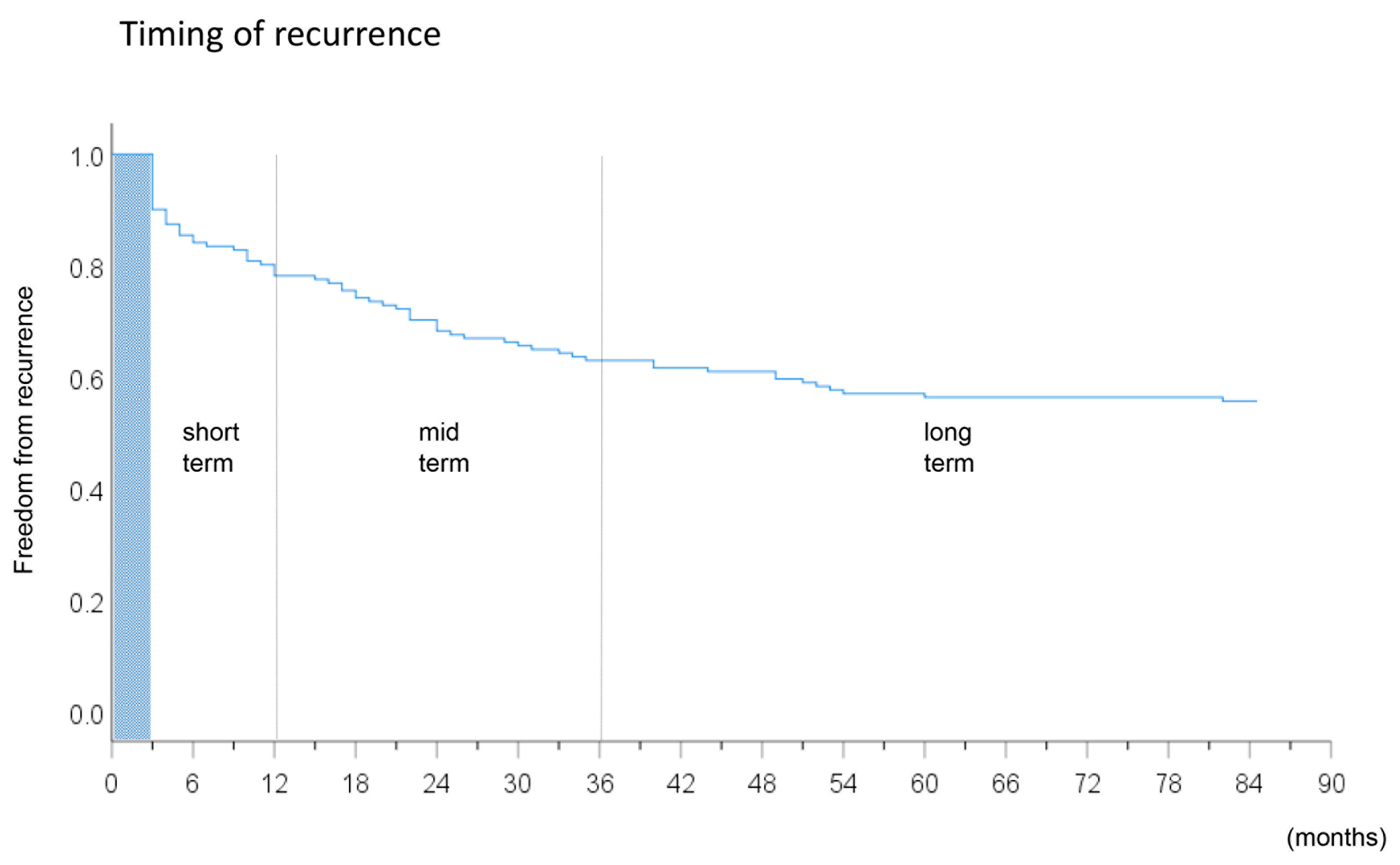
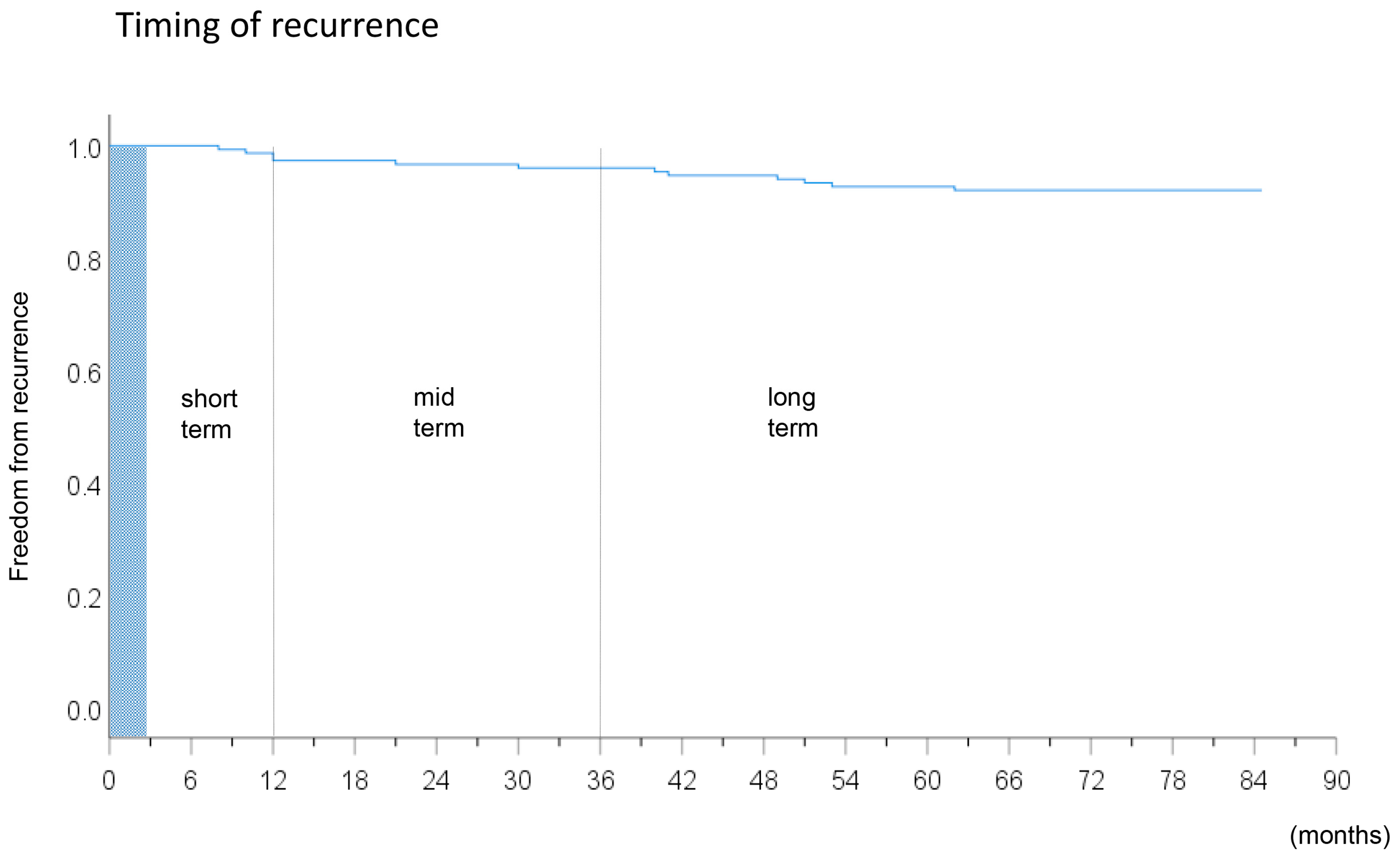
2.6. Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
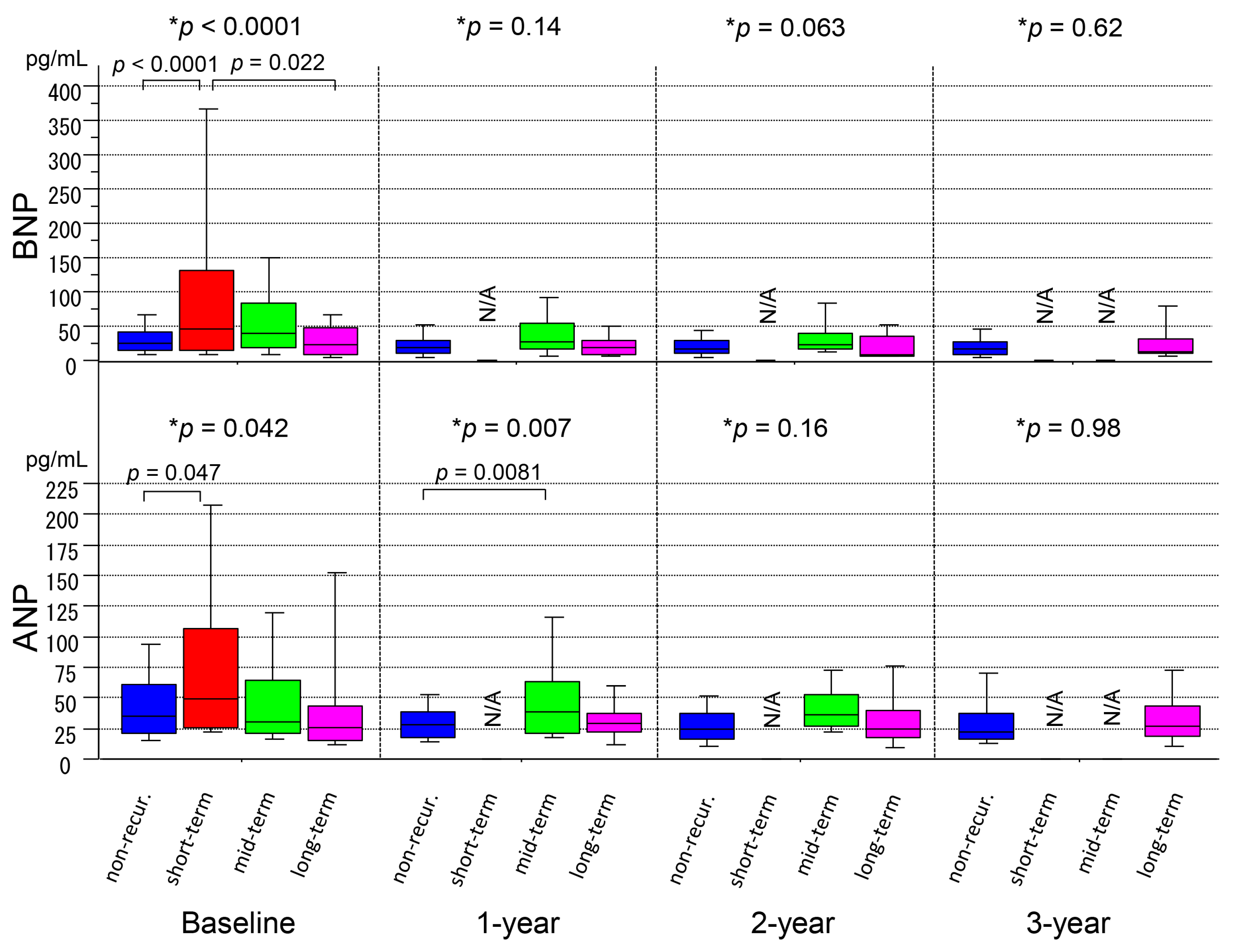
3.2. Serial Measurements of ANP/BNP Levels
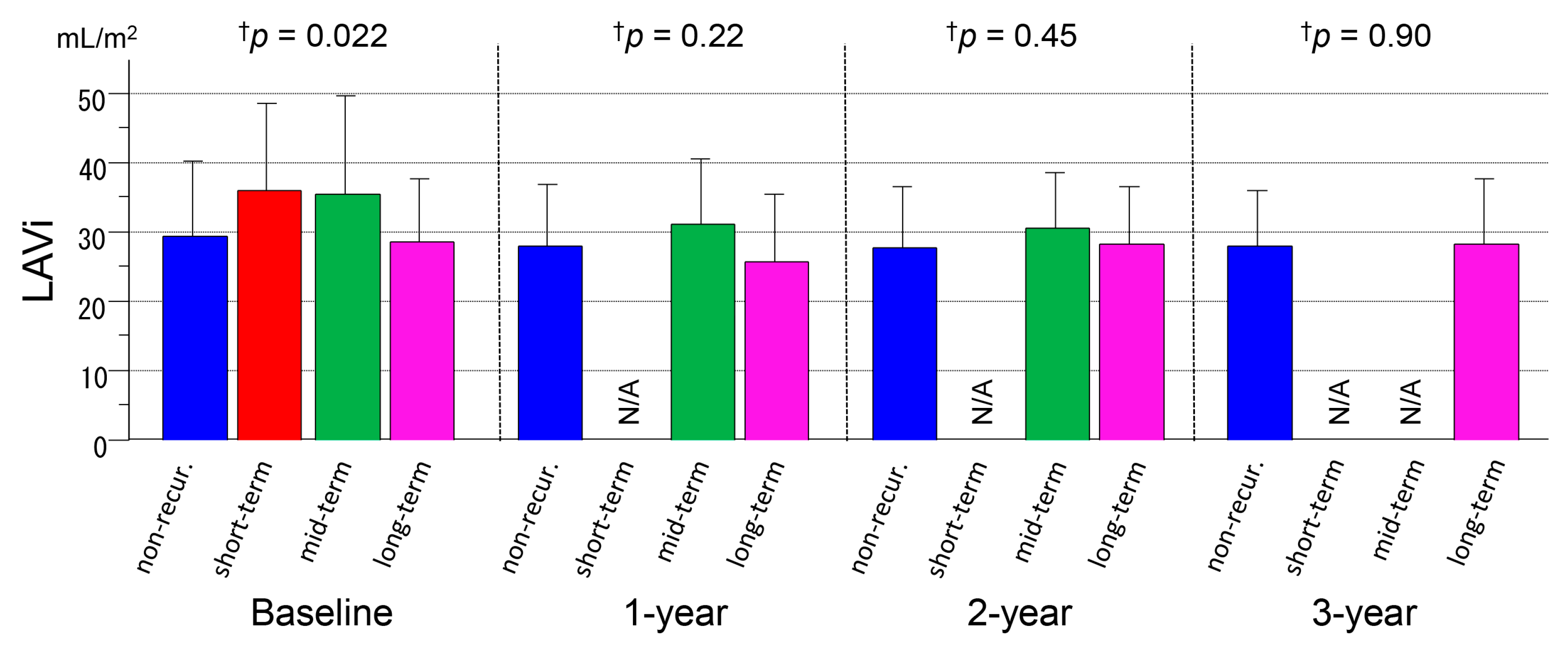
3.3. Serial Measurements of LAVi
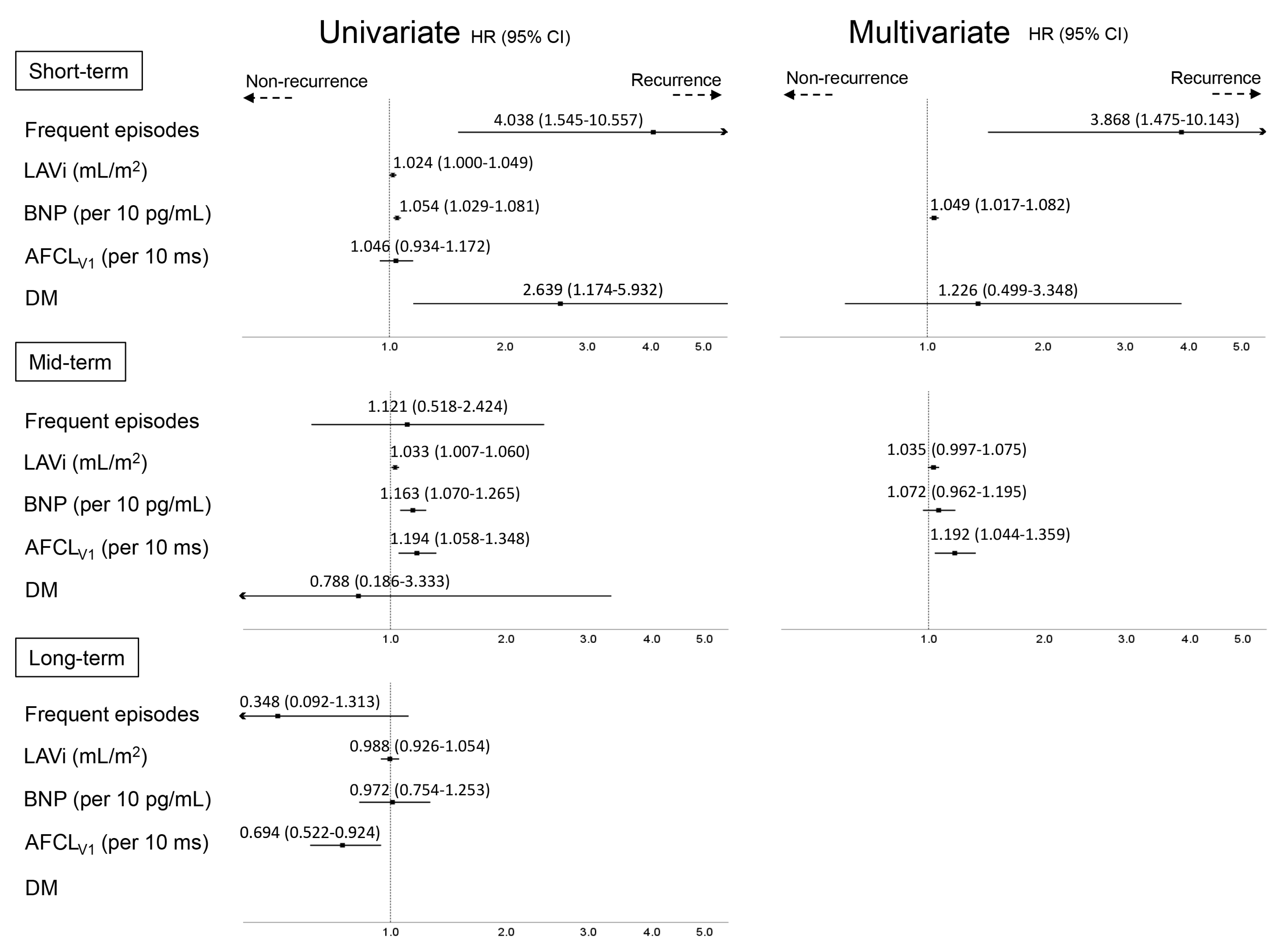
3.4. Predictors of AF Recurrence at Different Time-Points
3.5. PV Reconnections and Outcomes after 2nd Ablation Procedure
4. Discussion
4.1. Main Findings
- Patients in the short-term recurrence group more frequently suffered from AF episodes and diabetes mellitus than those in the other groups and had a higher BNP level at baseline than the non-recurrence group;
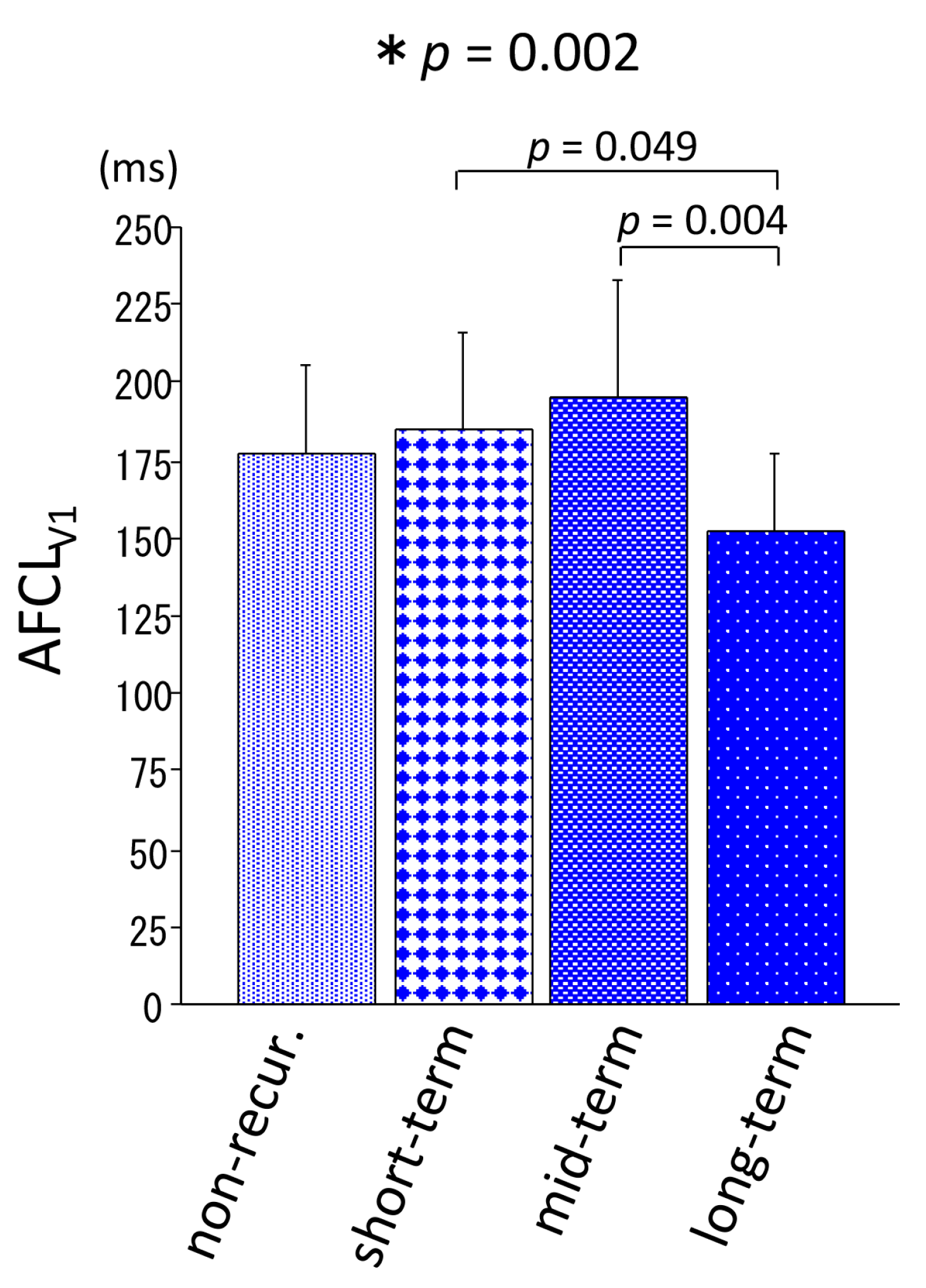
- Patients in the mid-term recurrence group had a larger LA and longer AFCLV1 at baseline compared with the other groups;
- Patients in the long-term recurrence group were hemodynamically stable throughout the long follow-up period, as were those in the non-recurrence group, and had a shorter AFCLV1 at baseline;
- The overall success rate after one or two procedure(s) was satisfactory regardless of the timing of recurrence, indicating that an ablation strategy including PV isolation followed by ablation for non-PV ectopies, mainly SVC isolation, is adequately effective as a treatment of paroxysmal AF.
4.2. Short-Term Recurrence Group
4.3. Mid-Term Recurrence Group
4.4. Long-Term Recurrence Group
4.5. Prior Studies
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; Neven, K.; Kokturk, B.; Konstantinidou, M.; Metzner, A.; et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: Lessons from a 5-year follow-up. Circulation 2010, 122, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Pratola, C.; Baldo, E.; Notarstefano, P.; Toselli, T.; Ferrari, R. Radiofrequency ablation of atrial fibrillation: Is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation 2008, 117, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Khairy, P.; Andrade, J.G.; Hoffmann, B.A.; Levesque, S.; Verma, A.; Weerasooriya, R.; Novak, P.; Arentz, T.; Deisenhofer, I.; et al. Redefining the Blanking Period After Catheter Ablation for Paroxysmal Atrial Fibrillation: Insights From the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) Trial. Circ. Arrhythmia Electrophysiol. 2016, 9, e003909. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kholmovski, E.; Ghafoori, E.; Kamali, R.; Kwan, E.; Lichter, J.; MacLeod, R.; Dosdall, D.J.; Ranjan, R. Characterization of edema after cryo and radiofrequency ablations based on serial magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2019, 30, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Usui, E.; Miyazaki, S.; Taniguchi, H.; Ichihara, N.; Kanaji, Y.; Takagi, T.; Iwasawa, J.; Kuroi, A.; Nakamura, H.; Hachiya, H.; et al. Recurrence after “long-term success” in catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm 2015, 12, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Barakat, A.F.; Saliba, W.I.; Abdur Rehman, K.; Tarakji, K.G.; Rickard, J.; Bassiouny, M.; Baranowski, B.; Tchou, P.; Bhargava, M.; et al. Recurrent Atrial Fibrillation After Initial Long-Term Ablation Success: Electrophysiological Findings and Outcomes of Repeat Ablation Procedures. Circ. Arrhythmia Electrophysiol. 2018, 11, e005785. [Google Scholar] [CrossRef]
- Takigawa, M.; Takahashi, A.; Kuwahara, T.; Okubo, K.; Takahashi, Y.; Watari, Y.; Takagi, K.; Fujino, T.; Kimura, S.; Hikita, H.; et al. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: The incidence of recurrence and progression of atrial fibrillation. Circ. Arrhythm Electrophysiol. 2014, 7, 267–273. [Google Scholar] [CrossRef]
- Baba, M.; Yoshida, K.; Ieda, M. Clinical Applications of Natriuretic Peptides in Heart Failure and Atrial Fibrillation. Int. J. Mol. Sci. 2019, 20, 2824. [Google Scholar] [CrossRef]
- Ogawa, K.; Yoshida, K.; Uehara, Y.; Ebine, M.; Kimata, A.; Nishina, H.; Takeyasu, N.; Noguchi, Y.; Ieda, M.; Aonuma, K.; et al. Mechanistic implication of decreased plasma atrial natriuretic peptide level for transient rise in the atrial capture threshold early after ICD or CRT-D implantation. J. Interv. Card. Electrophysiol. 2018, 53, 131–140. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2018. [Google Scholar] [CrossRef]
- Matsuo, S.; Lellouche, N.; Wright, M.; Bevilacqua, M.; Knecht, S.; Nault, I.; Lim, K.T.; Arantes, L.; O’Neill, M.D.; Platonov, P.G.; et al. Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J. Am. Coll. Cardiol. 2009, 54, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, H.; Yoshida, K.; Iida, M.; Hatano, N.; Muramatsu, T.; Aonuma, K. Right-to-left frequency gradient during atrial fibrillation initiated by right atrial ectopies and its augmentation by adenosine triphosphate: Implications of right atrial fibrillation. Heart Rhythm 2016, 13, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Ejima, K.; Kato, K.; Iwanami, Y.; Henmi, R.; Yagishita, D.; Manaka, T.; Fukushima, K.; Arai, K.; Ashihara, K.; Shoda, M.; et al. Impact of an Empiric Isolation of the Superior Vena Cava in Addition to Circumferential Pulmonary Vein Isolation on the Outcome of Paroxysmal Atrial Fibrillation Ablation. Am. J. Cardiol. 2015, 116, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hattori, A.; Tsuneoka, H.; Tsumagari, Y.; Yui, Y.; Kimata, A.; Ito, Y.; Ebine, M.; Uehara, Y.; Koda, N.; et al. Electrophysiological relation between the superior vena cava and right superior pulmonary vein in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2017, 28, 1117–1126. [Google Scholar] [CrossRef]
- Arruda, M.; Mlcochova, H.; Prasad, S.K.; Kilicaslan, F.; Saliba, W.; Patel, D.; Fahmy, T.; Morales, L.S.; Schweikert, R.; Martin, D.; et al. Electrical isolation of the superior vena cava: An adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J. Cardiovasc. Electrophysiol. 2007, 18, 1261–1266. [Google Scholar] [CrossRef]
- Yoshida, K.; Baba, M.; Hasebe, H.; Shinoda, Y.; Harunari, T.; Ebine, M.; Uehara, Y.; Watabe, H.; Takeyasu, N.; Horigome, H.; et al. Structural relation between the superior vena cava and pulmonary veins in patients with atrial fibrillation. Heart Vessel. 2019, 34, 2052–2058. [Google Scholar] [CrossRef]
- Stiles, M.K.; John, B.; Wong, C.X.; Kuklik, P.; Brooks, A.G.; Lau, D.H.; Dimitri, H.; Roberts-Thomson, K.C.; Wilson, L.; De Sciscio, P.; et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: Characterizing the “second factor”. J. Am. Coll. Cardiol. 2009, 53, 1182–1191. [Google Scholar] [CrossRef]
- Hussein, A.A.; Saliba, W.I.; Martin, D.O.; Shadman, M.; Kanj, M.; Bhargava, M.; Dresing, T.; Chung, M.; Callahan, T.; Baranowski, B.; et al. Plasma B-type natriuretic peptide levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. Circulation 2011, 123, 2077–2082. [Google Scholar] [CrossRef]
- Yoshida, K.; Tada, H.; Ogata, K.; Sekiguchi, Y.; Inaba, T.; Ito, Y.; Sato, Y.; Sato, A.; Seo, Y.; Kandori, A.; et al. Electrogram organization predicts left atrial reverse remodeling after the restoration of sinus rhythm by catheter ablation in patients with persistent atrial fibrillation. Heart Rhythm 2012, 9, 1769–1778. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C. Type 2 diabetes mellitus and atrial fibrillation: From mechanisms to clinical practice. Arch. Cardiovasc. Dis. 2015, 108, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Creta, A.; Providência, R.; Adragão, P.; de Asmundis, C.; Chun, J.; Chierchia, G.; Defaye, P.; Schmidt, B.; Anselme, F.; Finlay, M.; et al. Impact of Type-2 Diabetes Mellitus on the Outcomes of Catheter Ablation of Atrial Fibrillation (European Observational Multicentre Study). Am. J. Cardiol. 2020, 125, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Otake, H.; Suzuki, H.; Honda, T.; Maruyama, Y. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int. Heart J. 2009, 50, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Kurotobi, T.; Shimada, Y.; Kino, N.; Ito, K.; Tonomura, D.; Yano, K.; Tanaka, C.; Yoshida, M.; Tsuchida, T.; Fukumoto, H. Features of intrinsic ganglionated plexi in both atria after extensive pulmonary isolation and their clinical significance after catheter ablation in patients with atrial fibrillation. Heart Rhythm 2015, 12, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Lai, L.P.; Hwang, J.J.; Lin, J.L.; Chiang, F.T. Molecular genetics of atrial fibrillation. J. Am. Coll. Cardiol. 2008, 52, 241–250. [Google Scholar] [CrossRef]
- Fatkin, D.; Santiago, C.F.; Huttner, I.G.; Lubitz, S.A.; Ellinor, P.T. Genetics of Atrial Fibrillation: State of the Art in 2017. Heart Lung Circ. 2017, 26, 894–901. [Google Scholar] [CrossRef]
| Variables | No Recurrence (n = 84) | Short-Term Recurrence (n = 30) | Mid-Term Recurrence (n = 26) | Long-term Recurrence (n = 11) | p Value |
|---|---|---|---|---|---|
| Time to recurrence (mo.) | 4 (3–6) | 22 (17–26) | 51 (45–54) | NA | |
| Female | 17 (20.2%) | 6 (20.0%) | 6 (23.1%) | 2 (18.0%) | 0.99 |
| Age (years) | 64 ± 8 | 63 ± 9 | 66 ± 7 | 61 ± 12 | 0.44 |
| Age at onset (years) | 60 ± 11 | 57 ± 11 | 62 ± 8 | 55 ± 16 | 0.20 |
| Body mass index (kg/m2) | 24.4 ± 3.1 | 24.7 ± 2.7 | 24.7 ± 3.8 | 23.2 ± 2.3 | 0.55 |
| AF history (months) | 24 (7–60) | 24 (12–66) | 24 (9–60) | 36 (8–105) | 0.54 |
| AF triggers | 0.34 | ||||
| Sympathetic activity | 76 (90.5%) | 30 (100%) | 23 (88.5%) | 10 (90.9%) | |
| Parasympathetic activity | 8 (9.5%) | 0 | 3 (11.5%) | 1 (9.0%) | |
| AF episodes ≥ 1/week | 45 (53.6%) | 25 (83.3%) | 14 (53.9%) | 3 (27.3%) | 0.005 |
| AF at baseline lab. tests | 11 (13.1%) | 13 (43.3%) | 7 (22.6%) | 0 | 0.001 |
| AF at ablation | 12 (14.3%) | 9 (30.0%) | 4 (15.4%) | 0 | 0.092 |
| Atrial flutter | 11 (13.1%) | 4 (13.3%) | 3 (11.5%) | 0 | 0.65 |
| NYHA class II | 16 (19.0%) | 11 (36.7%) | 10 (38.5%) | 0 | 0.018 |
| Intense exercise (≥5 h/week) | 5 (6.0%) | 0 | 2 (7.7%) | 2 (18.2%) | 0.17 |
| Hypertension | 48 (57.1%) | 14 (46.7%) | 14 (53.8%) | 4 (36.4%) | 0.51 |
| Dyslipidemia | 28 (33.3%) | 14 (46.7%) | 8 (30.8%) | 3 (27.3%) | 0.50 |
| Diabetes mellitus | 9 (10.7%) | 8 (26.7%) | 2 (7.7%) | 0 | 0.049 |
| Hypoglycemic agents | |||||
| DPP 4 inhibitor | 8 (9.5%) | 7 (23.3%) | 2 (7.7%) | 0 | |
| Biguanide | 3 (3.5%) | 2 (6.7%) | 0 | 0 | |
| Sulphonylurea | 1 (1.2%) | 1 (3.3%) | 0 | 0 | |
| Pioglitazone | 1 (1.2%) | 1 (3.3%) | 0 | 0 | |
| α-glucosidase inhibitor | 0 | 3 (10.0%) | 0 | 0 | |
| Hemoglobin A1c (%) | 6.0 ± 0.7 | 6.4 ± 1.7 | 6.1 ± 0.6 | 5.7 ± 0.5 | 0.37 |
| Hemoglobin A1c in patients with diabetes mellitus (%) | 7.0 ± 1.0 | 7.5 ± 2.4 | 6.9 ± 0.2 | NA | 0.82 |
| SSS (HR at rest < 60 bpm or sinus pauses of > 3.0 s) | 13 (15.5%) | 10 (33.3%) | 6 (23.1%) | 2 (18.2%) | 0.22 |
| CHADS2 score | 1.0 ± 1.0 | 1.2 ± 1.0 | 1.1 ± 1.0 | 0.5 ± 0.7 | 0.20 |
| COPD | 0 | 0 | 3 (11.5%) | 0 | 0.002 |
| Former smoker | 53 (63.1%) | 20 (66.7% | 14 (53.8%) | 4 (36.4%) | 0.28 |
| Current smoker | 10 (11.9%) | 2 (6.7%) | 1 (3.8%) | 0 | 0.38 |
| History of PCI | 1 (1.2%) | 2 (6.7%) | 0 | 0 | 0.23 |
| Sleep apnea syndrome | 5 (6.0%) | 1 (3.3%) | 1 (3.8%) | 0 | 0.80 |
| Cancer | 6 (7.1%) | 1 (3.3%) | 2 (7.7%) | 1 (9.1%) | 0.87 |
| Collagen disease | 1 (1.2%) | 0 | 0 | 0 | 0.85 |
| C-reactive protein (mg/dL) | 0.12 ± 0.21 | 0.09 ± 0.12 | 0.11 ± 0.09 | 0.06 ± 0.02 | 0.70 |
| eGFR (mL/min/1.73 m2) | 71 ± 14 | 71 ± 16 | 63 ± 15 | 74 ± 16 | 0.072 |
| LVDd (mm) | 47.9 ± 6.2 | 48.8 ± 5.0 | 50.5 ± 6.8 | 48.9 ± 4.6 | 0.30 |
| Ejection fraction (%) | 68 ± 6 | 65 ± 8 | 65 ± 10 | 67 ± 9 | 0.39 |
| E/e’ | 7.5 ± 4.2 | 9.1 ± 4.8 | 8.8 ± 3.3 | 7.9 ± 3.8 | 0.30 |
| LAVi ≥ 34.0 mm/m2 | 21 (26.6%) | 11 (40.7%) | 11 (42.3%) | 2 (22.2%) | 0.30 |
| TRPG (mmHg) | 18.0 ± 6.0 | 19.7 ±6.1 | 24.6 ± 8.3 | 18.0 ± 7.4 | 0.0006 |
| Anti-arrhythmic drugs | |||||
| Amiodarone | 11 (13%) | 5 (17%) | 6 (23%) | 3 (27%) | 0.49 |
| Bepridil | 3 (4%) | 1 (3%) | 1 (4%) | 0 | 0.94 |
| Sotalol | 0 | 1 (3%) | 1 (4%) | 0 | 0.33 |
| Cibenzoline | 4 (5%) | 2 (7%) | 2 (8%) | 0 | 0.78 |
| Disopyramide | 9 (11%) | 2 (7%) | 1 (4%) | 1 (9%) | 0.71 |
| Flecainide | 9 (11%) | 6 (20%) | 3 (12%) | 2 (18%) | 0.58 |
| Pilsicainide | 16 (19%) | 8 (27%) | 5 (19%) | 1 (9%) | 0.63 |
| Propafenone | 8 (10%) | 4 (13%) | 3 (12%) | 1 (9%) | 0.94 |
| Aprindine | 3 (4%) | 0 | 1 (4%) | 0 | 0.67 |
| Other drugs | |||||
| Beta blocker | 49 (58.3%) | 15 (50.0%) | 13 (50.0%) | 7 (63.6%) | 0.74 |
| ACEI | 6 (7.1%) | 1 (3.3%) | 3 (11.5%) | 1 (9.1%) | 0.69 |
| ARB | 27 (32.1%) | 10 (33.3%) | 9 (34.6%) | 3 (27.2%) | 0.98 |
| Baseline | 1-Year | 2-Year | 3-Year | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Recur | Short-Term | Mid-Term | Long-Term | Non-Recur | Short-Term | Mid-Term | Long-Term | Non-Recur | Short-Term | Mid-Term | Long-Term | Non-Recur | Short-Term | Mid-Term | Long-Term | |
| BNP (pg/mL) | 26 (15–42) | 46 (14–132) | 40 (19–83) | 23 (9–47) | 19 (11–30) | NA | 28 (17–55) | 19 (9–28) | 17 (9–30) | NA | 22 (17–39) | 8 (6–35) | 16 (9–27) | NA | NA | 13 (10–31) |
| ANP (pg/mL) | 36 (22–61) | 49 (27–106) | 31 (22–64) | 26 (15–43) | 28 (18–38) | NA | 39 (21–64) | 30 (22–37) | 24 (16–37) | NA | 36 (27–53) | 25 (18–40) | 22 (16–38) | NA | NA | 27 (18–43) |
| LAVi (mL/m2) | 29.6 ± 10.7 | 36.2 ± 12.6 | 35.5 ± 14.3 | 28.5 ± 9.2 | 27.9 ± 8.9 | NA | 31.2 ± 9.4 | 25.8 ± 9.8 | 27.7 ± 9.0 | NA | 30.8 ± 7.9 | 28.4 ± 8.4 | 28.0 ± 8.1 | NA | NA | 28.5 ± 9.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba, M.; Yoshida, K.; Naruse, Y.; Hattori, A.; Yui, Y.; Kimata, A.; Ito, Y.; Tsumagari, Y.; Tsuneoka, H.; Shinoda, Y.; et al. Predictors of Recurrence after Catheter Ablation of Paroxysmal Atrial Fibrillation in Different Follow-Up Periods. Medicina 2020, 56, 465. https://doi.org/10.3390/medicina56090465
Baba M, Yoshida K, Naruse Y, Hattori A, Yui Y, Kimata A, Ito Y, Tsumagari Y, Tsuneoka H, Shinoda Y, et al. Predictors of Recurrence after Catheter Ablation of Paroxysmal Atrial Fibrillation in Different Follow-Up Periods. Medicina. 2020; 56(9):465. https://doi.org/10.3390/medicina56090465
Chicago/Turabian StyleBaba, Masako, Kentaro Yoshida, Yoshihisa Naruse, Ai Hattori, Yoshiaki Yui, Akira Kimata, Yoko Ito, Yasuaki Tsumagari, Hidekazu Tsuneoka, Yasutoshi Shinoda, and et al. 2020. "Predictors of Recurrence after Catheter Ablation of Paroxysmal Atrial Fibrillation in Different Follow-Up Periods" Medicina 56, no. 9: 465. https://doi.org/10.3390/medicina56090465
APA StyleBaba, M., Yoshida, K., Naruse, Y., Hattori, A., Yui, Y., Kimata, A., Ito, Y., Tsumagari, Y., Tsuneoka, H., Shinoda, Y., Harunari, T., Hanaki, Y., Hasebe, H., Misaki, M., Abe, D., Nogami, A., Ieda, M., & Takeyasu, N. (2020). Predictors of Recurrence after Catheter Ablation of Paroxysmal Atrial Fibrillation in Different Follow-Up Periods. Medicina, 56(9), 465. https://doi.org/10.3390/medicina56090465





