Are Force Enhancement after Stretch and Muscle Fatigue Due to Effects of Elevated Inorganic Phosphate and Low Calcium on Cross Bridge Kinetics?
Abstract
1. Introduction
2. Methods
2.1. Muscle Samples
2.2. Solutions
2.3. Preparation of Single Fibres
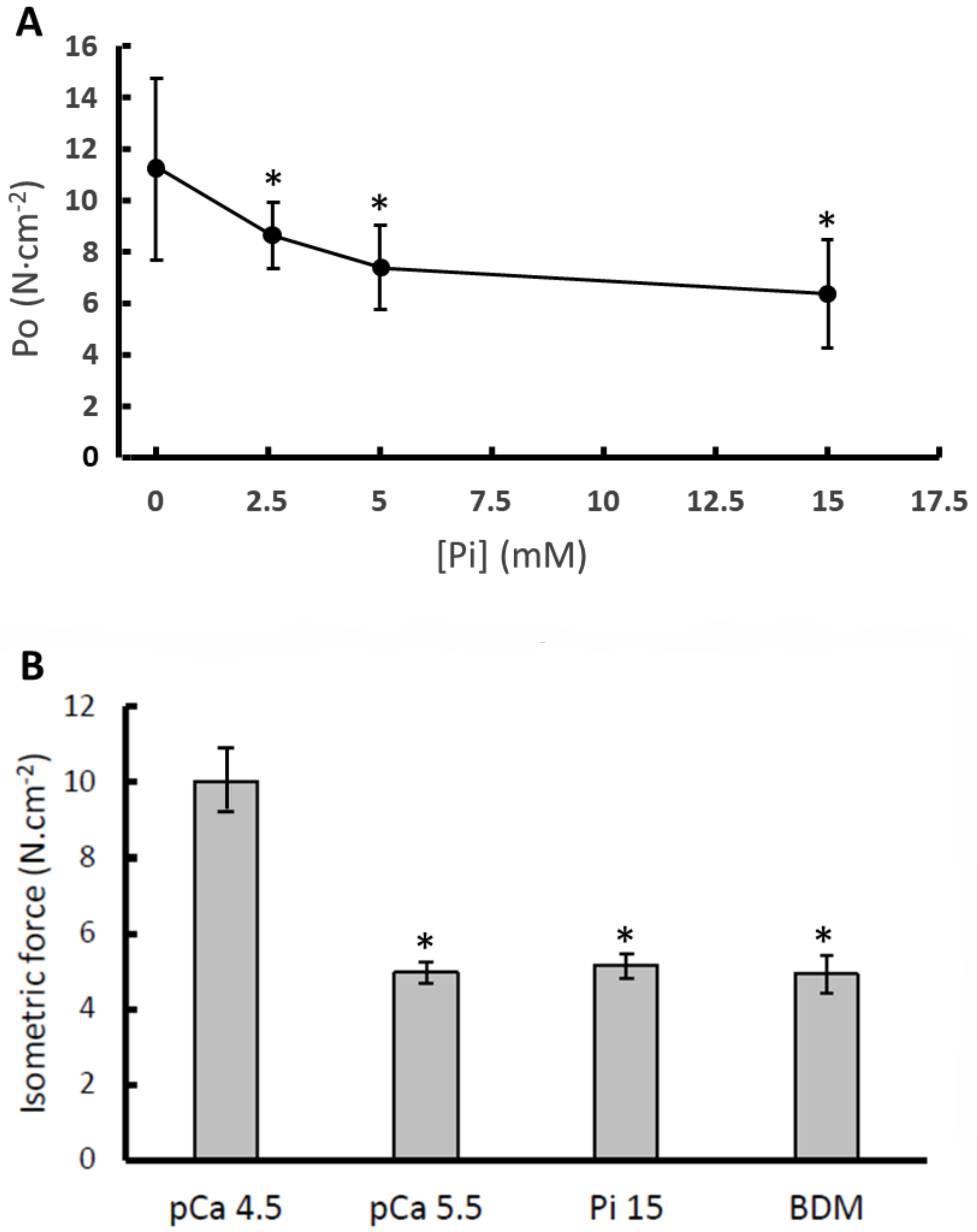
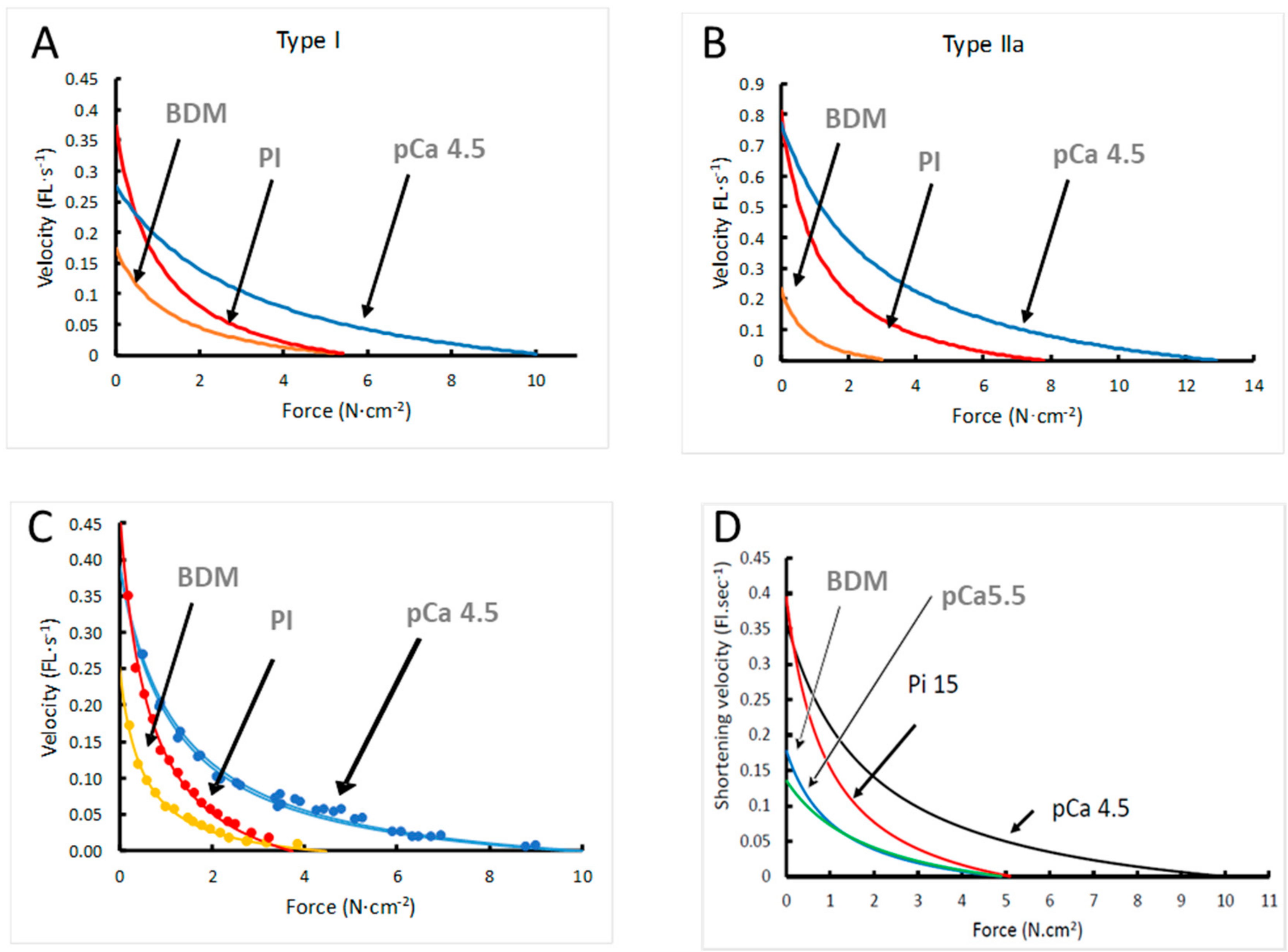
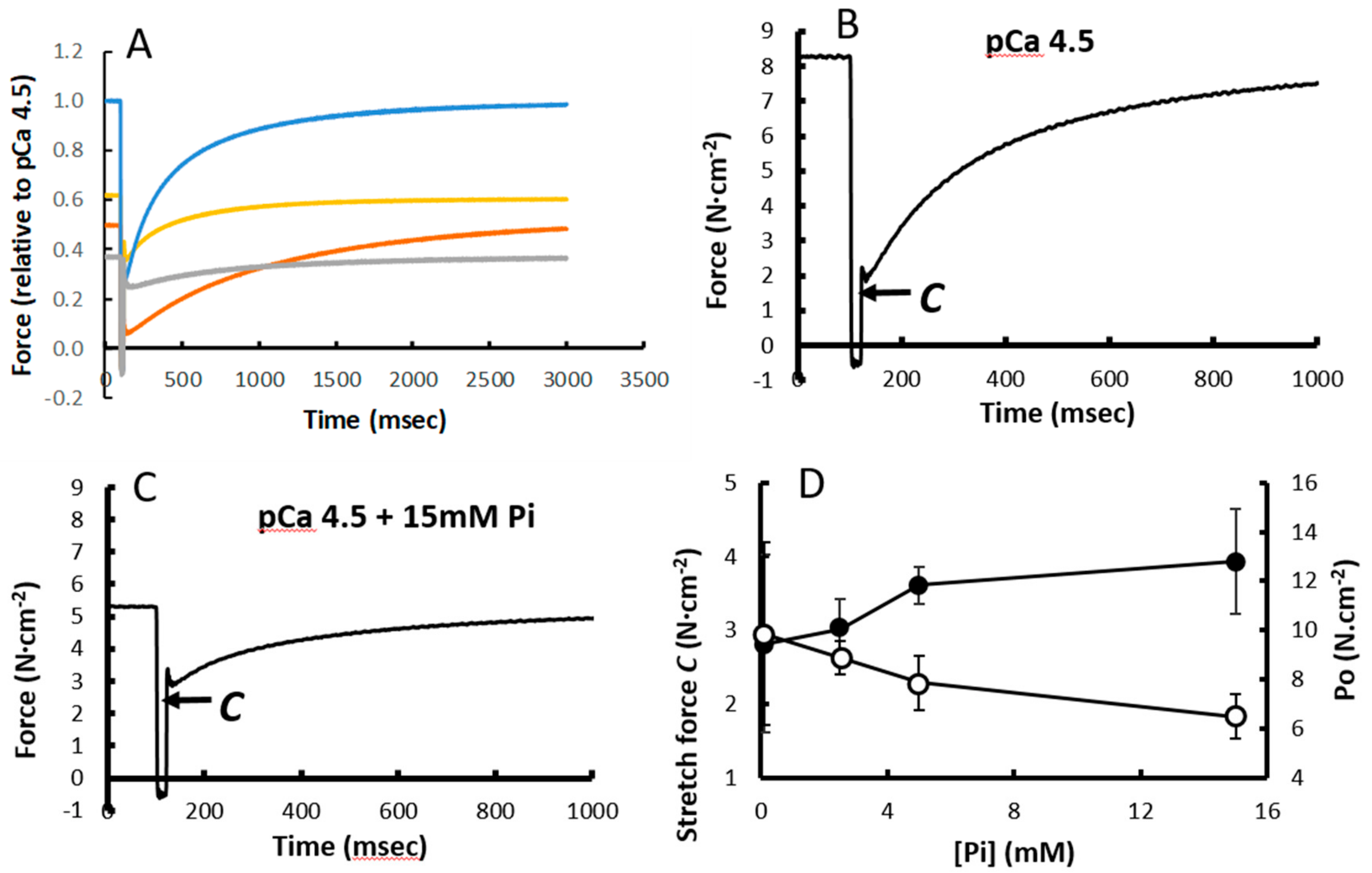
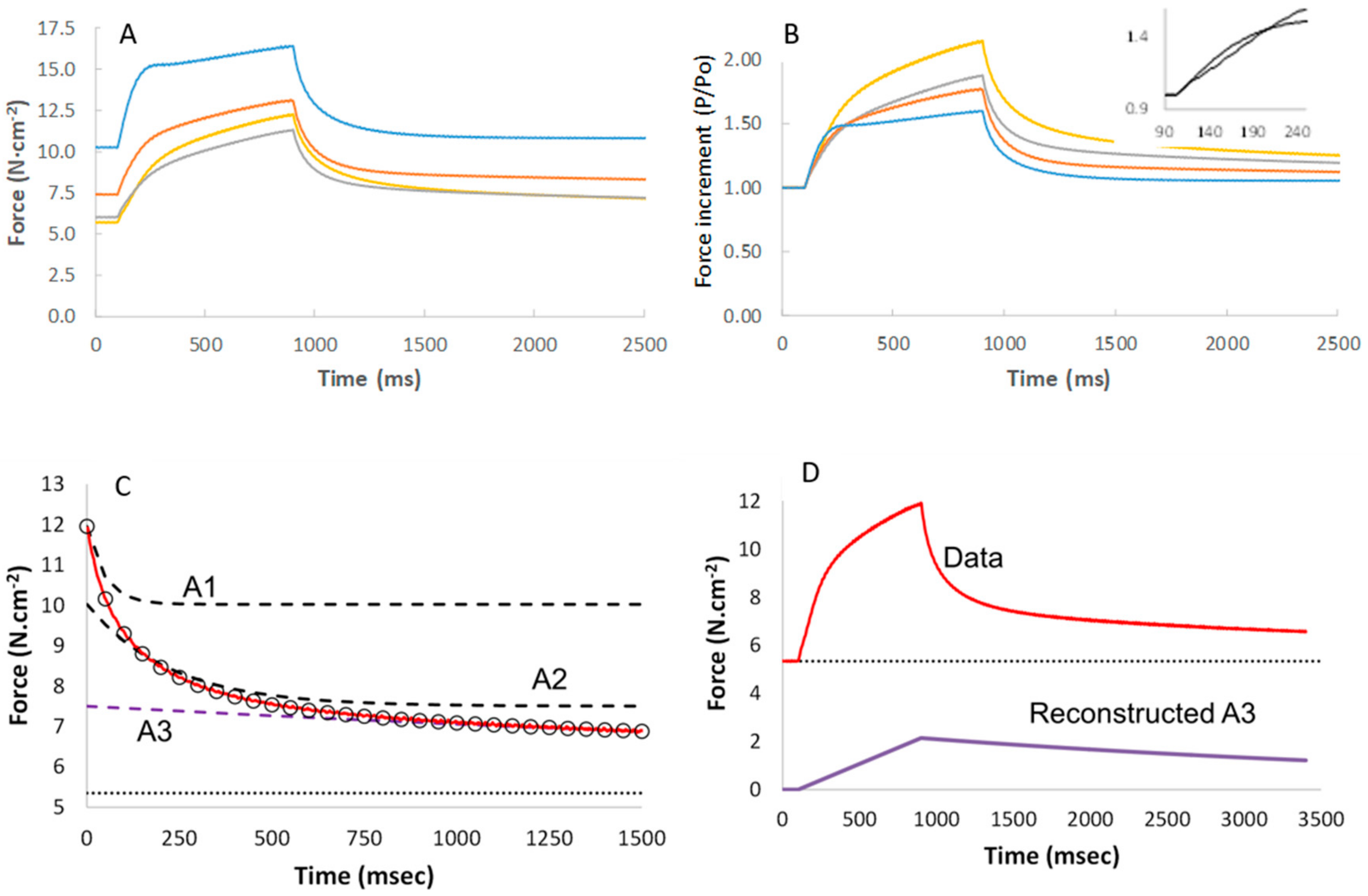
3. Results
3.1. Isometric Force
3.2. Force–Velocity Relationship
3.3. Ktr
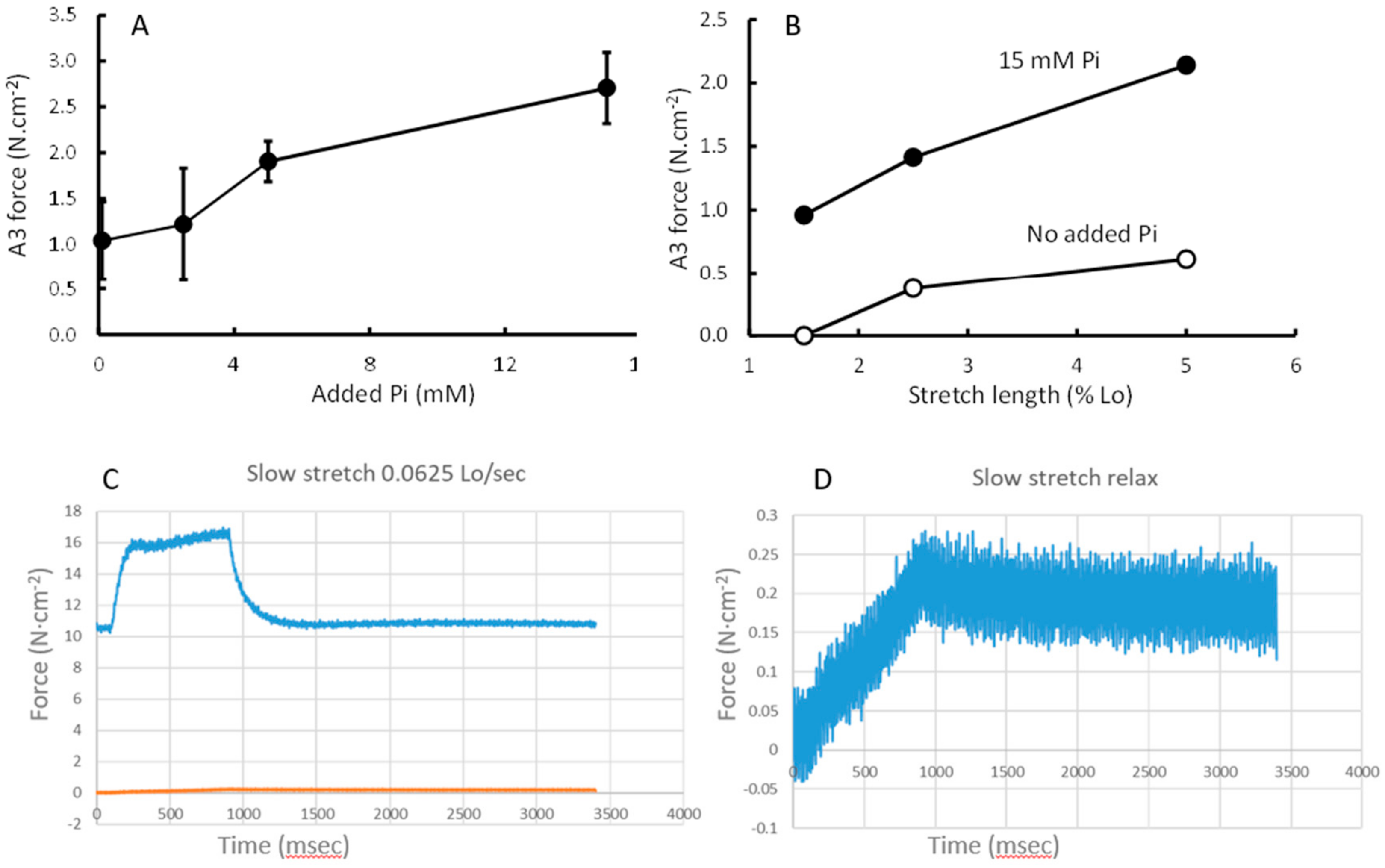
3.4. Force Increment During a 1% Stretch
3.5. Stretching
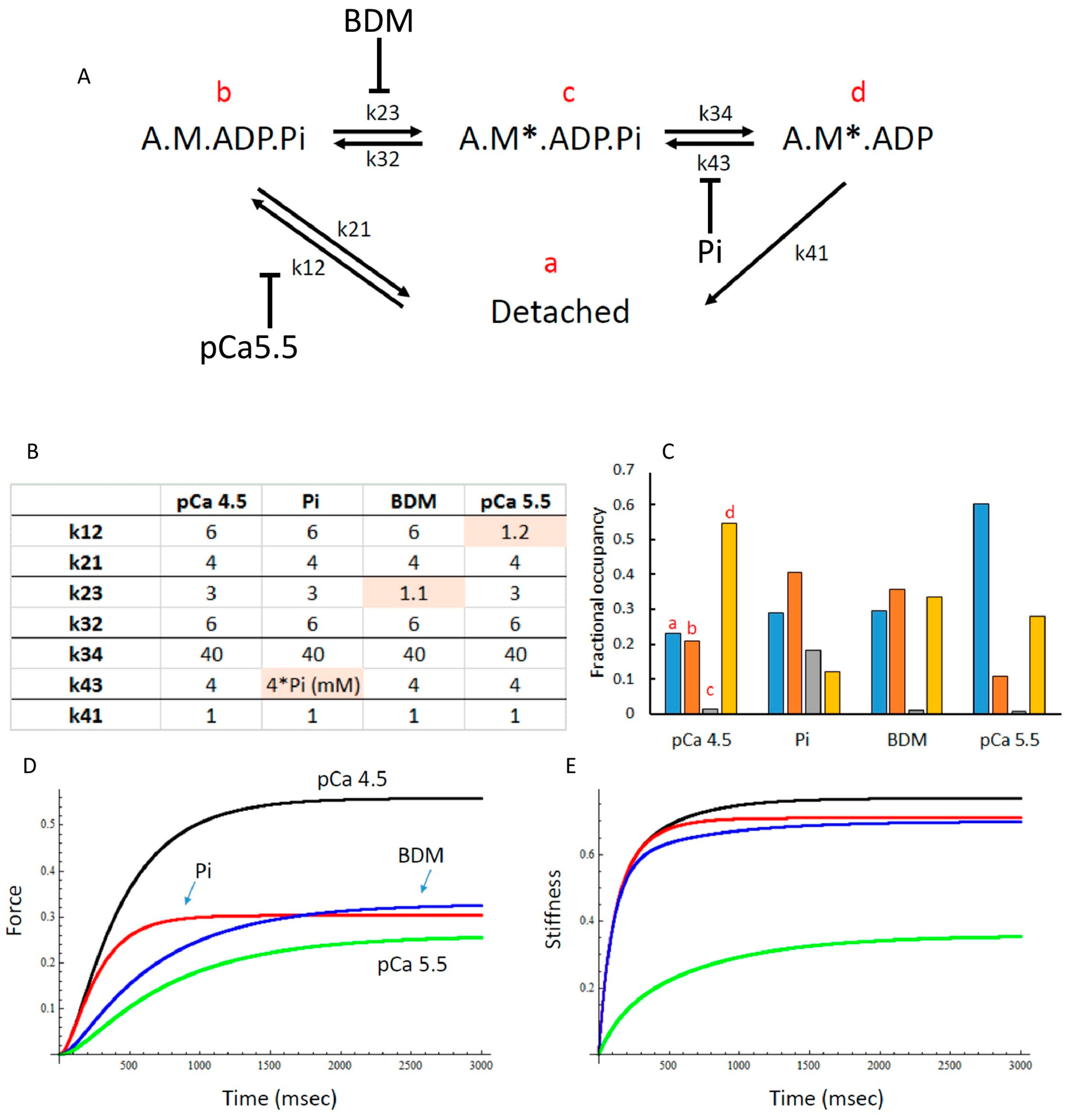
3.6. Modelling of Cross Bridge States
4. Discussion
4.1. Low Ca2+
4.2. Effects of Elevated Pi and BDM
4.3. Force Enhancement at the End of a Stretch
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Degens, H.; Veerkamp, J.H. Changes in oxidative capacity and fatigue resistance in skeletal muscle. Int. J. Biochem. 1994, 26, 871–878. [Google Scholar] [CrossRef]
- Jones, D.A. Changes in the force–velocity relationship of fatigued muscle: Implications for power production and possible causes. J. Physiol. 2010, 588, 2977–2986. [Google Scholar] [CrossRef]
- Westerblad, H.; Allen, D.G. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J. Physiol. 1993, 468, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; de Ruiter, C.J.; de Haan, A. Change in contractile properties of human muscle in relationship to the loss of power and slowing of relaxation seen with fatigue. J. Physiol. 2006, 576, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.M.; Nielsen, O.B.; Pedersen, T.H.; Overgaard, K. Fatiguing stimulation increases curvature of the force–velocity relationship in isolated fast-twitch and slow-twitch rat muscles. J. Exp. Boil. 2019, 222, jeb204545. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, C.J.; Didden, W.J.M.; Jones, D.A.; De Haan, A. The force–velocity relationship of human adductor pollicis muscle during stretch and the effects of fatigue. J. Physiol. 2000, 526, 671–681. [Google Scholar] [CrossRef]
- Flitney, F.W.; Jones, D.A. The effects of stretch on force production in fresh and fatigued skeletal muscle. J. Muscle Res. Cell Motil. 1990, 11, 75–76. [Google Scholar]
- Jones, D.A.; Turner, D.; McIntyre, D.B.; Newham, D. Energy turnover in relation to slowing of contractile properties during fatiguing contractions of the human anterior tibialis muscle. J. Physiol. 2009, 587, 4329–4338. [Google Scholar] [CrossRef]
- Bosutti, A.; Mulder, E.; Zange, J.; Bühlmeier, J.; Ganse, B.; Degens, H. Effects of 21 days of bed rest and whey protein supplementation on plantar flexor muscle fatigue resistance during repeated shortening contractions. Eur. J. Appl. Physiol. 2020, 120, 969–983. [Google Scholar] [CrossRef]
- Stienen, G.J.; Versteeg, P.G.; Papp, Z.; Elzinga, G. Mechanical properties of skinned rabbit psoas and soleus muscle fibres during lengthening: Effects of phosphate and Ca2+. J. Physiol. 1992, 451, 503–523. [Google Scholar] [CrossRef]
- Nocella, M.; Cecchi, G.; Colombini, B. Phosphate increase during fatigue affects crossbridge kinetics in intact mouse muscle at physiological temperature. J. Physiol. 2017, 595, 4317–4328. [Google Scholar] [CrossRef] [PubMed]
- Degens, H.; Bosutti, A.; Gilliver, S.F.; Slevin, M.; Van Heijst, A.F.; Wüst, R.C.I. Changes in contractile properties of skinned single rat soleus and diaphragm fibres after chronic hypoxia. Pflüger Archiv für die Gesammte Physiologie des Menschen und der Thiere 2010, 460, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Larsson, L. Contractile studies of single human skeletal muscle fibers: A comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve 1997, 20, 948–952. [Google Scholar] [CrossRef]
- Gilliver, S.F.; Rittweger, J.; Degens, H.; Jones, D.A. Effects of submaximal activation on the determinants of power of chemically skinned rat soleus fibres. Exp. Physiol. 2010, 96, 171–178. [Google Scholar] [CrossRef][Green Version]
- Degens, H.; Yu, F.; Li, X.; Larsson, L. Effects of age and gender on shortening velocity and myosin isoforms in single rat muscle fibres. Acta Physiol. Scand. 1998, 163, 33–40. [Google Scholar] [CrossRef]
- Brenner, B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys. J. 1983, 41, 99–102. [Google Scholar] [CrossRef]
- Brenner, B.; Eisenberg, E. Rate of force generation in muscle: Correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. USA 1986, 83, 3542–3546. [Google Scholar] [CrossRef]
- Ford, L.E.; Huxley, A.F.; Simmons, R.M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J. Physiol. 1981, 311, 219–249. [Google Scholar] [CrossRef]
- Rassier, D.E.; Herzog, W. Active force inhibition and stretch-induced force enhancement in frog muscle treated with BDM. J. Appl. Physiol. (1985) 2004, 97, 1395–1400. [Google Scholar] [CrossRef]
- Plas, R.L.C.; Degens, H.; Meijer, J.P.; De Wit, G.M.J.; Philippens, I.H.C.H.M.; Bobbert, M.F.; Jaspers, R.T. Muscle contractile properties as an explanation of the higher mean power output in marmosets than humans during jumping. J. Exp. Biol. 2015, 218, 2166–2173. [Google Scholar] [CrossRef]
- Tesi, C.; Colomo, F.; Piroddi, N.; Poggesi, C. Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J. Physiol. 2002, 541, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-J.; Qin, Z.; Wang, P.-Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef] [PubMed]
- Karatzaferi, C.; Franks-Skiba, K.; Cooke, R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R948–R955. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, C.; Hunter, S.K.; Trappe, S.W.; Smith, C.S.; Fitts, R. Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: Implications for muscle fatigue in humans. J. Physiol. 2018, 596, 3993–4015. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Morris, C.; Homsher, E. Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am. J. Physiol. 1995, 269, C1532–C1539. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.M.; Nielsen, O.B.; Overgaard, K. Effects of manipulating tetanic calcium on the curvature of the force–velocity relationship in isolated rat soleus muscles. Acta Physiol. 2018, 222, e12977. [Google Scholar] [CrossRef]
- Iwamoto, H. Thin filament cooperativity as a major determinant of shortening velocity in skeletal muscle fibers. Biophys. J. 1998, 74, 1452–1464. [Google Scholar] [CrossRef]
- Caremani, M.; Dantzig, J.; Goldman, Y.E.; Lombardi, V.; Linari, M. Effect of Inorganic Phosphate on the Force and Number of Myosin Cross bridges During the Isometric Contraction of Permeabilized Muscle Fibers from Rabbit Psoas. Biophys. J. 2008, 95, 5798–5808. [Google Scholar] [CrossRef]
- Caremani, M.; Melli, L.; Dolfi, M.; Lombardi, V.; Linari, M. The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site. J. Physiol. 2013, 591, 5187–5205. [Google Scholar] [CrossRef]
- Coupland, M.E.; Puchert, E.; Ranatunga, K.W. Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: Effect of inorganic phosphate. J. Physiol. 2001, 536, 879–891. [Google Scholar] [CrossRef]
- Metzger, J.M.; Moss, R.L. Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science 1990, 247, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Ušaj, M.; Rassier, D.E.; Mansson, A. Blebbistatin Effects Expose Hidden Secrets in the Force-Generating Cycle of Actin and Myosin. Biophys. J. 2018, 115, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Freundt, J.K.; Linke, W.A. Titin as a force-generating muscle protein under regulatory control. J. Appl. Physiol. 2019, 126, 1474–1482. [Google Scholar] [CrossRef]

| pCa 4.5 | 15 mM Pi | 1 mM BDM | |
|---|---|---|---|
| Type I | |||
| Po (N·cm−2) | 10.9 ± 1.0 (25) | 5.8 ± 0.64 (21) c | 5.8 ± 0.88 (15) c |
| Vmax (FL·s−1) | 0.30 ± 0.02 (25) | 0.40 ± 0.04 (21) c | 0.19 ± 0.02 (15) c,p |
| a/Po | 0.31 ± 0.04 (25) | 0.19 ± 0.02 (21) c | 0.24 ± 0.03 (15) c |
| Type IIa | |||
| Po (N·cm−2) | 12.0 ± 1.0 (19) | 7.4 ± 0.6 (14) c | 3.3 ± 0.4 (11) c,p |
| Vmax (FL·s−1) | 0.74 ± 0.07 (19) | 0.81 ± 0.09 (14) | 0.24 ± 0.03 (11) c,p |
| a/Po | 0.26 ± 0.03 (19) | 0.15 ± 0.02 (14) c | 0.25 ± 0.02 (11) |
| Force | Shortening Velocity | a/Po | Muscle Stiffness | |
|---|---|---|---|---|
| Fatigue | Decrease | Decrease | Decrease | Maintained |
| pCa 5.5 | Decrease | Decrease | Increase | Decrease |
| Pi | Decrease | Increase | Decrease | Maintained |
| BDM | Decrease | Decrease | Maintained | Maintained |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degens, H.; Jones, D.A. Are Force Enhancement after Stretch and Muscle Fatigue Due to Effects of Elevated Inorganic Phosphate and Low Calcium on Cross Bridge Kinetics? Medicina 2020, 56, 249. https://doi.org/10.3390/medicina56050249
Degens H, Jones DA. Are Force Enhancement after Stretch and Muscle Fatigue Due to Effects of Elevated Inorganic Phosphate and Low Calcium on Cross Bridge Kinetics? Medicina. 2020; 56(5):249. https://doi.org/10.3390/medicina56050249
Chicago/Turabian StyleDegens, Hans, and David A. Jones. 2020. "Are Force Enhancement after Stretch and Muscle Fatigue Due to Effects of Elevated Inorganic Phosphate and Low Calcium on Cross Bridge Kinetics?" Medicina 56, no. 5: 249. https://doi.org/10.3390/medicina56050249
APA StyleDegens, H., & Jones, D. A. (2020). Are Force Enhancement after Stretch and Muscle Fatigue Due to Effects of Elevated Inorganic Phosphate and Low Calcium on Cross Bridge Kinetics? Medicina, 56(5), 249. https://doi.org/10.3390/medicina56050249





