Correlation between Preoperative Coronary Artery Stenosis Severity Measured by Instantaneous Wave-Free Ratio and Intraoperative Transit Time Flow Measurement of Attached Grafts
Abstract
:1. Introduction
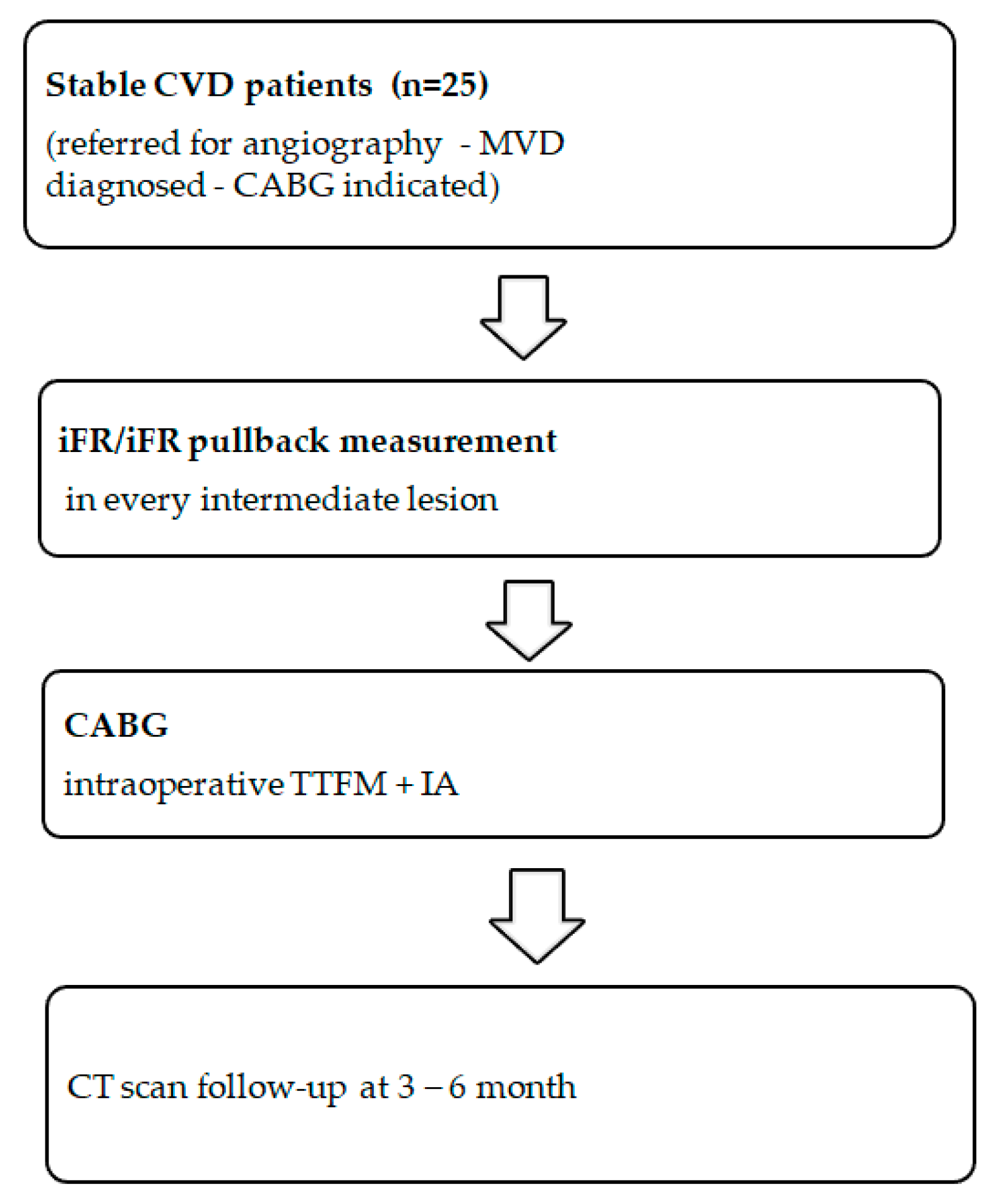
2. Materials and Methods
2.1. Study Groups
2.2. iFR Measurement
2.3. Revascularization and Graft Flow Measurement
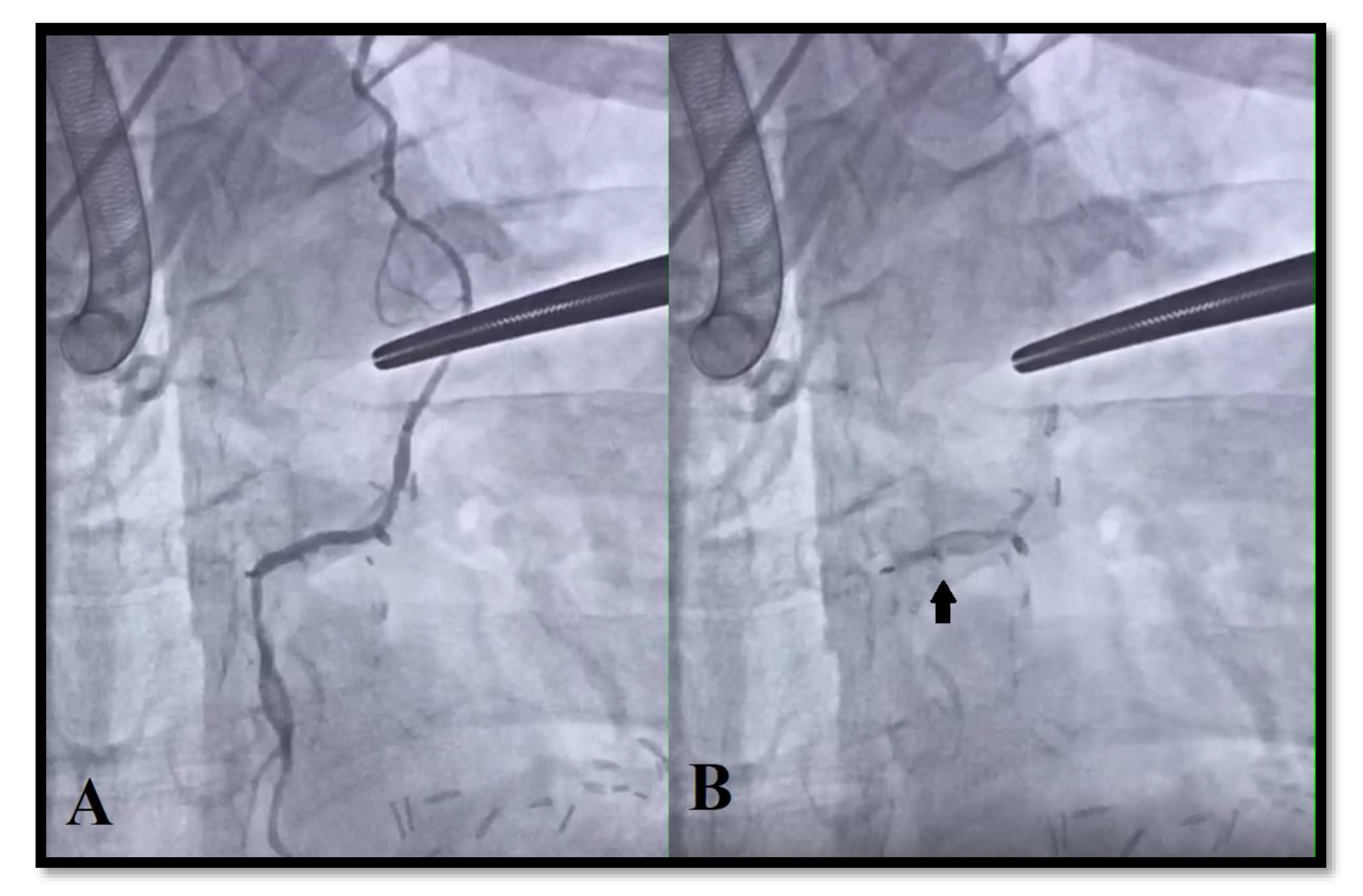
2.4. Intraoperative Angiography
2.5. Statistical Analysis
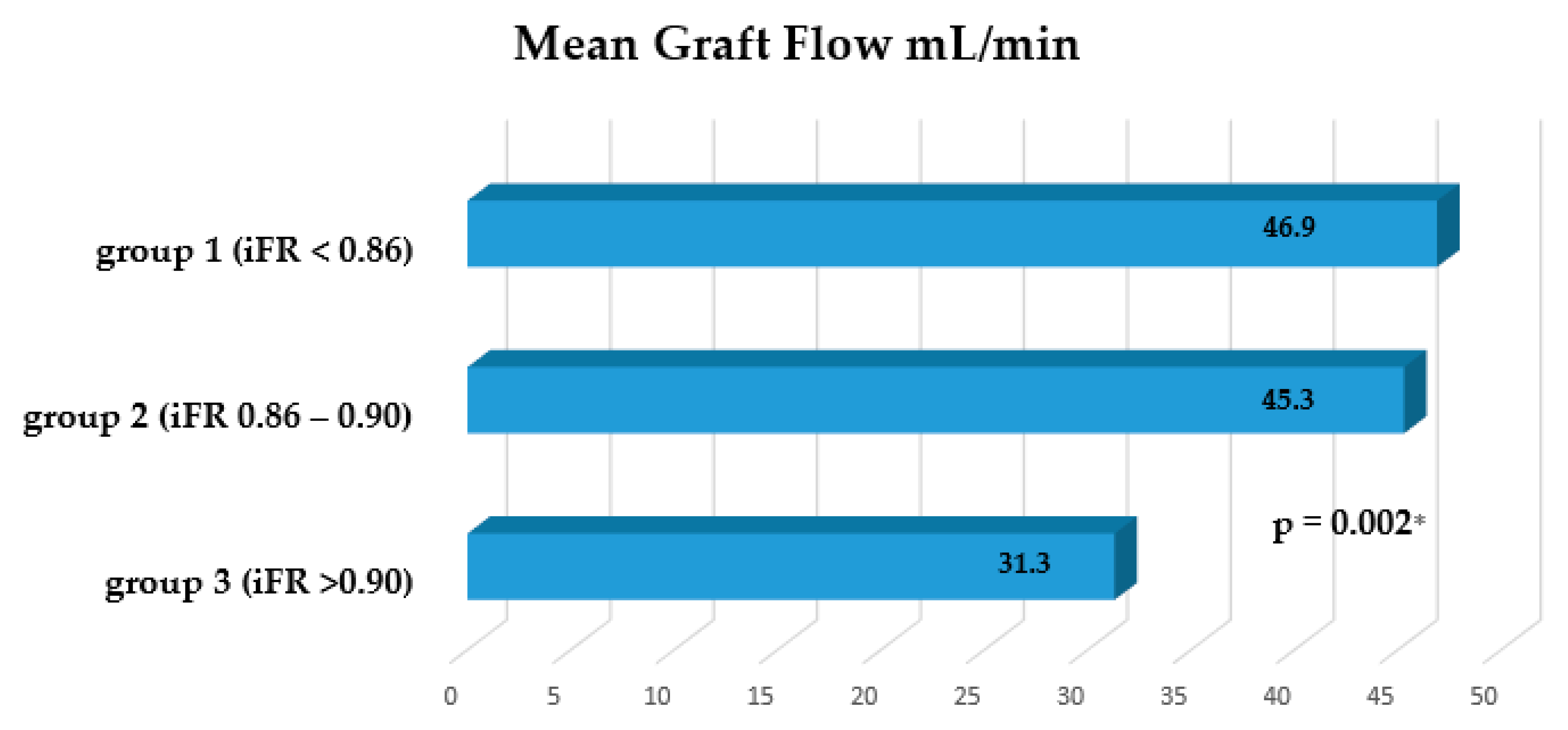
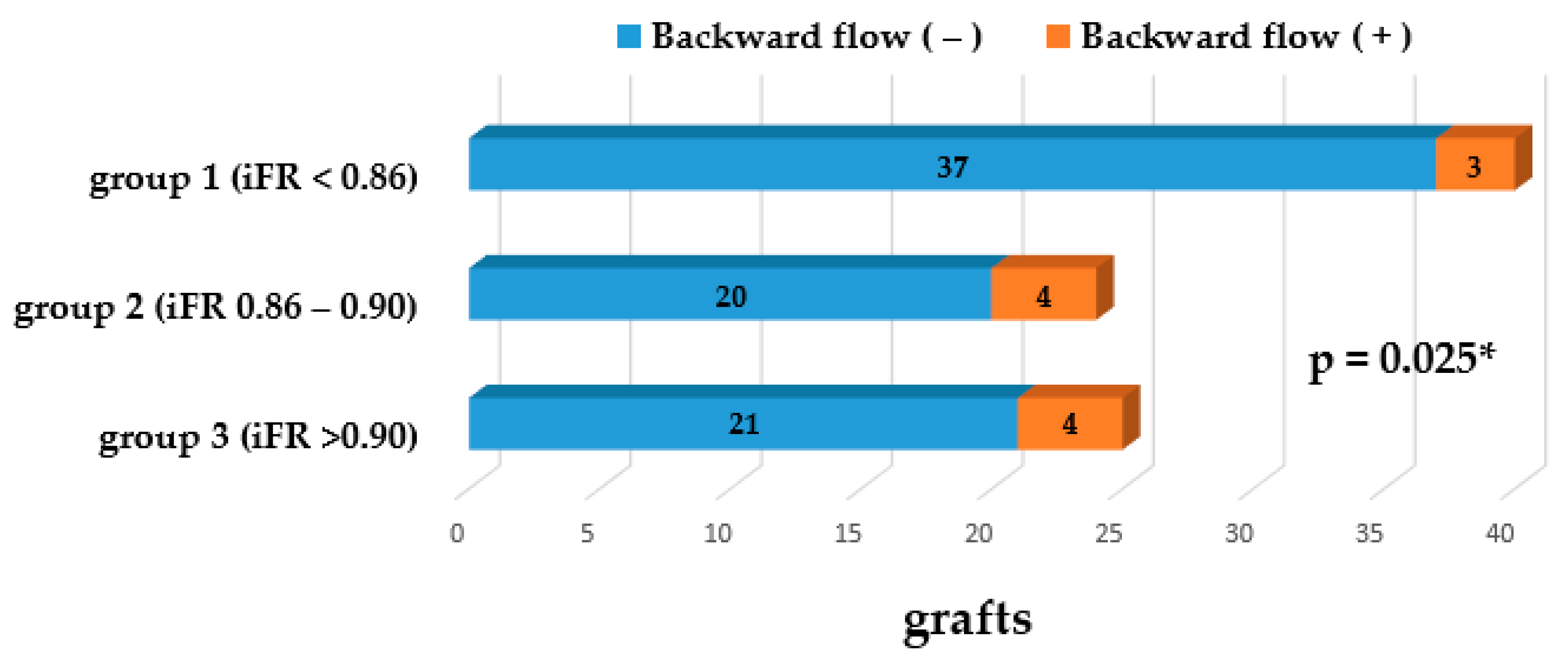
3. Results
Graft Flow Assessment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sabik, J.F.; Blackstone, E.H. Coronary Artery Bypass Graft Patency and Competitive Flow**Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology. J. Am. Coll. Cardiol. 2008, 51, 126–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Kobayashi, J.; Toda, K.; Fujita, T.; Shimahara, Y.; Kasahara, Y.; Kitamura, S. A 10-year angiographic follow-up of competitive flow in sequential and composite arterial grafts. Eur. J. Cardio-Thorac. Surg. 2011, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Jüni, P. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution. Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [PubMed]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; Tarkin, J.; Petraco, R.; Broyd, C.; Jabbour, R.; et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary waveintensity analysis: Results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardio-Thorac. Surg. 2019, 55, 4–90. [Google Scholar]
- Götberg, M.; Cook, C.M.; Sen, S.; Nijjer, S.; Escaned, J.; Davies, J.E. The Evolving Future of Instantaneous Wave-Free Ratio and Fractional Flow Reserve. J. Am. Coll. Cardiol. 2017, 70, 1379–1402. [Google Scholar] [CrossRef] [PubMed]
- Götberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Öhagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.E.; Sen, S.; Dehbi, H.M.; Al-Lamee, R.; Petraco, R.; Nijjer, S.S.; Bhindi, R.; Lehman, S.J.; Walters, D.; Sapontis, J.; et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N. Engl. J. Med. 2017, 376, 1824–1834. [Google Scholar] [CrossRef] [Green Version]
- Balacumaraswami, L.; Taggart, D.P. Intraoperative Imaging Techniques to Assess Coronary Artery Bypass Graft Patency. Ann. Thorac. Surg. 2007, 83, 2251–2257. [Google Scholar] [CrossRef]
- Honda, K.; Okamura, Y.; Nishimura, Y.; Uchita, S.; Yuzaki, M.; Kaneko, M.; Yamamoto, N.; Kubo, T.; Akasaka, T. Graft flow assessment using a transit time flow meter in fractional flow reserve-guided coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2015, 149, 1622–1628. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, A.; Maehara, A.; Généreux, P.; Asrress, K.N.; Berry, C.; De Bruyne, B.; Davies, J.E.; Escaned, J.; Fearon, W.F.; Gould, K.L.; et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pawith fractional flow reserve: The RESOLVE study. J. Am. Coll. Cardiol. 2014, 63, 1253–1261. [Google Scholar] [CrossRef] [Green Version]
- Modi, B.N.; Rahman, H.; Kaier, T.; Ryan, M.; Williams, R.; Briceno, N.; Ellis, H.; Pavlidis, A.; Redwood, S.; Clapp, B.; et al. Revisiting the Optimal FFR and iFR Thresholds for Predicting the Physiological Significance of Coronary Artery Disease. Circ. Cardiovasc. Interv. 2018, 11, e007041. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Pinho-Gomes, A.C.; Taggart, D.P. Relationship of Intraoperative Transit Time Flowmetry Findings to Angiographic Graft Patency at Follow-Up. Ann. Thorac. Surg. 2016, 101, 1996–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieser, T.M.; Rose, S.; Kowalewski, R.; Belenkie, I. Transit-time flow predicts outcomes in coronary artery bypass graft patients: A series of 1000 consecutive arterial grafts. Eur. J. Cardio-Thorac. Surg. 2010, 38, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Developed with the Special Contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI); Wijns, W.; Kolh, P.; Danchin, N.; Di Mario, C.; Falk, V.; Folliguet, T.; Garg, S.; Huber, K.; James, S.; et al. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2010, 31, 2501–2555. [Google Scholar] [PubMed]
- Fukui, T. Intraoperative graft assessment during coronary artery bypass surgery. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 123–130. [Google Scholar] [CrossRef]
- Ohmes, L.B.; Di Franco, A.; Di Giammarco, G.; Rosati, C.M.; Lau, C.; Girardi, L.N.; Massetti, M.; Gaudino, M. Techniques for intraoperative graft assessment in coronary artery bypass surgery. J. Thorac. Dis. 2017, 9 (Suppl. 4), S327–S332. [Google Scholar] [CrossRef] [Green Version]
- Summers, M.R.; Patel, M.R. Appropriateness of percutaneous coronary intervention: A review. Curr. Cardiol. Rep. 2013, 15, 53–61. [Google Scholar] [CrossRef]
- Kitamura, S.; Kawachi, K.; Seki, T.; Sawabata, N.; Morita, R.; Kawata, T. Angiographic demonstration of no-flow anatomical patency of internal thoracic-coronary artery bypass grafts. Ann. Thorac. Surg. 1992, 53, 156–159. [Google Scholar] [CrossRef]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A. Patencies of 2127 arterial to coronary conduits over 15 years. Ann. Thorac. Surg. 2004, 77, 93–101. [Google Scholar] [CrossRef]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L.; et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Song, K.; Shen, J.; Wang, Y.; Yang, Y.; Ding, W.; Xia, L.; Wang, C. Long-term patency rate of radial artery conduits in chinese patients undergoing off-pump coronary artery bypass grafting. Int. Heart J. 2019, 60, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Hayward, P.A.; Gordon, I.R.; Hare, D.L.; Matalanis, G.; Horrigan, M.L.; Rosalion, A.; Buxton, B.F. Comparable patencies of the radial artery and right internal thoracic artery or saphenous vein beyond 5 years: Results from the Radial Artery Patency and Clinical Outcomes trial. J. Thorac. Cardiovasc. Surg. 2010, 139, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akita, S.; Tajima, K.; Kato, W.; Tanaka, K.; Goto, Y.; Yamamoto, R.; Yazawa, T.; Kozakai, M.; Usui, A. The long-term patency of a gastroepiploic artery bypass graft deployed in a semiskeletonized fashion: Predictors of patency. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 868–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, N.L.; Hockmuth, D.R.; Everson, C.T.; Robart, C.C.; Mitchell, R.S. Right gastroepiploic artery used for coronary artery bypass grafting: Evaluation of flow characteristics and size. J. Thorac. Cardiovasc. Surg. 1993, 106, 579–586. [Google Scholar] [CrossRef]
- Hashimoto, H.; Isshiki, T.; Ikari, Y.; Hara, K.; Saeki, F.; Tamura, T.; Yamaguchi, T.; Suma, H. Effects of competitive blood flow on arterial graft patency and diameter: Medium-term postoperative follow-up. J. Thorac. Cardiovasc. Surg. 1996, 111, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Tinica, G.; Chistol, R.O.; Enache, M.; Constantin, M.M.L.; Ciocoiu, M.; Furnica, C. Long-term graft patency after coronary artery bypass grafting: Effects of morphological and pathophysiological factors. Anatol. J. Cardiol. 2018, 20, 275–282. [Google Scholar] [CrossRef]
- D’Ancona, G.; Karamanoukian, H.L.; Ricci, M.; Schmid, S.; Bergsland, J.; Salerno, T.A. Graft revision after transit time flow measurement in off-pump coronary artery bypass grafting. Eur. J. Cardio-Thorac. Surg. 2000, 17, 287–293. [Google Scholar] [CrossRef] [Green Version]


| Variables | Value |
|---|---|
| Age | 63.8 ± 8.9 (48–78) |
| Body mass index (kg/m2) | 27.2 ± 4.79 |
| Sex | |
| Male | 23 (92%) |
| Hypertension | 23 (92%) |
| Diabetes mellitus | 2 (8%) |
| Dyslipidemia | 22 (88%) |
| Arrhythmia | 4 (16%) |
| Previous PCI | 9 (36%) |
| Previous MI | 15 (60%) |
| History of smoking | 15 (60%) |
| LV EF% | 47.12 ± 6.4 |
| NYHA | |
| II | 19 (76%) |
| III | 6 (24%) |
| Euro Score II | 1.38 ± 0.75 |
| Syntax score | 31.38 ± 4.33 |
| Distal anastomoses per 1 pt. | 3.56 ± 0.82 (2–5) |
| Variables | Group 1 (iFR < 0.86) | Group 2 (iFR 0.86–0.90) | Group 3 (iFR > 0.90) |
|---|---|---|---|
| Distal anastomosis | 40 | 24 | 25 |
| Grafts | 36 | 22 | 23 |
| SVG to RCA | 7 (17.5%) | 4 (16.6%) | 3 (12%) |
| SVG to PDA | 8 (20%) | 5 (20.8%) | 2 (8%) |
| SVG to OM | 11 (27.5%) | 6 (25%) | 9 (36%) |
| SVG to diagonal | 3 (7.5%) | 2 (8%) | 4 (16%) |
| LIMA to LAD | 11 (27.5%) | 7 (29%) | 7 (28%) |
| Sequential grafts | 4 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolegenuly, A.; Ordiene, R.; Mamedov, A.; Unikas, R.; Benetis, R. Correlation between Preoperative Coronary Artery Stenosis Severity Measured by Instantaneous Wave-Free Ratio and Intraoperative Transit Time Flow Measurement of Attached Grafts. Medicina 2020, 56, 714. https://doi.org/10.3390/medicina56120714
Tolegenuly A, Ordiene R, Mamedov A, Unikas R, Benetis R. Correlation between Preoperative Coronary Artery Stenosis Severity Measured by Instantaneous Wave-Free Ratio and Intraoperative Transit Time Flow Measurement of Attached Grafts. Medicina. 2020; 56(12):714. https://doi.org/10.3390/medicina56120714
Chicago/Turabian StyleTolegenuly, Almas, Rasa Ordiene, Arslan Mamedov, Ramunas Unikas, and Rimantas Benetis. 2020. "Correlation between Preoperative Coronary Artery Stenosis Severity Measured by Instantaneous Wave-Free Ratio and Intraoperative Transit Time Flow Measurement of Attached Grafts" Medicina 56, no. 12: 714. https://doi.org/10.3390/medicina56120714
APA StyleTolegenuly, A., Ordiene, R., Mamedov, A., Unikas, R., & Benetis, R. (2020). Correlation between Preoperative Coronary Artery Stenosis Severity Measured by Instantaneous Wave-Free Ratio and Intraoperative Transit Time Flow Measurement of Attached Grafts. Medicina, 56(12), 714. https://doi.org/10.3390/medicina56120714






