The Impact of Obesity on Nighttime Blood Pressure Dipping
Abstract
1. Introduction
2. Patients and Methods
- Group 1: patients with BMI ≥ 30 kg/m2 and <40 kg/m2;
- Group 2: patients with BMI ≥ 40 kg/m2.
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abarca-Gómez:, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Zdrojewski, T.; Rutkowski, M.; Bandosz, P. Epidemiologia palenia papierosów oraz innych czynników ryzyka chorób układu krążenia w Polsce-badanie NATPOL 2011. In Proceedings of the IV konferencja “Tytoń albo Zdrowie”, Warszawa, Poland, 9 December 2011. [Google Scholar]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Stamler, R.; Stamler, J.; Riedlinger, W.F.; Algera, G.; Roberts, R.H. Weight and blood pressure: Findings in hypertension screening of 1 million Americans. JAMA 1978, 240, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.R.; Elliott, P.; Shipley, M. Body mass index versus height and weight in relation to blood pressure. Finding for the 10,079 persons in the INTERSALT Study. Am. J. Epidemiol. 1990, 131, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef]
- WHO (2020): Obesity and Overweight. Fact Sheets Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 December 2020).
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redán, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2013, 31, 1925–1938. [Google Scholar] [CrossRef]
- Dolan, E.; Stanton, A.; Thijs, L.; Hinedi, K.; Atkins, N.; McClory, S. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 2005, 46, 156–161. [Google Scholar] [CrossRef]
- Rothwell, P.M. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010, 375, 938–948. [Google Scholar] [CrossRef]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Fedecostante, M.; Spannella, F.; Giulietti, F.; Espinosa, E.; Dessί-Fulgheri, P.; Sarzani, R. Associations Between Body Mass Index, Ambulatory Blood Pressure Findings, and Changes in Cardiac Structure: Relevance of Pulse and Nighttime Pressures. J. Clin. Hypertens. 2015, 17, 147–153. [Google Scholar] [CrossRef]
- Wrzosek, M.; Wiśniewska, K.; Sawicka, A.; Tałałaj, M.; Nowicka, G. Early Onset of Obesity and Adult Onset of Obesity as Factors Affecting Patient Characteristics Prior to Bariatric Surgery. Obes. Surg. 2018, 28, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Cicero, A.F.; Derosa, G. Are there mild and serious metabolic syndromes? The need for a graded diagnosis. J. Cardiovasc. Med. 2014, 15, 759–760. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Strazzullo, P. Excess Body Weight, Insulin Resistance and Isolated Systolic Hypertension: Potential Pathophysiological Links. High Blood Press. Cardiovasc. Prev. 2018, 25, 17–23. [Google Scholar] [CrossRef]
- Son, W.M.; Kim, D.Y.; Kim, Y.S.; Ha, M.S. Effect of Obesity on Blood Pressure and Arterial Stiffness in Middle-Aged Korean Women. Osong Public Health Res. Perspect. 2017, 8, 369–372. [Google Scholar] [CrossRef]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef]
- Cuspidi, C.; Negri, F.; Sala, C.; Valerio, C.; Mancia, G. Association of left atrial enlargement with left ventricular hypertrophy and diastolic dysfunction: A tissue Doppler study in echocardiographic practice. Blood Press. 2012, 21, 24–30. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, Y.M.; Lee, Y.; Lim, Y.H.; Shin, J. A reverse dipping pattern predicts cardiovascular mortality in a clinical cohort. J. Korean Med. Sci. 2013, 28, 1468–1473. [Google Scholar] [CrossRef]
- Kim, B.K.; Lim, Y.H.; Lee, H.T.; Lee, J.U.; Kim, K.S.; Kim, S.G. Non-Dipper Pattern is a Determinant of the Inappropriateness of Left Ventricular Mass in Essential Hypertensive Patients. Korean Circ. J. 2011, 41, 191–197. [Google Scholar] [CrossRef]
- Salles, G.F.; Reboldi, G.; Fagard, R.H.; Cardoso, C.R.; Pierdomenico, S.D.; Verdecchia, P.; Eguchi, K.; Kario, K.; Hoshide, S.; Polonia, J.; et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: The ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension 2016, 67, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H.; Thijs, L.; Staessen, J.A.; Clement, D.L.; De Buyzere, M.L.; De Bacquer, D.A. Night–day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J. Hum. Hypertens. 2009, 23, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Bouhanick, B.; Bongard, V.; Amar, J.; Bousquel, S.; Chamontin, B. Prognostic value of nocturnal blood pressure and reverse-dipping status on the occurrence of cardiovascular events in hypertensive diabetic patients. Diabetes Metab. 2008, 34, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.; Steffen, P.R.; Blumenthal, J.A.; Kuhn, C.; Hinderliter, A.L. Nighttime blood pressure dipping: The role of the sympathetic nervous system. Am. J. Hypertens. 2002, 15, 111–118. [Google Scholar] [CrossRef]
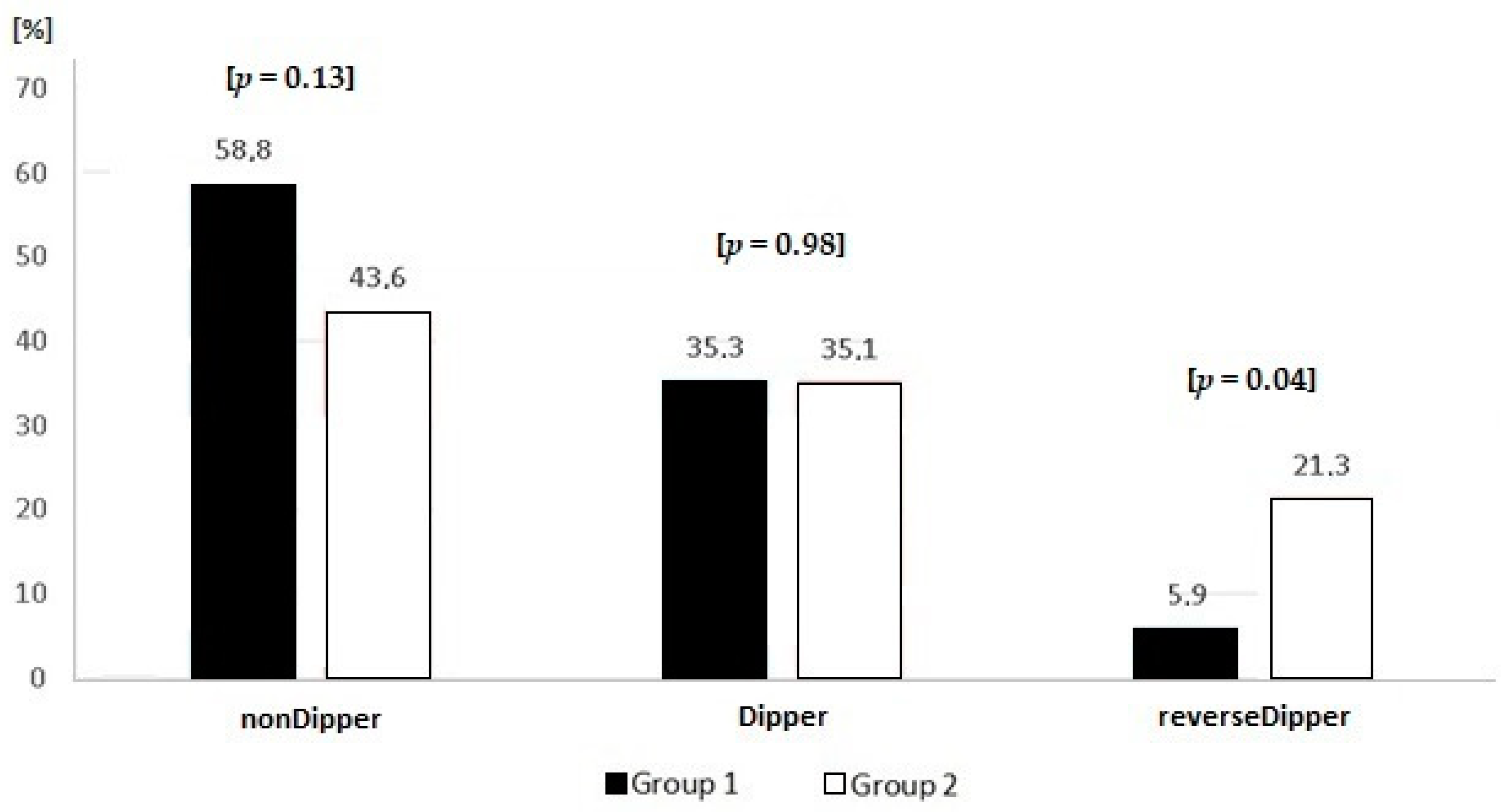
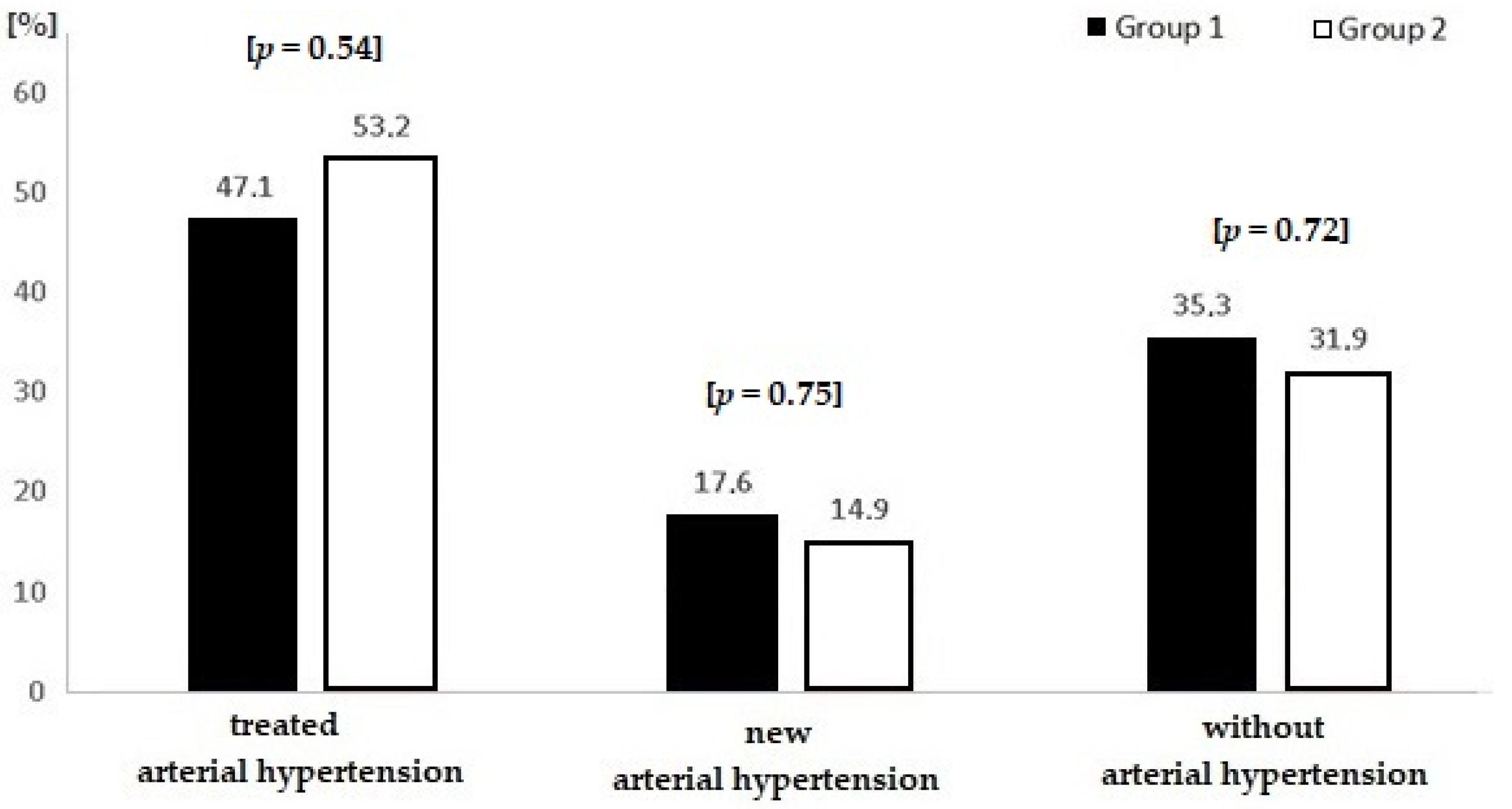
| Parameter | Groups | ||||
|---|---|---|---|---|---|
| Group 1 < 40 (n = 34) | Group 2 ≥ 40 (n = 94) | ||||
| n = 128 | median | (25–75% quartile) | median | (25–75% quartile) | p |
| Age (years old) | 46.5 | (39.0–58.0) | 39.0 | (33.0–48.0) | 0.004 |
| Total cholesterol (mg/dL) | 196.5 | (177.0–212.0) | 192.0 | (167.0–211.0) | 0.43 |
| Low-density lipoprotein (mg/dL) | 119.5 | (97.0–133.0) | 115.5 | (86.0–131.0) | 0.52 |
| High-density lipoprotein (mg/dL) | 51.5 | (43.0–62.0) | 44.0 | (40.0–51.0) | 0.006 |
| Triglycerides (mg/dL) | 121.0 | (105.0–162.0) | 147.0 | (119.0–200.0) | 0.05 |
| Uric acid (mg/dL) | 5.85 | (5.4–6.9) | 6.25 | (5.6–7.1) | 0.23 |
| C-reactive protein (mg/L) | 2.45 | (1.0–5.3) | 5.95 | (3.6–8.8) | 0.0003 |
| Aspartate transaminase (U/L) | 22.5 | (18.0–28.0) | 22 | (19.0–28.0) | 0.83 |
| Alanine transaminase (U/L) | 27.5 | (19.0–37.0) | 28.0 | (23.0–42.0) | 0.55 |
| Thyroid-stimulating hormone (µIU/mL) | 1.65 | (1.19–2.53) | 1.59 | (1.19–2.15) | 0.58 |
| Glucose (mg/dL) | 95.0 | (89.0–102.0) | 92.5 | (88.0–113.0) | 0.85 |
| Creatinine (mg/dL) | 0.77 | (0.68–0.87) | 0.65 | (0.61–0.79) | 0.002 |
| Parameter | Groups | ||||
|---|---|---|---|---|---|
| Group 1 < 40 (n = 34) | Group 2 ≥ 40 (n = 94) | ||||
| n = 128 | median | (25–75% quartile) | median | (25–75% quartile) | p |
| Systolic blood pressure 24-h (mmHg) | 127.0 | (116.0–134.0) | 132.0 | (127.0–140.0) | 0.004 |
| Diastolic blood pressure 24-h (mmHg) | 75.0 | (72.0–82.0) | 84.0 | (76.0–90.0) | 0.0003 |
| Systolic blood pressure daytime (mmHg) | 129.5 | (119.0–138.0) | 134.0 | (128.0–141.0) | 0.008 |
| Diastolic blood pressure daytime (mmHg) | 77.5 | (74.0–86.0) | 86.0 | (78.0–93.0) | 0.0007 |
| Systolic blood pressure nighttime (mmHg) | 117.0 | (108.0–126.0) | 124.0 | (117.0–136.0) | 0.003 |
| Diastolic blood pressure nighttime (mmHg) | 68.5 | (63.0–74.0) | 75.5 | (66.0–83.0) | 0.002 |
| Dipping % | 8.95 | (5.3–11.4) | 7.4 | (1.2–12.4) | 0.42 |
| Heart rate 24-h (beats per minute) | 67.5 | (63.0–72.0) | 76.0 | (69.0–81.0) | 0.00005 |
| Drug | Groups | ||||
|---|---|---|---|---|---|
| Group 1 < 40 (n = 34) | Group 2 ≥ 40 (n = 94) | ||||
| n = 128 | n | % | n | % | p |
| β-blocker | 14 | 41.2 | 27 | 28.7 | 0.18 |
| ACE-I/ARB | 21 | 61.8 | 49 | 52.1 | 0.33 |
| Diuretic | 13 | 38.2 | 33 | 35.1 | 0.74 |
| Ca-blocker | 12 | 35.3 | 31 | 33 | 0.81 |
| Other | 1 | 2.9 | 4 | 4.3 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moczulska, B.; Zechowicz, M.; Leśniewska, S.; Osowiecka, K.; Gromadziński, L. The Impact of Obesity on Nighttime Blood Pressure Dipping. Medicina 2020, 56, 700. https://doi.org/10.3390/medicina56120700
Moczulska B, Zechowicz M, Leśniewska S, Osowiecka K, Gromadziński L. The Impact of Obesity on Nighttime Blood Pressure Dipping. Medicina. 2020; 56(12):700. https://doi.org/10.3390/medicina56120700
Chicago/Turabian StyleMoczulska, Beata, Maciej Zechowicz, Sylwia Leśniewska, Karolina Osowiecka, and Leszek Gromadziński. 2020. "The Impact of Obesity on Nighttime Blood Pressure Dipping" Medicina 56, no. 12: 700. https://doi.org/10.3390/medicina56120700
APA StyleMoczulska, B., Zechowicz, M., Leśniewska, S., Osowiecka, K., & Gromadziński, L. (2020). The Impact of Obesity on Nighttime Blood Pressure Dipping. Medicina, 56(12), 700. https://doi.org/10.3390/medicina56120700





