Structural Connectivity-Based Parcellation of the Dopaminergic Midbrain in Healthy Subjects and Schizophrenic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls Selection
2.2. Data Acquisition
2.3. Data Preprocessing
2.4. Post-Processing
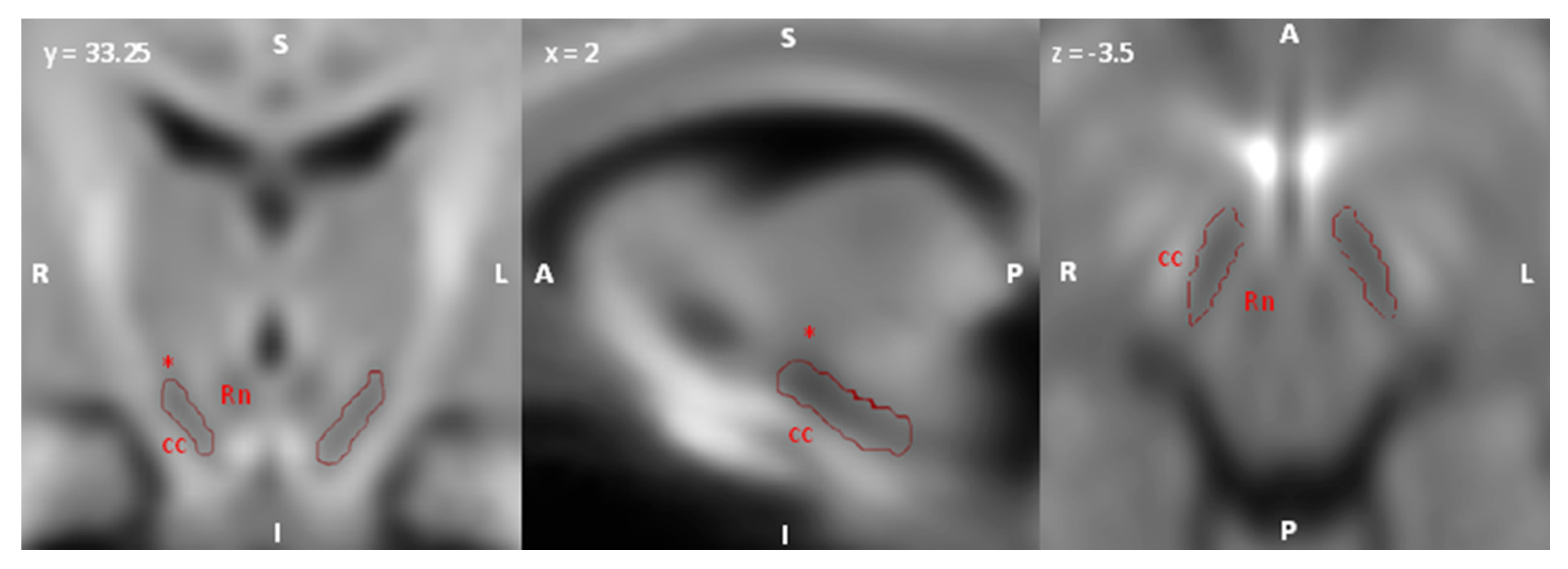
2.5. Regions of Interest (ROI) Selection
2.6. Connectivity-Based Parcellation and Tractography
- Seed-based tractography: 5000 streamlines were reconstructed seeding from seed ROIs (striatum, SNc/VTA) to the ipsilateral target ROIs (cortical groups) with the following parameters: algorithm IFod2 [65] step size 1.25, maximum angle 30. Target ROIs were used as inclusion mask; streamlines reaching each of the target masks were counted as connecting the source voxel to that target, and then immediately terminated. No exclusion masks were employed.
- Tractogram-to-voxel mapping: by applying the track-density-imaging (TDI) framework, each tractogram was mapped to an image in which intensity is defined as the number of streamlines passing through a given grid element [66], corresponding in dimension and voxel size to seed ROI; track-density streamline maps were then multiplied to binarized seed ROIs to obtain tractograms endpoint distribution.
- Classification: each map obtained from the previous step was normalized by dividing each voxel’s intensity to the mean intensity of the map, in order to obtain comparable intensity values: then, each voxel in the seed ROI was classified using a hard segmentation algorithm (find_the_biggest command on FSL) that assigns it to the map showing higher intensity.
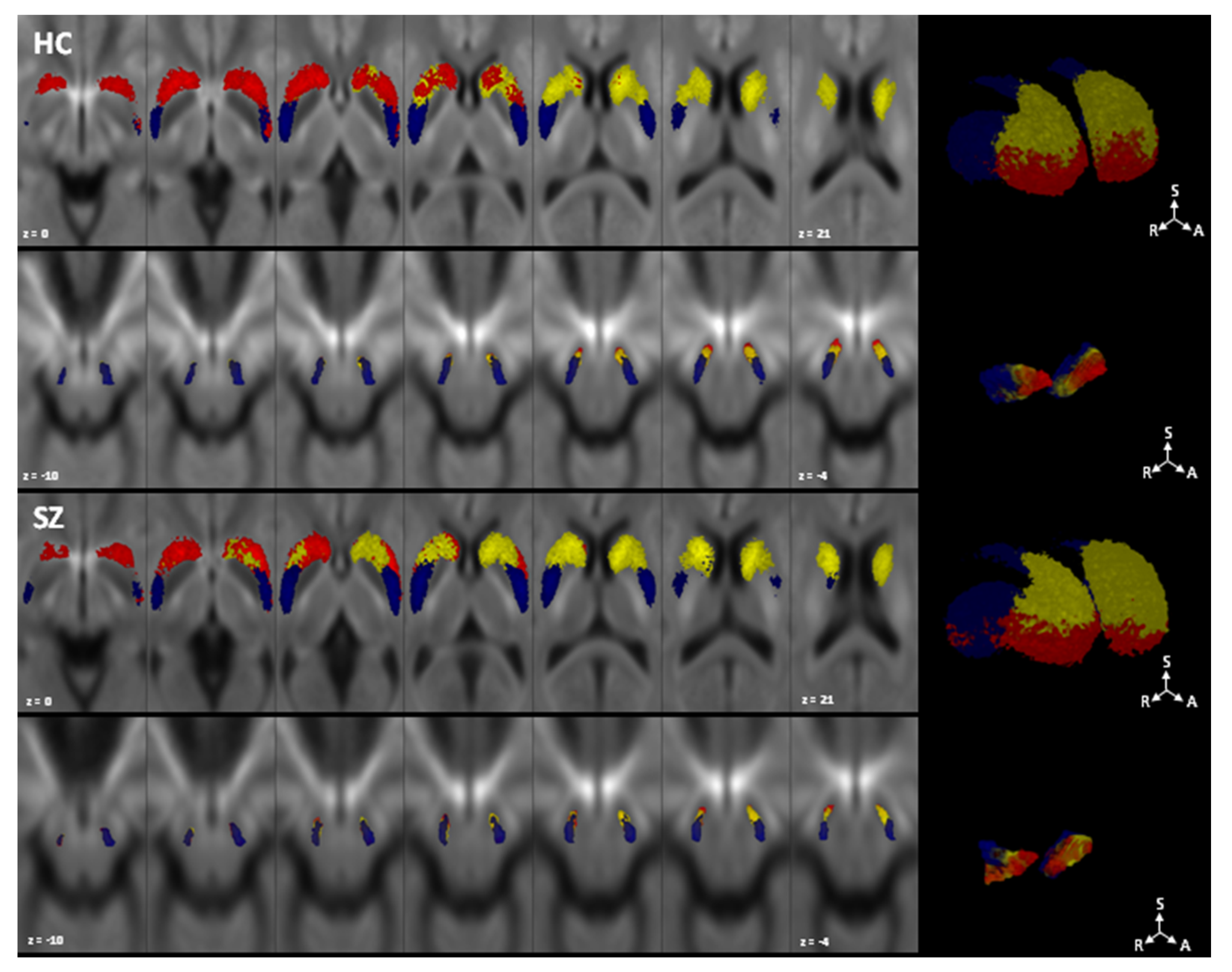
- Maximum probability maps reconstruction: for visualization purposes, each individual map was non-linearly coregistered to the corresponding study-specific FOD templates, binarized and summed up across the whole group to obtain maximum probability maps of each parcel at the group level. A threshold of 50% percent was assigned to show only voxels overlapping in at least half of the sample.
2.7. Quantitative Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- Weinberger, D.R. Implications of Normal Brain Development for the Pathogenesis of Schizophrenia. Arch. Gen. Psychiatry 1987, 44, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Nour, M.M. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry 2016, 15, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S. Psychosis as a State of Aberrant Salience: A Framework Linking Biology, Phenomenology, and Pharmacology in Schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Mizrahi, R.; Li, M. From dopamine to salience to psychosis—Linking biology, pharmacology and phenomenology of psychosis. Schizophr. Res. 2005, 79, 59–68. [Google Scholar] [CrossRef]
- Strauss, G.P.; Waltz, J.A.; Gold, J.M. A Review of Reward Processing and Motivational Impairment in Schizophrenia. Schizophr. Bull. 2013, 40, S107–S116. [Google Scholar] [CrossRef]
- Murray, G.K.; Corlett, P.R.; Clark, L.; Pessiglione, M.; Blackwell, A.D.; Honey, G.; Jones, P.B.; Bullmore, E.T.; Robbins, T.W.; Fletcher, P.C. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry 2007, 13, 267–276. [Google Scholar] [CrossRef]
- Yoon, J.H.; Westphal, A.J.; Minzenberg, M.J.; Niendam, T.; Ragland, J.D.; Lesh, T.; Solomon, M.; Carter, C.S. Task-evoked substantia nigra hyperactivity associated with prefrontal hypofunction, prefrontonigral disconnectivity and nigrostriatal connectivity predicting psychosis severity in medication naïve first episode schizophrenia. Schizophr. Res. 2014, 159, 521–526. [Google Scholar] [CrossRef]
- Yoon, J.H.; Minzenberg, M.J.; Raouf, S.; D’Esposito, M.; Carter, C.S. Impaired Prefrontal-Basal Ganglia Functional Connectivity and Substantia Nigra Hyperactivity in Schizophrenia. Biol. Psychiatry 2013, 74, 122–129. [Google Scholar] [CrossRef]
- White, D.M.; Kraguljac, N.V.; Reid, M.A.; Lahti, A.C. Contribution of substantia nigra glutamate to prediction error signals in schizophrenia: A combined magnetic resonance spectroscopy/functional imaging study. NPJ Schizophr. 2015, 1, 14001. [Google Scholar] [CrossRef]
- Vaillancourt, D.E.; Spraker, M.B.; Prodoehl, J.; Abraham, I.; Corcos, D.M.; Zhou, X.J.; Comella, C.L.; Little, D.M. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009, 72, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. Brain 1999, 122, 1421–1436. [Google Scholar] [CrossRef] [PubMed]
- Goschke, T.; Bolte, A. Emotional modulation of control dilemmas: The role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia 2014, 62, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef]
- Düzel, E.; Bunzeck, N.; Guitart-Masip, M.; Düzel, S. NOvelty-related Motivation of Anticipation and exploration by Dopamine (NOMAD): Implications for healthy aging. Neurosci. Biobehav. Rev. 2010, 34, 660–669. [Google Scholar] [CrossRef]
- Krebs, R.M.; Schott, B.H.; Düzel, E. Personality Traits Are Differentially Associated with Patterns of Reward and Novelty Processing in the Human Substantia Nigra/Ventral Tegmental Area. Biol. Psychiatry 2009, 65, 103–110. [Google Scholar] [CrossRef]
- D’Ardenne, K.; Eshel, N.; Luka, J.; Lenartowicz, A.; Nystrom, L.E.; Cohen, J.D. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc. Natl. Acad. Sci. USA 2012, 109, 19900–19909. [Google Scholar] [CrossRef]
- Haber, S.N.; Ryoo, H.; Cox, C.; Lu, W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: Comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J. Comp. Neurol. 1995, 362, 400–410. [Google Scholar] [CrossRef]
- Haber, S.N.; Knutson, B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Haber, S.N.; Behrens, T.E. The Neural Network Underlying Incentive-Based Learning: Implications for Interpreting Circuit Disruptions in Psychiatric Disorders. Neuron 2014, 83, 1019–1039. [Google Scholar] [CrossRef]
- Haber, S.N. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 2014, 282, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lynd-Balta, E.; Haber, S. The organization of midbrain projections to the striatum in the primate: Sensorimotor-related striatum versus ventral striatum. Neuroscience 1994, 59, 625–640. [Google Scholar] [CrossRef]
- Haber, S.N.; Fudge, J.L.; McFarland, N.R. Striatonigrostriatal Pathways in Primates Form an Ascending Spiral from the Shell to the Dorsolateral Striatum. J. Neurosci. 2000, 20, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Lambert, C.; Dolan, R.J.; Düzel, E. Parcellation of the human substantia nigra based on anatomical connectivity to the striatum. Neuroimage 2013, 81, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Larcher, K.; Misic, B.; Dagher, A. Anatomical and functional organization of the human substantia nigra and its connections. eLife 2017, 6, e26653. [Google Scholar] [CrossRef] [PubMed]
- Tziortzi, A.C.; Haber, S.N.; Searle, G.E.; Tsoumpas, C.; Long, C.J.; Shotbolt, P.; Douaud, G.; Jbabdi, S.; Behrens, T.E.J.; Rabiner, E.A.; et al. Connectivity-Based Functional Analysis of Dopamine Release in the Striatum Using Diffusion-Weighted MRI and Positron Emission Tomography. Cereb. Cortex 2014, 24, 1165–1177. [Google Scholar] [CrossRef]
- Mamiya, P.C.; Richards, T.; Corrigan, N.M.; Kuhl, P.K. Strength of Ventral Tegmental Area Connections With Left Caudate Nucleus Is Related to Conflict Monitoring. Front. Psychol. 2020, 10. [Google Scholar] [CrossRef]
- Gunn, R.N.; Jenkinson, M.; Rabinner, E.A.; Behrens, T.; Shotbolt, P.; Long, C.; Tsoumpas, C.; Searle, G.; Haber, S.; Tziortzi, A. Quantification of dopamine release within the connectivity-derived functional subdivision of striatum. J. Cereb. Blood Flow Metab. 2012, 32, S166. [Google Scholar]
- Wang, L.; Alpert, K.I.; Calhoun, V.D.; Cobia, D.J.; Keator, D.B.; King, M.D.; Kogan, A.; Landis, D.; Tallis, M.; Turner, M.D.; et al. SchizConnect: Mediating neuroimaging databases on schizophrenia and related disorders for large-scale integration. Neuroimage 2016, 124, 1155–1167. [Google Scholar] [CrossRef]
- Wang, L.; Alpert, K.; Calhoun, V.; Keator, D.; King, M.; Kogan, A. Schizconnect: A one-stop web-based resource for large-scale schizophrenia neuroimaging data integration. Schizophr. Bull. 2015, 41. [Google Scholar] [CrossRef]
- Gollub, R.L.; Shoemaker, J.M.; King, M.D.; White, T.; Ehrlich, S.; Sponheim, S.R.; Clark, V.P.; Turner, J.A.; Mueller, B.A.; Magnotta, V.; et al. The MCIC collection: A shared repository of multi-modal, multi-site brain image data from a clinical investigation of schizophrenia. Neuroinformatics 2013, 11, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, A.; Milardi, D.; Bertino, S.; Basile, G.A.; Calamuneri, A.; Chillemi, G.; Rizzo, G.; Anastasi, G.; Quartarone, A. Structural connectivity-based topography of the human globus pallidus: Implications for therapeutic targeting in movement disorders. Mov. Disord. 2019, 34, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, A.; Milardi, D.; Basile, G.A.; Bertino, S.; Calamuneri, A.; Chillemi, G.; Paladina, G.; Impellizzeri, F.; Trimarchi, F.; Anastasi, G.; et al. The cortico-rubral and cerebello-rubral pathways are topographically organized within the human red nucleus. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Ethirion, B.; Varoquaux, G.; Bzdok, D. Connectivity-based parcellation: Critique and implications. Hum. Brain Mapp. 2015, 36, 4771–4792. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.-D.; Yeh, C.-H.; Calamante, F.; Cho, K.-H.; Connelly, A.; Lin, C. Resolving crossing fibres using constrained spherical deconvolution: Validation using diffusion-weighted imaging phantom data. Neuroimage 2008, 42, 617–625. [Google Scholar] [CrossRef]
- Jeurissen, B.; Tournier, J.-D.; Dhollander, T.; Connelly, A.; Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 2014, 103, 411–426. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Flaum, M.; Arndt, S. The Comprehensive Assessment of Symptoms and History (CASH). Arch. Gen. Psychiatry 1992, 49, 615–623. [Google Scholar] [CrossRef]
- Andreasen, N.; Grove, W. Positive and Negative Symptoms in Schizophrenia. Psychiatr. Psychobiol. 1986, 1, 108–121. [Google Scholar] [CrossRef]
- Andreasen, N.C. Scale for the Assessment of Negative Symptoms (SANS). Br. J. Psychiatry 1989, 155, 49–52. [Google Scholar] [CrossRef]
- Simpson, G.M.; Angus, J.W.S. A Rating Scale for Extrapyramidal Side Effects. Acta Psychiatr. Scand. 1970, 45, 11–19. [Google Scholar] [CrossRef]
- Barnes, T.R.E. A Rating Scale for Drug-Induced Akathisia. Br. J. Psychiatry 1989, 154, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-Aron, B.; Sijbers, J.; Fieremans, E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016, 142, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, C.; Haldar, J.P.; Joshi, A.A.; Leahy, R.M. Correcting susceptibility-induced distortion in diffusion-weighted {MRI} using constrained nonrigid registration. IEEE Trans Signal Inf Process 2012. [Google Scholar] [CrossRef]
- Gholipour, A.; Kehtarnavaz, N.; Gopinath, K.; Briggs, R.W.; Devous, M.D.; Haley, R.W. Distortion Correction via Non-rigid Registration of Functional to Anatomical Magnetic Resonance Brain Images. In Proceedings of the 2006 International Conference on Image Processing, Atlanta, GA, USA, 8–11 October 2006. [Google Scholar]
- Maes, F.; Collignon, A.; Vandermeulen, D.; Marchal, G.; Suetens, P. Multimodality image registration by maximization of mutual information. IEEE Trans. Med. Imaging 1997, 16, 187–198. [Google Scholar] [CrossRef]
- Modat, M.; Cash, D.M.; Daga, P.; Winston, G.P.; Duncan, J.S.; Ourselin, S. Global image registration using a symmetric block-matching approach. J. Med. Imaging 2014, 1, 024003. [Google Scholar] [CrossRef]
- Tournier, J.D.; Smith, R.; Raffelt, D.; Tabbara, R.; Dhollander, T.; Pietsch, M.; Christiaens, D.; Jeurissen, B.; Yeh, C.-H.; Connelly, A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019, 202, 116137. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Smith, S. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011, 56, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Smith, S.; Jenkinson, M. FNIRT-FMRIB’s non-linear image registration tool. In Proceedings of the 14Th Annual Meeting of the Organization for Human Brain Mapping (OHBM), Melbourne, Australia, 15–19 June 2008. [Google Scholar]
- Basser, P.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Veraart, J.; Sijbers, J.; Sunaert, S.; Leemans, A.; Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. Neuroimage 2013, 81, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Westin, C.-F.; Maier, S.; Mamata, H.; Nabavi, A.; Jolesz, F.; Kikinis, R. Processing and visualization for diffusion tensor MRI. Med. Image Anal. 2002, 6, 93–108. [Google Scholar] [CrossRef]
- Dhollander, T.; Raffelt, D.; Connelly, A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. In Proceedings of the ISMRM Workshop on Breaking the Barriers of Diffusion MRI, Lisbon, Portugal, 11–16 September 2016. [Google Scholar]
- Tournier, J.D.; Calamante, F.; Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage 2007, 35, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Pauli, W.M.; Nili, A.N.; Tyszka, J.M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 2018, 5, 180063. [Google Scholar] [CrossRef]
- Pietsch, M.; Raffelt, D.; Dhollander, T.; Tournier, J.-D. Multi-Contrast Diffeomorphic Non-Linear Registration of Orientation Density Functions. In Proceedings of the ISMRM 25th Annual Meeting & Exhibition, Honolulu, HI, USA, 22–27 April 2017; p. 3522. [Google Scholar]
- Raffelt, D.; Tournier, J.-D.; Fripp, J.; Crozier, S.; Connelly, A.; Salvado, O. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage 2011, 56, 1171–1180. [Google Scholar] [CrossRef]
- Raffelt, D.; Tournier, J.-D.; Crozier, S.; Connelly, A.; Salvado, O. Reorientation of fiber orientation distributions using apodized point spread functions. Magn. Reson. Med. 2012, 67, 844–855. [Google Scholar] [CrossRef]
- Patriat, R.; Cooper, S.E.; Duchin, Y.; Niederer, J.; Lenglet, C.; Aman, J.; Park, M.C.; Vitek, J.L.; Harel, N. Individualized tractography-based parcellation of the globus pallidus pars interna using 7T MRI in movement disorder patients prior to DBS surgery. Neuroimage 2018, 178, 198–209. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Calamante, F.; Connelly, A. Improved Probabilistic Streamlines Tractography by 2nd Order Integration over Fibre Orientation Distributions. In Proceedings of the 2010 ISMRM Annual Meeting, Stockholm, Sweden, 1–7 May 2010. [Google Scholar]
- Calamante, F.; Tournier, J.-D.; Jackson, G.D.; Connelly, A. Track-density imaging (TDI): Super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage 2010, 53, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Theisen, F.; Leda, R.; Pozorski, V.; Oh, J.M.; Adluru, N.; Wong, R.; Okonkwo, O.; Dean, I.D.C.; Bendlin, B.B.; Johnson, S.C.; et al. Evaluation of striatonigral connectivity using probabilistic tractography in Parkinson’s disease. Neuroimage Clin. 2017, 16, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Bertino, S.; Basile, G.A.; Bramanti, A.; Anastasi, G.P.; Quartarone, A.; Milardi, D.; Cacciola, A. Spatially coherent and topographically organized pathways of the human globus pallidus. Hum. Brain Mapp. 2020, 41, 4641–4661. [Google Scholar] [CrossRef] [PubMed]
- Bertino, S.; Basile, G.A.; Anastasi, G.; Bramanti, A.; Fonti, B.; Cavallaro, F.; Bruschetta, D.; Milardi, D.; Cacciola, A. Anatomical Characterization of the Human Structural Connectivity between the Pedunculopontine Nucleus and Globus Pallidus via Multi-Shell Multi-Tissue Tractography. Medicina 2020, 56, 452. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. The primate basal ganglia: Parallel and integrative networks. J. Chem. Neuroanat. 2003, 26, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.; Kunishio, K.; Mizobuchi, M.; Lynd-Balta, E. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995, 15, 4851–4867. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; Kherif, F.; Klöppel, S.; Cook, P.A.; Alexander, D.C.; Parker, G.J.M.; Deichmann, R.; Ashburner, J.; Frackowiak, R.S.J. Evidence for Segregated and Integrative Connectivity Patterns in the Human Basal Ganglia. J. Neurosci. 2008, 28, 7143–7152. [Google Scholar] [CrossRef]
- Milardi, D.; Quartarone, A.; Bramanti, A.; Anastasi, G.; Bertino, S.; Basile, G.A.; Buonasera, P.; Pilone, G.; Celeste, G.; Rizzo, G.; et al. The Cortico-Basal Ganglia-Cerebellar Network: Past, Present and Future Perspectives. Front. Syst. Neurosci. 2019, 13, 61. [Google Scholar] [CrossRef]
- Chung, H.-W.; Chou, M.-C.; Chen, C.-Y. Principles and Limitations of Computational Algorithms in Clinical Diffusion Tensor MR Tractography. Am. J. Neuroradiol. 2010, 32, 3–13. [Google Scholar] [CrossRef]
- Cacciola, A.; Bertino, S.; Basile, G.A.; Di Mauro, D.; Calamuneri, A.; Chillemi, G.; Duca, A.; Bruschetta, D.; Flace, P.; Favaloro, A.; et al. Mapping the structural connectivity between the periaqueductal gray and the cerebellum in humans. Brain Struct. Funct. 2019, 224, 2153–2165. [Google Scholar] [CrossRef]
- Gradin, V.B.; Kumar, P.; Waiter, G.; Ahearn, T.; Stickle, C.; Milders, M.; Reid, I.; Hall, J.; Steele, J.D. Expected value and prediction error abnormalities in depression and schizophrenia. Brain 2011, 134, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Schachtzabel, C.; Wagner, G.; Schikora, J.; Schultz, C.; Reichenbach, J.R.; Sauer, H.; Schlösser, R.G. Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. NeuroImage 2010, 50, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, M.; Kegeles, L.S.; Abi-Dargham, A. Glutamate, Dopamine, and Schizophrenia. Ann. N. Y. Acad. Sci. 2003, 1003, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Alba-Ferrara, L.; De Erausquin, G.A. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front. Integr. Neurosci. 2013, 7, 9. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kubicki, M.; Asami, T.; Seidman, L.J.; Goldstein, J.M.; Mesholam-Gately, R.I.; McCarley, R.W.; Shenton, M.E. Extensive white matter abnormalities in patients with first-episode schizophrenia: A diffusion tensor imaging (DTI) study. Schizophr. Res. 2013, 143, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kawasaki, Y.; Takahashi, T.; Furuichi, A.; Noguchi, K.; Seto, H.; Suzuki, M. Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: A voxel-based diffusion tensor imaging study. Psychiatry Res. Neuroimaging 2012, 202, 233–238. [Google Scholar] [CrossRef]
- Kelly, S.; Jahanshad, N.; Zalesky, A.; Kochunov, P.; Agartz, I.; Alloza, C.; Andreassen, O.A.; Arango, C.; Banaj, N.; Bouix, S.; et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 2018, 23, 1261–1269. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef]
- Winston, G.P. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant. Imaging Med. Surg. 2012, 2, 254–265. [Google Scholar]
| HC | SZ | |||
|---|---|---|---|---|
| Mean | StD | Mean | StD | |
| Age | 31.75 | 11.76 | 32.56 | 10.50 |
| Males | 14 | / | 22 | / |
| Females | 10 | / | 8 | / |
| Handedness (R/L/Both) | 23/1/0 | / | 26/3/1 | / |
| Mean Illness Duration (years) | / | / | 9.26 | 8.73 |
| Extrapyramidal Signs (SAS) | / | / | 2.39 | 2.79 |
| Negative Symptoms (SANS) | / | / | 7.21 | 2.81 |
| Positive Symptoms (SAPS) | / | / | 4.82 | 2.25 |
| Disorganized symptoms (SAPS) | / | / | 2.04 | 2.23 |
| Striatal SDI | SNc/VTA SDI | FA | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | StD | Mean | StD | Mean | StD | |||
| Limbic | HC | Left | 32.020 | 3.83 | 23.922 | 9.999 | 0.467 | 0.050 |
| Right | 39.671 | 3.39 | 27.298 | 7.225 | 0.468 | 0.058 | ||
| SZ | Left | 30.902 | 3.24 | 22.016 | 8.494 | 0.438 | 0.139 | |
| Right | 38.903 | 2.03 | 26.889 | 10.791 | 0.436 | 0.146 | ||
| Prefrontal | HC | Left | 24.725 | 1.95 | 26.050 | 11.162 | 0.474 | 0.047 |
| Right | 32.462 | 2.9 | 23.838 | 8.271 | 0.436 | 0.146 | ||
| SZ | Left | 25.503 | 2.03 | 29.570 | 7.817 | 0.488 | 0.061 | |
| Right | 32.78 | 3.21 | 28.228 | 12.131 | 0.440 | 0.144 | ||
| Sensorimotor | HC | Left | 34.171 | 3.71 | 44.516 | 11.582 | 0.477 | 0.045 |
| Right | 30.121 | 2.22 | 41.457 | 12.972 | 0.487 | 0.050 | ||
| SZ | Left | 31.932 | 3.68 | 43.388 | 9.056 | 0.445 | 0.140 | |
| Right | 31.131 | 2.25 | 36.982 | 11.084 | 0.447 | 0.149 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, G.A.; Bramanti, A.; Bertino, S.; Cutroneo, G.; Bruno, A.; Tisano, A.; Paladina, G.; Milardi, D.; Anastasi, G. Structural Connectivity-Based Parcellation of the Dopaminergic Midbrain in Healthy Subjects and Schizophrenic Patients. Medicina 2020, 56, 686. https://doi.org/10.3390/medicina56120686
Basile GA, Bramanti A, Bertino S, Cutroneo G, Bruno A, Tisano A, Paladina G, Milardi D, Anastasi G. Structural Connectivity-Based Parcellation of the Dopaminergic Midbrain in Healthy Subjects and Schizophrenic Patients. Medicina. 2020; 56(12):686. https://doi.org/10.3390/medicina56120686
Chicago/Turabian StyleBasile, Gianpaolo Antonio, Alessia Bramanti, Salvatore Bertino, Giuseppina Cutroneo, Antonio Bruno, Adriana Tisano, Giuseppe Paladina, Demetrio Milardi, and Giuseppe Anastasi. 2020. "Structural Connectivity-Based Parcellation of the Dopaminergic Midbrain in Healthy Subjects and Schizophrenic Patients" Medicina 56, no. 12: 686. https://doi.org/10.3390/medicina56120686
APA StyleBasile, G. A., Bramanti, A., Bertino, S., Cutroneo, G., Bruno, A., Tisano, A., Paladina, G., Milardi, D., & Anastasi, G. (2020). Structural Connectivity-Based Parcellation of the Dopaminergic Midbrain in Healthy Subjects and Schizophrenic Patients. Medicina, 56(12), 686. https://doi.org/10.3390/medicina56120686




