Gastric Calcifying Fibrous Tumor: An Easy Misdiagnosis as Gastrointestinal Stromal Tumor–A Systemic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Information Sources
2.3. Study Selection
2.4. Synthesis of Results
2.5. Statistical Analysis
3. Results
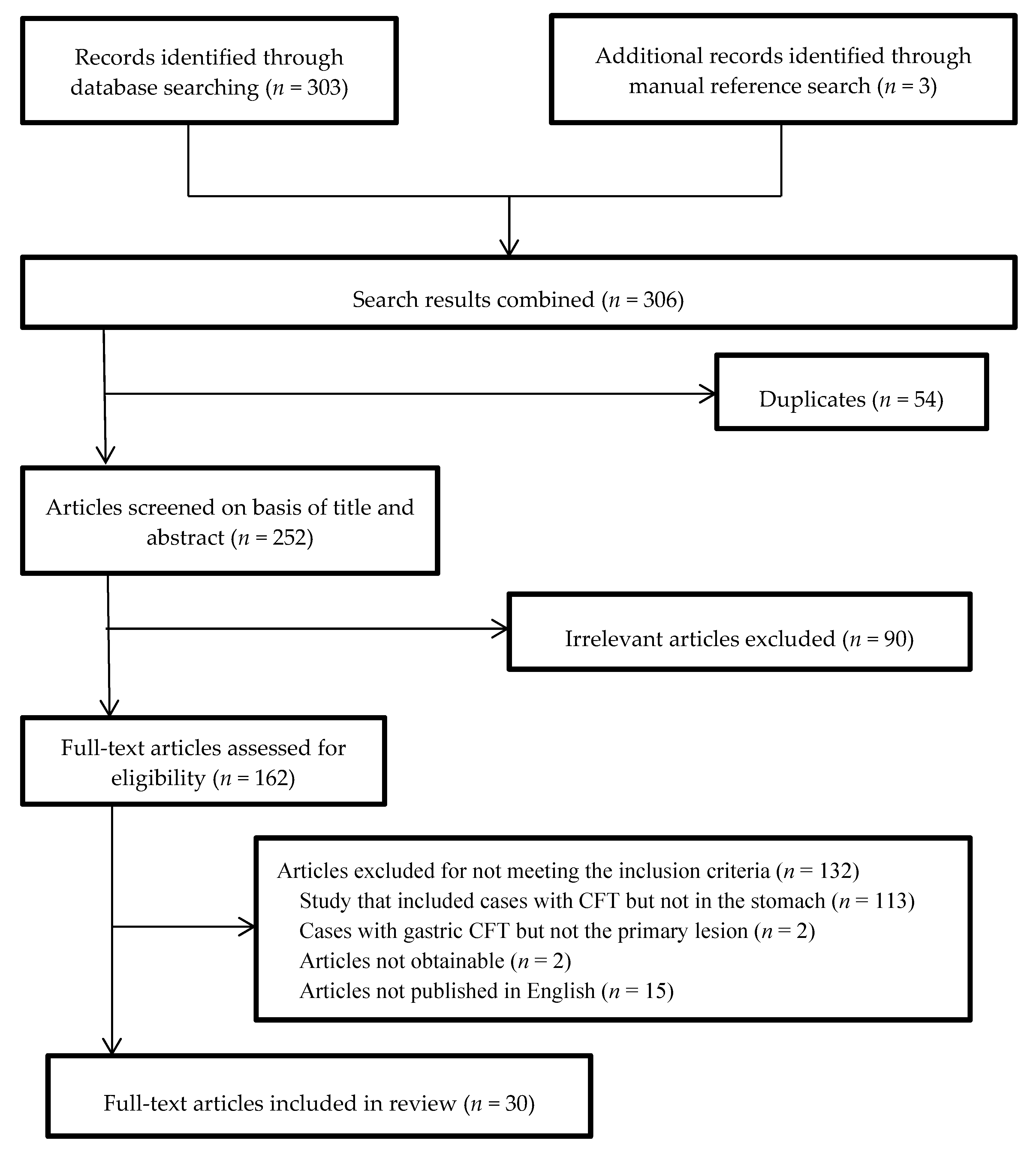
3.1. Study Selection Process
3.2. Clinical Characteristics of All Patients
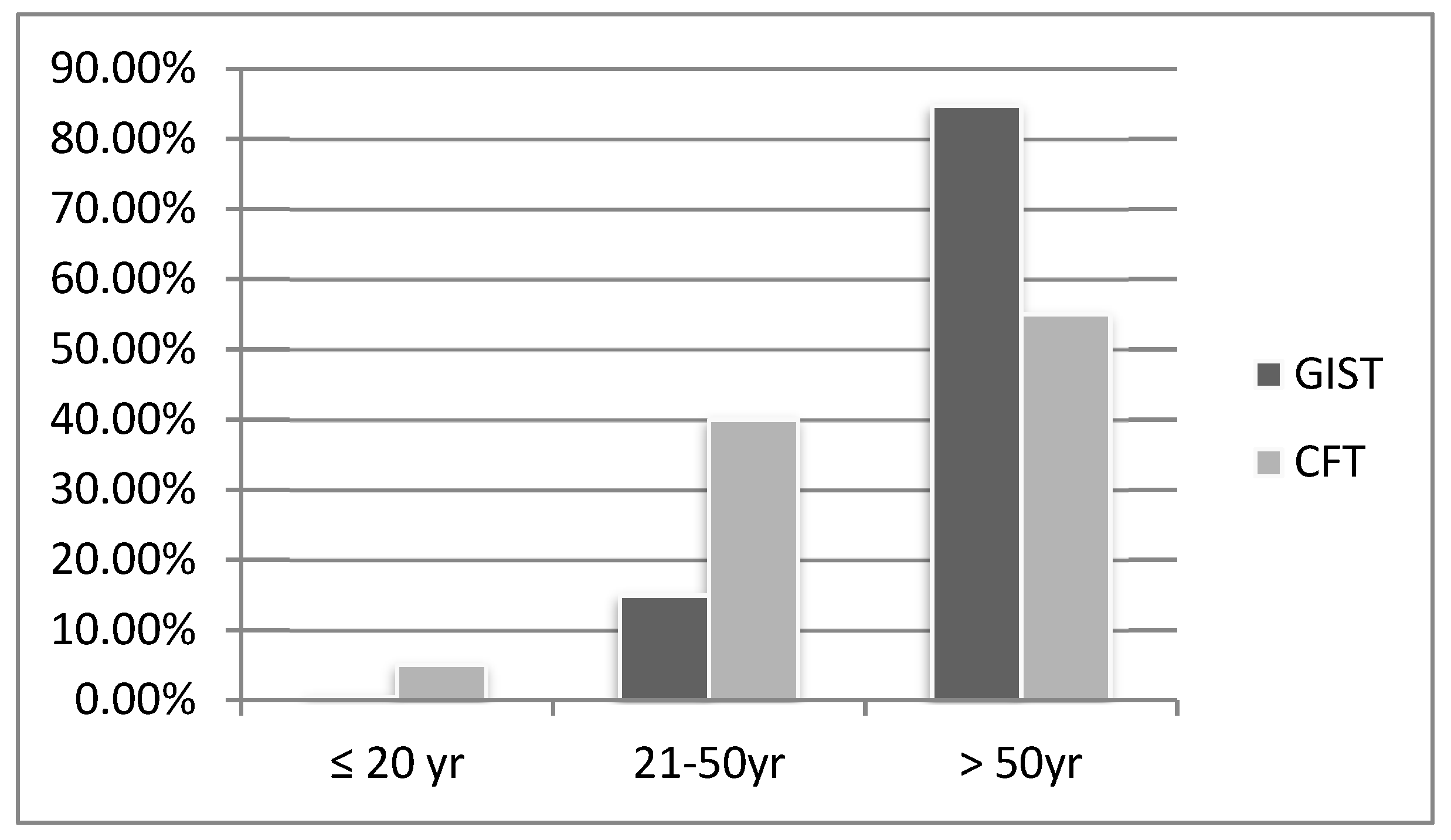
3.3. Age, Children Ratio, and Size
3.4. Symptoms
3.5. Location and Layer of the Stomach
3.6. Endoscopic Ultrasound
3.7. Computed Tomography
3.8. Calcification
3.9. Choice of Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polkowski, M. Endoscopic Ultrasound and Endoscopic Ultrasound-Guided Fine-Needle Biopsy for the Diagnosis of Malignant Submucosal Tumors. Endoscopy 2005, 37, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Okasha, H.; Wifi, M.N.; El Nady, M.; Mahdy, R.; Al-Gemeie, E.; Al-Nabawy, W.; Aref, W.; El-Naggar, A.; Essam, K.; Hamdy, A. Role of endoscopic ultrasound and endoscopic-ultrasound-guided fine-needle aspiration in endoscopic biopsy negative gastrointestinal lesions. Endosc. Ultrasound 2017, 6, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors—Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001, 438, 1–12. [Google Scholar] [CrossRef]
- Chorti, A.; Papavramidis, T.S.; Michalopoulos, A. Calcifying Fibrous Tumor: Review of 157 Patients Reported in International Literature. Medicine 2016, 95, e3690. [Google Scholar] [CrossRef]
- Okoronkwo, N.; Ghavimi, S.; Chokshi, R.; Peters, S.; Ahlawat, S. Gastric calcifying fibrous tumor mimicking GI stromal tumor. Gastrointest. Endosc. 2018, 88, 556–558. [Google Scholar] [CrossRef]
- Kocova, L.; Michal, M.; Sulc, M.; Zamecnik, M. Calcifying fibrous pseudotumour of visceral peritoneum. Histopathology 1997, 31, 182–184. [Google Scholar] [CrossRef]
- Nascimento, A.F.; Ruiz, R.; Hornick, J.L.; Fletcher, C.D. Calcifying fibrous ‘pseudotumor’: Clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int. J. Surg. Pathol. 2002, 10, 189–196. [Google Scholar] [CrossRef]
- Elpek, G.O.; Kupesiz, G.Y.; Ogus, M. Incidental calcifying fibrous tumor of the stomach presenting as a polyp. Pathol. Int. 2006, 56, 227–231. [Google Scholar] [CrossRef]
- Lee, D.; Suh, Y.-L.; Lee, S.-K. Calcifying fibrous pseudotumour arising in a gastric inflammatory myofibroblastic tumour. Pathol. J. RCPA 2006, 38, 588–591. [Google Scholar] [CrossRef]
- Ogasawara, N.; Izawa, S.; Mizuno, M.; Tanabe, A.; Ozeki, T.; Noda, H.; Takahashi, E.; Sasaki, M.; Yokoi, T.; Kasugai, K. Gastric calcifying fibrous tumor removed by endoscopic submucosal dissection. World J. Gastrointest. Endosc. 2013, 5, 457–460. [Google Scholar] [CrossRef]
- Agaimy, A.; Bihl, M.P.; Tornillo, L.; Wunsch, P.H.; Hartmann, A.; Michal, M. Calcifying fibrous tumor of the stomach: Clinicopathologic and molecular study of seven cases with literature review and reappraisal of histogenesis. Am. J. Surg. Pathol. 2010, 34, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Vasilakaki, T.; Skafida, E.; Tsavari, A.; Arkoumani, E.; Koulia, K.; Myoteri, D.; Grammatoglou, X.; Moustou, E.; Firfiris, N.; Zisis, D. Gastric calcifying fibrous tumor: A very rare case report. Case Rep. Oncol. 2012, 5, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Attila, T.; Chen, D.; Gardiner, G.W.; Ptak, T.W.; Marcon, N.E. Gastric calcifying fibrous tumor. Can. J. Gastroenterol. 2006, 20, 487–489. [Google Scholar] [CrossRef]
- Piccinni, G.; Nacchiero, M. Management of narrower anastomotic colonic strictures. Case report and proposal technique. Surg. Endosc. 2001, 15, 1227. [Google Scholar] [CrossRef]
- Fan, S.F.; Yang, H.; Li, Z.; Teng, G.J. Gastric calcifying fibrous pseudotumour associated with an ulcer: Report of one case with a literature review. Br. J. Radiol. 2010, 83, e188–e191. [Google Scholar] [CrossRef]
- Delbecque, K.; Legrand, M.; Boniver, J.; Lauwers, G.Y.; de Leval, L. Calcifying fibrous tumour of the gastric wall. Histopathology 2004, 44, 399–400. [Google Scholar] [CrossRef]
- George, S.A.; Abdeen, S. Gastric Calcifying Fibrous Tumor Resembling Gastrointestinal Stromal Tumor: A Case Report. Iran. J. Pathol. 2015, 10, 306–309. [Google Scholar]
- Sato, S.; Ooike, N.; Yamamoto, T.; Wada, M.; Miyamoto, A.; Matsukawa, M.; Morohoshi, T. Rare gastric calcifying fibrous pseudotumor removed by endoscopic submucosal dissection. Dig. Endosc. 2008, 20, 84–86. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, J.; Ren, W.; Sun, S.; Tang, S.; Xie, L. A gastric calcifying fibrous pseudotumor detected by transabdominal ultrasound after oral administration of an echoic cellulose-based gastrointestinal ultrasound contrast agent. Ultraschall der Medizin 2014, 35, 181–183. [Google Scholar] [CrossRef]
- Jang, K.Y.; Park, H.S.; Moon, W.S.; Lee, H.; Kim, C.Y. Calcifying fibrous tumor of the stomach: A case report. J. Korean Surg. Soc. 2012, 83, 56–59. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, M.D.; Chen, T.; Zhong, Y.S.; Zhou, P.H.; Wu, H.F.; Yao, L.Q. Endoscopic diagnosis and treatment of calcifying fibrous tumors. Turk. J. Gastroenterol. 2014, 25, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, Z.; Ding, S. Gastric calcifying fibrous tumor: A case of suspected immunoglobulin G4-related gastric disease. Saudi J. Gastroenterol. 2015, 21, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, S.; Ryu, Y.; Takata, H.; Asaumi, Y.; Sakatoku, M.; Seike, T.; Okamura, T.; Inamura, K.; Kawai, H.; Okuno, N.; et al. Imaging findings of gastric calcifying fibrous tumour. BJR Case Rep. 2016, 2, 20160064. [Google Scholar] [CrossRef] [PubMed]
- Pezhouh, M.K.; Rezaei, M.K.; Shabihkhani, M.; Ghosh, A.; Belchis, D.; Montgomery, E.A.; Voltaggio, L. Clinicopathologic study of calcifying fibrous tumor of the gastrointestinal tract: A case series. Hum. Pathol. 2017, 62, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Yang, X.D.; Chen, W.X.; Shi, Y.H.; Nie, Z.H.; Wu, J. Calcifying fibrous tumor of stomach: A case report. Medicine 2017, 96, e8882. [Google Scholar] [CrossRef]
- Lee, S.; Jahng, J.; Han, W. Gastric Calcifying Fibrous Tumor Manifesting as a Subepithelial Tumor. J. Gastrointest. Surg. 2018, 22, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, K.; Li, J. CT Features of Calcifying Fibrous Tumor of the Stomach. J. Gastrointest. Surg. 2018, 22, 1455–1456. [Google Scholar] [CrossRef]
- Tian, S.; Zeng, Z.; Peng, X.; Dong, W. Gastric calcifying fibrous tumor: A clinicopathological study of nine cases. Exp. Ther. Med. 2018, 16, 5137–5143. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Pinho, R.; Tente, D. Endoscopic Management of a Rare Entity: Gastric Calcifying Fibrous Tumor. GE Port. J. Gastroenterol. 2019, 26, 139–141. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, L.; Wu, S.; Li, R.; Yang, X.; Xu, H.; Zheng, S.; Wang, A.; Wang, C. Clinicopathologic Study of Calcifying Fibrous Tumor Emphasizing Different Anatomical Distribution and Favorable Prognosis. BioMed Res. Int. 2019, 2019, 5026860. [Google Scholar] [CrossRef]
- Azam, M.; Husen, Y.A.; Pervez, S. Calcifying fibrous pseudotumor in association with hyaline vascular type Castleman’s disease. Indian J. Pathol. Microbiol. 2009, 52, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; Broto, J.M.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv68–iv78. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Kawai, N.; Yamaguchi, S.; Nishida, Y. Submucosal tumors: Comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig. Endosc. 2013, 25, 479–489. [Google Scholar] [CrossRef]
- Kim, M.N.; Kang, S.J.; Kim, S.G.; Im, J.P.; Kim, J.S.; Jung, H.C.; Song, I.S. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography. Gut Liver 2013, 7, 642–647. [Google Scholar] [CrossRef]
- Izawa, N.; Sawada, T.; Abiko, R.; Kumon, D.; Hirakawa, M.; Kobayashi, M.; Obinata, N.; Nomoto, M.; Maehata, T.; Yamauchi, S.-I.; et al. Gastrointestinal stromal tumor presenting with prominent calcification. World J. Gastroenterol. 2012, 18, 5645–5648. [Google Scholar] [CrossRef]
- Salati, M.; Orsi, G.; Reggiani Bonetti, L.; Di Benedetto, F.; Longo, G.; Cascinu, S. Heavily calcified gastrointestinal stromal tumors: Pathophysiology and implications of a rare clinicopathologic entity. World J. Gastrointest. Oncol. 2017, 9, 135–141. [Google Scholar] [CrossRef]
- Bartolotta, T.V.; Taibbi, A.; Galia, M.; Cannella, I.; Lo Re, G.; Sparacia, G.; Midiri, M.; Lagalla, R. Gastrointestinal stromal tumour: 40-row multislice computed tomography findings. La Radiol. Med. 2006, 111, 651–660. [Google Scholar] [CrossRef] [PubMed]
- De Leo, C.; Memeo, M.; Spinelli, F.; Angelelli, G. Gastrointestinal stromal tumours: Experience with multislice CT. La Radiol. Med. 2006, 111, 1103–1114. [Google Scholar] [CrossRef]
- Levy, A.D.; Remotti, H.E.; Thompson, W.M.; Sobin, L.H.; Miettinen, M. Gastrointestinal stromal tumors: Radiologic features with pathologic correlation. Radiographics 2003, 23, 283–304, 456, quiz 532. [Google Scholar] [CrossRef]
- Da Ronch, T.; Modesto, A.; Bazzocchi, M. Gastrointestinal stromal tumour: Spiral computed tomography features and pathologic correlation. La Radiol. Med. 2006, 111, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Park, H.N.; Min, B.H.; Choi, D.; Kim, K.M.; Kim, S. Preoperative predictive factors for gastrointestinal stromal tumors: Analysis of 375 surgically resected gastric subepithelial tumors. J. Gastrointest. Surg. 2015, 19, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Scherubl, H.; Faiss, S.; Knoefel, W.T.; Wardelmann, E. Management of early asymptomatic gastrointestinal stromal tumors of the stomach. World J. Gastrointest. Endosc. 2014, 6, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Yegin, E.G.; Duman, D.G. Small EUS-suspected gastrointestinal stromal tumors of the stomach: An overview for the current state of management. Endosc. Ultrasound 2016, 5, 69–77. [Google Scholar] [CrossRef]
- Nazim, S.M.; Nusrat, A.; Kazmi, Z. Fibrous Pseudotumor of Tunica Albuginea Testis Mimicking Testicular Neoplasm in a Young Man. Case Rep. Surg. 2018, 2018, 9315864. [Google Scholar] [CrossRef]
- Kawahara, K.; Yasukawa, M.; Nakagawa, K.; Katsura, H.; Nagano, T.; Iwasaki, T. Multiple calcifying fibrous tumor of the pleura. Virchows Arch. 2005, 447, 1007–1008. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Criteria | % | Number of Cases |
|---|---|---|
| Sex | 100 | 60 |
| Age | 100 | 60 |
| Symptom | 100 | 60 |
| Location | 100 | 31 |
| Layer | 56.7 | 34 |
| Size | 98.3 | 59 |
| Image | ||
| EUS | 11.7 | 7 |
| CT | 15 | 9 |
| Choice of treatment | 83.3 | 50 |
| Follow-up | 70 | 42 |
| Recurrence | 70 | 42 |
| Criteria | (Number of Cases) |
|---|---|
| Sex (M:F) | 32:28 (60) |
| Age (year) | 49.2 ± 13.9 (60) |
| Men | 50.88 ± 14.04 (32) |
| Women | 47.25 ± 13.85 (28) |
| Children ratio a | 5% (3) |
| Symptom | n = 60 |
| Symptomatic | 53.3% (32) |
| Acute | 5% (3) |
| Chronic | 16.7 % (10) |
| Asymptomatic | 46.7% (28) |
| Location | n = 31 |
| Antrum | 6.6% (2) |
| Body | 74.2% (23) |
| Fundus | 19.4% (6) |
| Layer | n = 34 |
| 1st/2nd | 14.7% (5) |
| 3 | 55.9% (19) |
| 4 | 23.5% (8) |
| 5 | 5.9% (2) |
| Size (cm) | 2.4 ± 3.15 (59) |
| Image | |
| EUS | n = 7 |
| Heterogenous | 100% (7) |
| Iso-hypoechoic | 71.4% (5) |
| Hyperechoic | 28.6% (2) |
| Well-defined | 100% (7) |
| Acoustic shadowing (calcification) | 85.7% (6) |
| CT | n = 9 |
| Cannot detect tumor b | 21.4% (3) |
| Well-defined | 77.8% (7) |
| Irregular | 22.2% (2) |
| Homogeneous | 22.2% (2) |
| Heterogeneous | 77.8% (7) |
| Homo-hyperdense | 66.7% (6) |
| Hypodense | 33.3% (3) |
| Calcification | 77.8% (7) |
| Macrocalcification (coarse) | 85.7% (6) |
| Microcalcification (punctate) | 14.3% (1) |
| Overall calcification c | 85.7% (12/14) |
| Choice of treatment | n = 50 |
| Intervention | 36% (18) |
| Diagnostic treatment | 8% (4) |
| Minor operation (wedge resection) | 44% (22) |
| Major operation | 12% (6) |
| Follow-up (Mon) | 24.1 ± 39.1 (42) |
| Recurrence rate | 0% (0) |
| Symptoms | |
|---|---|
| Abdominal discomfort % (N) | 55.6% (25) |
| Abdominal pain % (N) | 17.8% (8) |
| Abdominal distention % (N) | 8.9% (4) |
| Constitutional symptoms % (N) | 4.4% (2) |
| Vomiting % (N) | 4.4% (2) |
| GI bleeding % (N) | 4.4% (2) |
| Retro-sternal burning sensation % (N) | 4.4% (2) |
| Disease | Size (cm) | Age (yr) | Children (%) | Symptoms | Calcification (%) | Layer | EUS | CT |
|---|---|---|---|---|---|---|---|---|
| CFT | 2.4 | 49.2 | 5 | Mostly Sx (abdominal discomfort) | 85.7 | 3rd |
|
|
| GIST | 6.0 | 65.0 | 0.5 | <2 cm: usually Asx >2 cm: epigastric pain and GIB | 3.6 | 4th |
|
|
| p value | <0.001 | <0.001 | 0.037 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-K.; Chen, H.-Y.; Chuang, M.-L.; Chen, C.-W.; Jong, G.-P. Gastric Calcifying Fibrous Tumor: An Easy Misdiagnosis as Gastrointestinal Stromal Tumor–A Systemic Review. Medicina 2020, 56, 541. https://doi.org/10.3390/medicina56100541
Tsai M-K, Chen H-Y, Chuang M-L, Chen C-W, Jong G-P. Gastric Calcifying Fibrous Tumor: An Easy Misdiagnosis as Gastrointestinal Stromal Tumor–A Systemic Review. Medicina. 2020; 56(10):541. https://doi.org/10.3390/medicina56100541
Chicago/Turabian StyleTsai, Meng-Ko, Hung-Yi Chen, Ming-Lung Chuang, Chun-Wen Chen, and Gwo-Ping Jong. 2020. "Gastric Calcifying Fibrous Tumor: An Easy Misdiagnosis as Gastrointestinal Stromal Tumor–A Systemic Review" Medicina 56, no. 10: 541. https://doi.org/10.3390/medicina56100541
APA StyleTsai, M.-K., Chen, H.-Y., Chuang, M.-L., Chen, C.-W., & Jong, G.-P. (2020). Gastric Calcifying Fibrous Tumor: An Easy Misdiagnosis as Gastrointestinal Stromal Tumor–A Systemic Review. Medicina, 56(10), 541. https://doi.org/10.3390/medicina56100541






