Incidence of Iron Deficiency and the Role of Intravenous Iron Use in Perioperative Periods
Abstract
1. Introduction
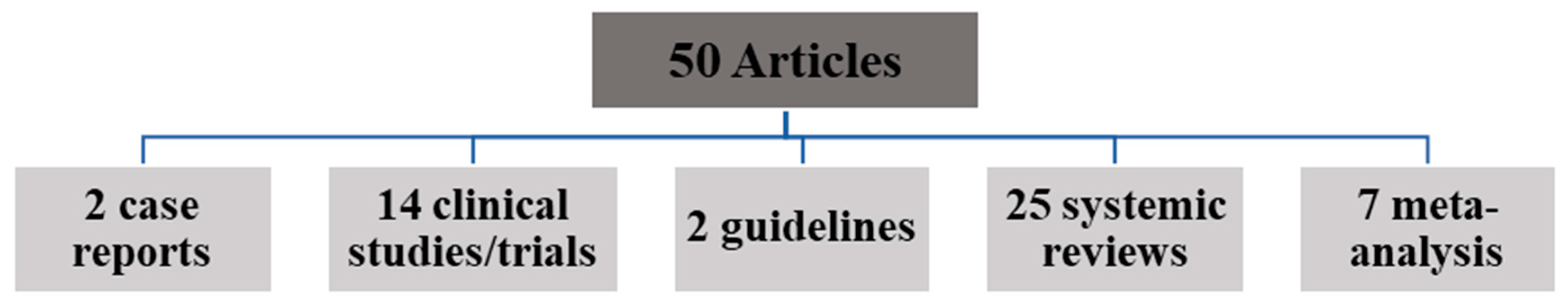
2. Materials and Methods
3. Results and Discussion
3.1. Iron Deficiency Diagnosis
3.2. Intravenous Iron Products–Short Presentation
3.3. The Role of Parenteral Iron Use in a Perioperative Period
3.4. The Overall Risks Associated with Intravenous Iron Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pasricha, S.R.; Flecknoe-Brown, S.C.; Allen, K.J.; Gibson, P.R.; McMahon, L.P.; Olynyk, J.K.; Roger, S.D.; Savoia, H.F.; Tampi, R.; Thomson, A.R.; et al. Diagnosis and management of iron deficiency anaemia: A clinical update. Med. J. Aust. 2010, 193, 525–532. [Google Scholar] [CrossRef]
- Munoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Elhenawy, A. Role of Preoperative Intravenous Iron Therapy to Correct Anemia before Major Surgery. Syst. Rev. 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.M.; Meland, E.; McGinn, S.; Anderson, J.H. Correction of iron-deficiency anaemia in colorectal surgery reduces perioperative transfusion rates: A before and after study. Int. J. Surg. 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef]
- Perelman, I.; Winter, R.; Sikora, L.; Martel, G.; Saidenberg, E.; Fergusson, D. The efficacy of postoperative iron therapy in improving clinical and patient-centered outcomes following surgery: A systematic review and meta-analysis. Transfus. Med. Rev. 2018, 32, 89–101. [Google Scholar] [CrossRef]
- Checherită, I.A.; David, C.; Ciocâlteu, A.; Lascăr, I. Management of the chronic renal patient undergoing surgery. Chirurgia (Bucharest, Romania: 1990) 2009, 104, 525–530. [Google Scholar]
- DeLoughery, T.G. Safety of Oral and Intravenous Iron. Acta Haematol. 2019, 142, 8–12. [Google Scholar] [CrossRef]
- Wolf, M.; Chertow, G.M.; Macdougall, I.C.; Kaper, R.; Krop, J.; Strauss, W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Goodnough, L.T.; Shander, A. Iron: The new advances in therapy. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Pope, M.; Lang, C.C.; Kalra, P.R. Iron deficiency in heart failure: Efficacy and safety of intravenous iron therapy. Cardiovasc. Ther. 2017, 35, e12301. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Nemeth, E.; Ganz, T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010, 116, 4754–4761. [Google Scholar] [CrossRef] [PubMed]
- Niculae, A.; David, C.; Dragomirescu, R.F.; Peride, I.; Turcu, F.L.; Petcu, L.C.; Covic, A.; Checherita, I.A. Correlation between recombinant human erythropoietin dose and inflammatory status in dialysed patients. Rev. Chim. Buchar. 2017, 68, 354–357. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Bisbe, E.; Shander, A.; Spahn, D.R.; Muñoz, M. Management of Perioperative Iron Deficiency Anemia. Acta Haematol. 2019, 142, 21–29. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematology 2015, 2015, 8–13. [Google Scholar] [CrossRef]
- Gupta, A.; Pratt, R.D.; Crumbliss, A.L. Ferrous iron content of intravenous iron formulations. Biometals 2016, 29, 411–415. [Google Scholar] [CrossRef]
- Worm, M.; Francuzik, W.; Renaudin, J.M.; Bilo, M.B.; Cardona, V.; Scherer Hofmeier, K.; Köhli, A.; Bauer, A.; Christoff, G.; Cichocka-Jarosz, E.; et al. Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry. Allergy 2018, 73, 1322–1330. [Google Scholar] [CrossRef]
- Auerbach, M.; Macdougall, I. The available intravenous iron formulations: History, efficacy, and toxicology. Hemodial. Int. 2017, 21, S83–S92. [Google Scholar] [CrossRef]
- Kei, T.; Mistry, N.; Curley, G.; Pavenski, K.; Shehata, N.; Tanzini, R.M.; Gauthier, M.F.; Thorpe, K.; Schweizer, T.A.; Ward, S.; et al. Efficacy and safety of erythropoietin and iron therapy to reduce red blood cell transfusion in surgical patients: A systematic review and meta-analysis. Can. J. Anesth./J. Can. D’anesth. 2019, 66, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Martin-Malo, A.; Borchard, G.; Flühmann, B.; Mori, C.; Silverberg, D.; Jankowska, E.A. Differences between intravenous iron products: Focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail. 2019, 6, 241–253. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208171s000lbl.pdf (accessed on 21 September 2020).
- Neiser, S.; Rentsch, D.; Dippon, U.; Kappler, A.; Weidler, P.G.; Göttlicher, J.; Steininger, R.; Wilhelm, M.; Braitsch, M.; Funk, F.; et al. Physico-chemical properties of the new generation IV iron preparations ferumoxytol, iron isomaltoside 1000 and ferric carboxymaltose. Biometals 2015, 28, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.S.; Coyne, D.W. Update on intravenous iron choices. Curr. Opin. Nephrol. Hypertens. 2014, 23, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Afif, W.; Knowles, S.; Lim, G.; Lin, Y.; Mothersill, C.; Nistor, I.; Rehman, F.; Song, C.; Xenodemetropoulos, T. Canadian expert consensus: Management of hypersensitivity reactions to intravenous iron in adults. Vox Sang. 2019, 114, 363–373. [Google Scholar] [CrossRef]
- Szebeni, J.; Fishbane, S.; Hedenus, M.; Howaldt, S.; Locatelli, F.; Patni, S.; Rampton, D.; Weiss, G.; Folkersen, J. Hypersensitivity to intravenous iron: Classification, terminology, mechanisms and management. British J. Pharmacol. 2015, 172, 5025–5036. [Google Scholar] [CrossRef]
- Muñoz, M.; Gómez-Ramírez, S.; Bhandari, S. The safety of available treatment options for iron-deficiency anemia. Expert Opin. Drug Saf. 2018, 17, 149–159. [Google Scholar] [CrossRef]
- Keating, G.M. Ferric carboxymaltose: A review of its use in iron deficiency. Drugs 2015, 75, 101–127. [Google Scholar] [CrossRef]
- Casteleyn, I.; Joosten, E. Evaluation of Parenteral Iron Therapy in Ambulatory Older Adults with Iron Deficiency Anaemia. Acta Haematol. 2017, 138, 221–222. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, K.J.; Lee, E.S.; Lee, K.H.; Lee, J.J.; Kim, T. Comparative efficacy and safety of intravenous ferric carboxymaltose and iron sucrose for the treatment of preoperative anemia in patients with menorrhagia: An open-label, multicenter, randomized study. J. Obstet. Gynaecol. Res. 2019, 45, 858–864. [Google Scholar] [CrossRef]
- Keeler, B.D.; Dickson, E.A.; Simpson, J.A.; Ng, O.; Padmanabhan, H.; Brookes, M.J.; Acheson, A.G.; IVICA Trial Group; Banerjea, A.; Walter, C.; et al. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: Outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia 2019, 74, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, S.; Maldonado-Ruiz, M.Á.; Campos-Garrigues, A.; Herrera, A.; Muñoz, M. Short-term perioperative iron in major orthopedic surgery: State of the art. Vox Sang. 2019, 114, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Breymann, C.; García-Erce, J.A.; Gómez-Ramírez, S.; Comin, J.; Bisbe, E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008, 94, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.J. Iron supplementation for anemia after hip fracture surgery: A randomized trial of 300 patients. JBJS 2010, 92, 265–269. [Google Scholar] [CrossRef]
- Schack, A.; Berkfors, A.A.; Ekeloef, S.; Gögenur, I.; Burcharth, J. The Effect of Perioperative Iron Therapy in Acute Major Non-cardiac Surgery on Allogenic Blood Transfusion and Postoperative Haemoglobin Levels: A Systematic Review and Meta-analysis. World J. Surg. 2019, 43, 1677–1691. [Google Scholar] [CrossRef]
- Schrier, S.L.; Bacon, B.R. Approach to the Patient with Suspected Iron Overload; Official Topic from UpToDate; UpToDate: Waltham, MA, USA, 2016. [Google Scholar]
- Çinarsoy, M.; Günes, A.K.; Gözden, H.E. ACUTE IRON OVERLOAD WITH IRON CARBOXYMALTOSE: CASE REPORT: PB2048. Hemasphere 2019, 3, 924. [Google Scholar] [CrossRef]
- Ramanathan, G.; Olynyk, J.K.; Ferrari, P. Diagnosing and preventing iron overload. Hemodial. Int. 2017, 21, S58–S67. [Google Scholar] [CrossRef]
- Bircher, A.J.; Auerbach, M. Hypersensitivity from intravenous iron products. Immunol. Allergy Clin. 2014, 34, 707–723. [Google Scholar] [CrossRef]
- Muñoz, M.; Gómez-Ramírez, S.; García-Erce, J.A. Intravenous iron in inflammatory bowel disease. World J. Gastroenterol. WJG 2009, 15, 4666. [Google Scholar] [CrossRef][Green Version]
- Silverstein, S.B.; Rodgers, G.M. Parenteral iron therapy options. Am. J. Hematol. 2004, 76, 74–78. [Google Scholar] [CrossRef]
- Achebe, M.; DeLoughery, T.G. Clinical data for intravenous iron–debunking the hype around hypersensitivity. Transfusion 2020. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Deloughery, T. Single-dose intravenous iron for iron deficiency: A new paradigm. Hematology 2016, 1, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, P.; Druais, P.L.; Waldvogel, S.; Favrat, B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: A randomized controlled trial. Cmaj 2012, 184, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Rampton, D.; Folkersen, J.; Fishbane, S.; Hedenus, M.; Howaldt, S.; Locatelli, F.; Patni, S.; Szebeni, J.; Weiss, G. Hypersensitivity reactions to intravenous iron: Guidance for risk minimization and management. Haematologica 2014, 99, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Leung, S.H. Safety profile of iron polymaltose infusions. Hosp. Pract. 2019, 47, 96–98. [Google Scholar] [CrossRef]
- Qunibi, W.Y. The efficacy and safety of current intravenous iron preparations for the management of iron-deficiency anaemia: A review. Arzneimittelforschung 2010, 60, 399–412. [Google Scholar] [CrossRef]
- Negoescu, I.; Niculescu, D.A.; David, C.; Peride, I.; Niculae, A.; Checherita, I.A.; Poiana, C. Biochemical determinants of aggressive behaviour–patho-physiological connections in esrdand dialysis. Farmacia 2018, 66, 925–929. [Google Scholar] [CrossRef]
- Anand, G.; Schmid, C. Severe hypophosphataemia after intravenous iron administration. Case Rep. 2017, 2017, bcr2016219160. [Google Scholar] [CrossRef]
- Zoller, H.; Schaefer, B.; Glodny, B. Iron-induced hypophosphatemia: An emerging complication. Curr. Opin. Nephrol. Hypertens. 2017, 26, 266–275. [Google Scholar] [CrossRef]
| “Gold Standard” Method | Usual Biomarkers | Other Biomarkers |
|---|---|---|
| Bone marrow biopsy | Serum ferritin | Mean corpuscular volume (MCV) |
| Transferrin saturation (TSAT) | Mean corpuscular haemoglobin concentration (MCHC) | |
| Serum iron | Red cell distribution width (RDW) | |
| C-reactive protein | Reticulocyte haemoglobin content (CHr) | |
| Zinc protoporphyrins (ZPP) in the red cell |
| Intravenous Iron Formula | Dosage and Minimum Administration Time |
|---|---|
| 1. Ferric carboxymaltose (Ferinject®, Injectafer®) | 1000 mg in 15 min |
| 2. Ferric derisomaltose (Monoferric®) | 1000 mg in at least 20 min (in patients > 50 kg) 20 mg/kg in at least 20 min (in patients < 50 kg) |
| 3. Iron sucrose (Venofer®) | 200 mg in 30 min |
| 4. Low molecular weight iron dextran (LMW dextran) (Cosmofer®, InFed®) | 20 mg/kg in 4–6 h |
| 5. Sodium feeric gluconate (Ferrlecit®) | 125 mg in 30–60 min |
| 6. Ferumoxytol (Feraheme®, Rienso®) | No longer in use |
| Adverse Reactions | Usual Treatment |
|---|---|
| 1. flushing |
|
| 2. urticaria | |
| 3. itching | |
| 4. joint pain | |
| 5. chest tightness |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țigliș, M.; Neagu, T.P.; Niculae, A.; Lascăr, I.; Grințescu, I.M. Incidence of Iron Deficiency and the Role of Intravenous Iron Use in Perioperative Periods. Medicina 2020, 56, 528. https://doi.org/10.3390/medicina56100528
Țigliș M, Neagu TP, Niculae A, Lascăr I, Grințescu IM. Incidence of Iron Deficiency and the Role of Intravenous Iron Use in Perioperative Periods. Medicina. 2020; 56(10):528. https://doi.org/10.3390/medicina56100528
Chicago/Turabian StyleȚigliș, Mirela, Tiberiu Paul Neagu, Andrei Niculae, Ioan Lascăr, and Ioana Marina Grințescu. 2020. "Incidence of Iron Deficiency and the Role of Intravenous Iron Use in Perioperative Periods" Medicina 56, no. 10: 528. https://doi.org/10.3390/medicina56100528
APA StyleȚigliș, M., Neagu, T. P., Niculae, A., Lascăr, I., & Grințescu, I. M. (2020). Incidence of Iron Deficiency and the Role of Intravenous Iron Use in Perioperative Periods. Medicina, 56(10), 528. https://doi.org/10.3390/medicina56100528





