Magnitude and Determinants of Patients at Risk of Developing Obstructive Sleep Apnea in a Non-Communicable Disease Clinic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Sample Size Estimation
2.5. Sampling Technique
2.6. Study Tool
2.7. Ethics
2.8. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spicuzza, L.; Caruso, D.; Di Maria, G. Obstructive sleep apnoea syndrome and its management. Ther. Adv. Chronic Dis. 2015, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Reddy, E.V.; Kadhiravan, T.; Mishra, H.K.; Sreenivas, V.; Handa, K.K.; Sinha, S.; Sharma, S.K. Prevalence and risk factors of obstructive sleep apnea among middle-aged urban Indians: A community-based study. Sleep Med. 2009, 10, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kumpawat, S.; Banga, A.; Goel, A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest 2006, 130, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.; Veasey, S.; Morgan, B.; O’Donnell, C. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Ahmad, A.N.; McLeod, G.; Al Zahrani, N.; Al Zahrani, H. Screening for high risk of sleep apnea in an ambulatory care setting in saudi arabia. Int. J. Environ. Res. Public Health 2019, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Vaessen, T.J.; Overeem, S.; Sitskoorn, M.M. Cognitive complaints in obstructive sleep apnea. Sleep Med. Rev. 2015, 19, 51–58. [Google Scholar] [CrossRef]
- Unnikrishnan, D.; Jun, J.; Polotsky, V. Inflammation in sleep apnea: An update. Rev. Endocr. Metab. Disord. 2015, 16, 25–34. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Ali, A. Obstructive sleep apnoea and type 2 diabetes. Eur. Endocrinol. 2014, 10, 43–50. [Google Scholar] [CrossRef]
- Kent, B.D.; McNicholas, W.T.; Ryan, S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J. Thorac. Dis. 2015, 7, 1343–1357. [Google Scholar]
- Yadav, D.; Cho, K.H. Total sleep duration and risk of type 2 diabetes: Evidence-based on clinical and epidemiological studies. Curr. Drug Metab. 2018, 19, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Ulualp, S.O. Snoring and obstructive sleep apnea. Med. Clin. N. Am. 2010, 94, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Khajehdehi, A.; Chung, F. A systematic review of screening questionnaires for obstructive sleep apnea. Can. J. Anaesth. J. Can. d–Anesthesie 2010, 57, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Senthilvel, E.; Auckley, D.; Dasarathy, J. Evaluation of sleep disorders in the primary care setting: History taking compared to questionnaires. J. Clin. Sleep Med. 2011, 7, 41–48. [Google Scholar] [PubMed]
- Sogebi, O.A.; Ogunwale, A. Risk factors of obstructive sleep apnea among Nigerian outpatients. Braz. J. Otorhinolaryngol. 2012, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Vasudev, C.; Sinha, S.; Banga, A.; Pandey, R.M.; Handa, K.K. Validation of the modified berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J. Med. Res. 2006, 124, 281–290. [Google Scholar] [PubMed]
- Yacoub, M.; Youssef, I.; Salifu, M.O.; McFarlane, S.I. Cardiovascular disease risk in obstructive sleep apnea: An update. J. Sleep Disord. Ther. 2017, 7, 283. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Makati, D.; Akbar, S. Review of and updates on hypertension in obstructive sleep apnea. Int. J. Hypertens. 2017, 2017, 1848375. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Wang, E.K.; Jeng, Y.-J. Adequate sleep among adolescents is positively associated with health status and health-related behaviors. BMC Public Health 2006, 6, 59. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Europe and the European Centre for Environment and Health Bonn Office. In Proceedings of the WHO Technical Meeting on Sleep and Health, Bonn, Germany, 22–24 January 2004. [Google Scholar]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Hillard, P.J.A.; Katz, E.S. National sleep foundation’s updated sleep duration recommendations. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Yadav, D.; Hyun, D.S.; Ahn, S.V.; Koh, S.B.; Kim, J.Y. A prospective study of the association between total sleep duration and incident hypertension. J. Clin. Hypertens. 2017, 19, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yadav, D.; Ahn, S.V.; Koh, S.B.; Park, J.T.; Yoon, J.; Yoo, B.S.; Lee, S.H. A prospective study of total sleep duration and incident metabolic syndrome: The arirang study. Sleep Med. 2015, 16, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Ramalingam, I.P.; Ramakrishnan, N. High prevalence of obstructive sleep apnea among people with type 2 diabetes mellitus in a tertiary care center. J. Assoc. Physicians India 2017, 65, 38–42. [Google Scholar] [PubMed]
- Rashid, R.; Ahmad, S.; Jaffar, A.; Ali, F.; Paidi, N. Determinants of patients at risk of developing obstructive sleep apnea in a primary care clinic. Res. Updates Med. Sci. 2014, 2, 70–74. [Google Scholar]
- Kang, K.; Seo, J.-G.; Seo, S.-H.; Park, K.-S.; Lee, H.-W. Prevalence and related factors for high-risk of obstructive sleep apnea in a large Korean population: Results of a questionnaire-based study. J. Clin. Neurol. 2014, 10, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Dixon-Williams, S.; Thornton, J.D. Where there is smoke… there is sleep apnea: Exploring the relationship between smoking and sleep apnea. Chest 2014, 146, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol and the risk of sleep apnoea: A systematic review and meta-analysis. Sleep Med. 2018, 42, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, B.M.; Stampfer, M.J.; Tworoger, S.S.; Hu, F.B.; Redline, S. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective us cohorts. Diabetes Care 2018, 41, 2111–2119. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010405. [Google Scholar] [CrossRef]
- McEvoy, R.D. Obstructive sleep apnoea and hypertension: The esada study. Eur. Respir. J. 2014, 44, 835–838. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.J.; Park, J.S.; Cho, Y.-J.; Yoon, H.I.; Lee, J.H.; Lee, C.-T.; Kim, S.J. Associations between obstructive sleep apnea severity and endoscopically proven gastroesophageal reflux disease. Sleep Breath. 2018, 22, 85–90. [Google Scholar] [CrossRef]
- Adedayo, A.M.; Olafiranye, O.; Smith, D.; Hill, A.; Zizi, F.; Brown, C.; Jean-Louis, G. Obstructive sleep apnea and dyslipidemia: Evidence and underlying mechanism. Sleep Breath. 2014, 18, 13–18. [Google Scholar] [CrossRef]
- Gündüz, C.; Basoglu, O.K.; Hedner, J.; Zou, D.; Bonsignore, M.R.; Hein, H.; Staats, R.; Pataka, A.; Barbe, F.; Sliwinski, P. Obstructive sleep apnoea independently predicts lipid levels: Data from the European sleep apnea database. Respirology 2018, 23, 1180–1189. [Google Scholar] [CrossRef]
- Kang, H.H.; Kang, J.Y.; Ha, J.H.; Lee, J.; Kim, S.K.; Moon, H.S.; Lee, S.H. The associations between anthropometric indices and obstructive sleep apnea in a Korean population. PLoS ONE 2014, 9, e114463. [Google Scholar] [CrossRef]
- Soylu, A.C.; Levent, E.; Sarıman, N.; Yurtlu, Ş.; Alparslan, S.; Saygı, A. Obstructive sleep apnea syndrome and anthropometric obesity indexes. Sleep Breath. 2012, 16, 1151–1158. [Google Scholar] [CrossRef]
- Subramanian, S.; Jayaraman, G.; Majid, H.; Aguilar, R.; Surani, S. Influence of gender and anthropometric measures on severity of obstructive sleep apnea. Sleep Breath. 2012, 16, 1091–1095. [Google Scholar] [CrossRef]
- Arokiasamy, P. India’s escalating burden of non-communicable diseases. Lancet Glob. Health 2018, 6, e1262–e1263. [Google Scholar] [CrossRef]
| Variable | High Risk for OSA (%) | Low Risk for OSA (%) | p Value # | Unadjusted OR (95% CI) |
|---|---|---|---|---|
| Gender | ||||
| Male | 64 (27.9) | 165 (72.1) | 0.299 | 1.244 (0.823–1.879) |
| Female | 58 (23.8) | 186 (76.2) | ||
| Age | ||||
| </=60 years | 84 (26.3) | 235 (73.7) | 0.700 | 1.091 (0.701–1.699) |
| >60 years | 38 (24.7) | 116 (75.3) | ||
| Marital status | ||||
| Others (unmarried/widow/divorce) | 20 (29.9) | 47 (70.1) | ||
| Married | 102 (25.1) | 304 (74.9) | 0.413 | 1.268 (0.718–2.241) |
| Occupation | ||||
| Sedentary worker | 66 (25.6) | 192 (74.4) | ||
| Moderate worker | 47 (26.4) | 131 (73.6) | 0.847 | 0.958 (0.620–1.480) Ref |
| Heavy worker | 9 (24.3) | 28 (75.7) | 0.870 | 1.069 (0.480–2.383) Ref |
| Smoking history | ||||
| Smoker | 38 (31.9) | 81 (68.1) | 0.077 | 1.508 (0.955–2.381) |
| Non-smoker | 84 (23.7) | 270 (76.3) | ||
| Alcohol history | ||||
| Drinker | 36 (31.0) | 80 (69.0) | 0.137 | 1.418 (0.893–2.251) |
| Non-drinker | 86 (24.1) | 271 (75.9) | ||
| History of diabetes mellitus | ||||
| Yes | 75(32.3) | 157 (67.7) | 0.001 | 1.972 (1.294–3.004) |
| No | 47 (19.5) | 194 (80.5) | ||
| History of hypertension | ||||
| Yes | 83 (31.0) | 185 (69.0) | 0.003 | 1.910 (1.237–2.948) |
| No | 39 (19.0) | 166 (81.0) | ||
| History of Dyslipidemia | ||||
| Yes | 32(45.1) | 39(54.9) | <0.001 | 2.844 (1.686–4.799) |
| No | 90(22.4) | 312(77.6) | ||
| History of Respiratory illness * | ||||
| Yes | 24(30.8) | 54(69.2) | 0.272 | 1.347 (0.791–2.294) |
| No | 98(24.8) | 297(75.2) | ||
| History of GERD | ||||
| Yes | 32(38.1) | 52(61.9) | 0.004 | 2.044 (1.241–3.369) |
| No | 90(23.1) | 299(76.9) | ||
| History of Sore throat | ||||
| Yes | 10(30.3) | 23(69.7) | 0.539 | 1.273 (0.588–2.758) |
| No | 112(25.5) | 328(74.5) |
| Variable | High Risk for OSA (N = 122) (Mean ± SD) | Low Risk for OSA (N = 351) (Mean ± SD) | p-Value $ | Mean Difference (95% CI) |
|---|---|---|---|---|
| Neck circumference (cm) | 37.26 (3.51) | 36.18 (3.34) | 0.003 | 1.080 (0.362–1.798) |
| Weight (kg) | 72.83 (10.75) | 63.83 (10.59) | <0.001 | 8.99 (6.77–11.21) |
| Height (cm) | 159.20 (8.27) | 158.08 (8.29) | 0.198 | 1.12 (−0.59–2.84) |
| BMI (kg/sq.m) | 28.81 (4.23) | 25.57 (4.03) | <0.001 | 3.24 (2.37–4.11) |
| Waist circumference (cm) | 95.03 (8.49) | 86.62 (8.87) | <0.001 | 8.41 (6.63–10.19) |
| Hip circumference (cm) | 104.47 (7.78) | 97.40 (7.79) | <0.001 | 7.06 (5.45–8.67) |
| Systolic blood pressure (mmHg) | 141.57 (14.86) | 133.77 (15.75) | <0.001 | 7.81 (4.68–10.93) |
| Diastolic blood pressure (mmHg) | 86.42 (9.00) | 83.07 (10.49) | 0.001 | 3.35 (1.40–5.30) |
| Waist Hip Ratio | 0.911 (0.067) | 0.892 (0.088) | 0.015 | 0.019 (0.0037–0.0338) |
| B | S.E. | Wald | p-Value | Adjusted Odds Ratio (95% CI) | |
|---|---|---|---|---|---|
| History of DM (No as reference) | 0.380 | 0.252 | 2.274 | 0.132 | 1.462 (0.892–2.394) |
| History of Dyslipidemia (No as reference) | 0.851 | 0.331 | 6.601 | 0.010 | 2.342 (1.224–4.482) |
| History of GERD (No as reference) | 0.093 | 0.309 | 0.092 | 0.762 | 1.098 (0.600–2.010) |
| Neck circumference | 0.017 | 0.037 | 0.210 | 0.647 | 1.017 (0.947–1.092) |
| Body mass index | 0.137 | 0.033 | 17.344 | 0.000 | 1.147 (1.075–1.223) |
| Waist circumference | 0.097 | 0.017 | 31.090 | 0.000 | 1.102 (1.065–1.141) |
| Systolic blood pressure | 0.019 | 0.010 | 3.748 | 0.053 | 1.019 (1.000–1.038) |
| Diastolic blood pressure | −0.006 | 0.014 | 0.166 | 0.683 | 0.994 (0.968–1.021) |
| Constant | −16.670 | 2.248 | 54.985 | 0.000 | 0.000 |
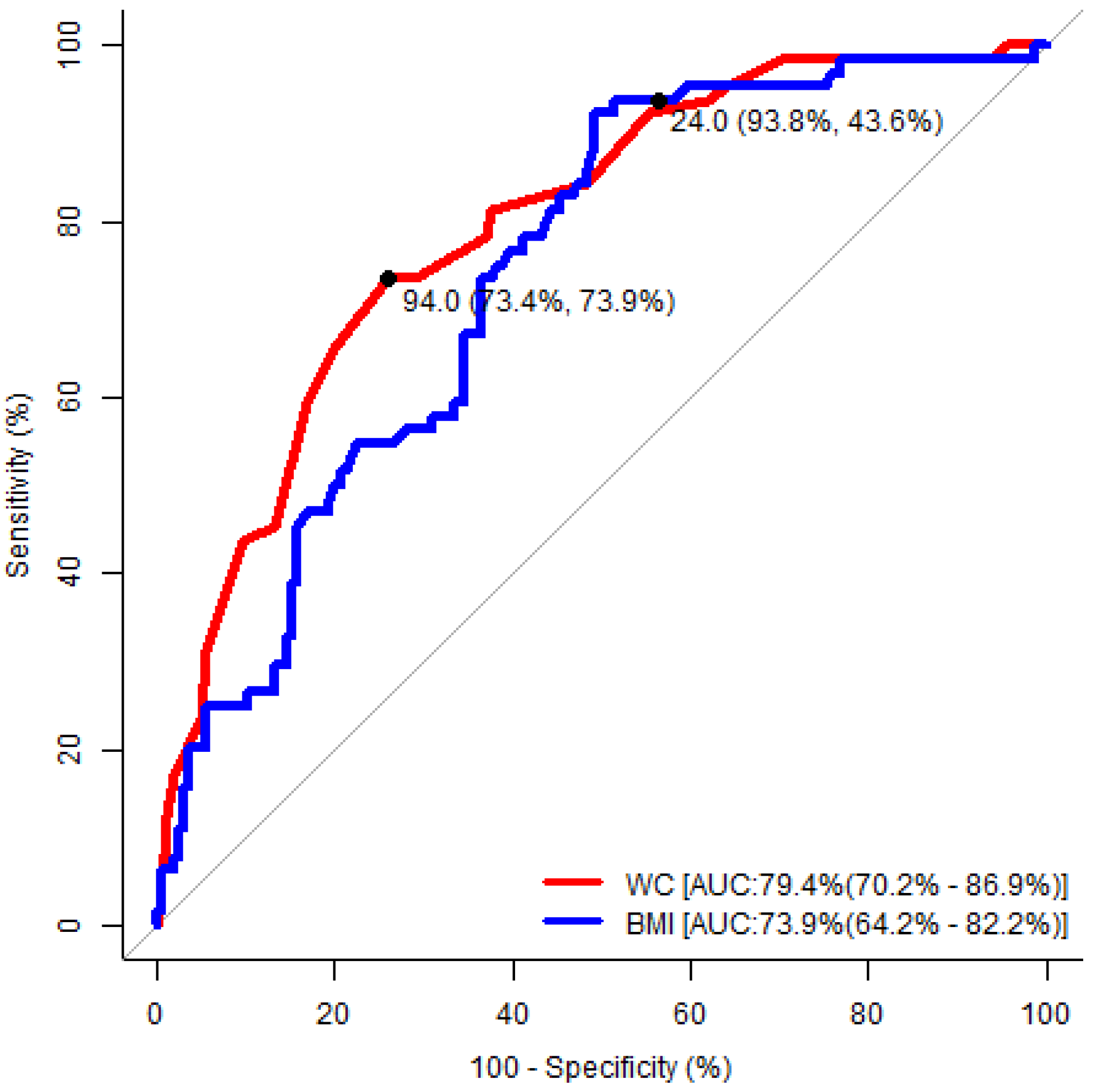
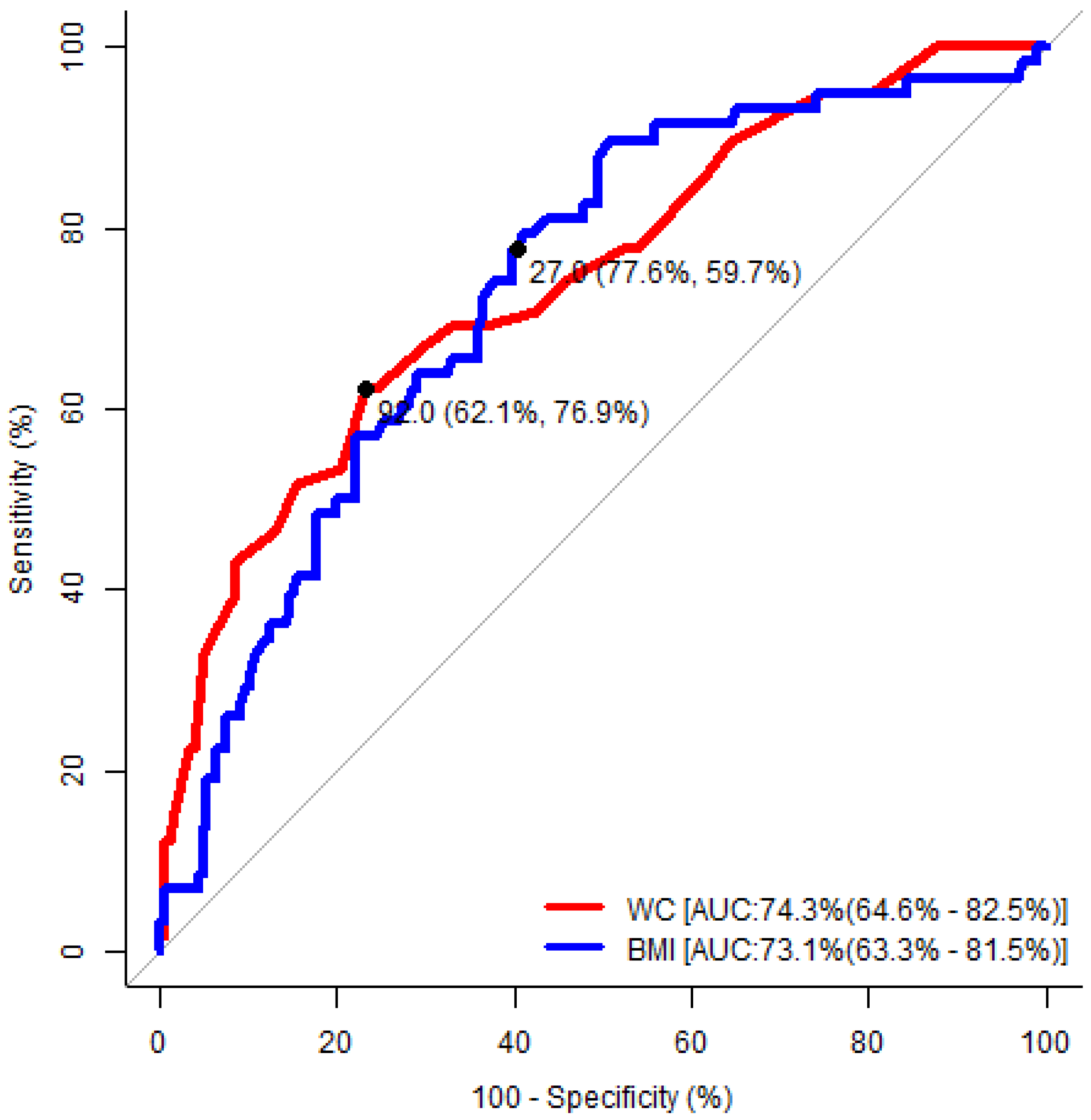
| Waist Circumference | Body Mass Index | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Area under the ROC curve (95% CI) | 0.79 (0.70–0.87) | 0.74 (0.65–0.83) | 0.74 (0.64–0.82) | 0.73 (0.63–0.82) |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Cut-off value | 94.0 cm | 92.0 cm | 24.0 kg/m2 | 27.0 kg/m2 |
| Sensitivity | 73% | 62% | 94% | 78% |
| Specificity | 74% | 77% | 44% | 60% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathiyalagen, P.; Govindasamy, V.; Rajagopal, A.; Vasudevan, K.; Gunasekaran, K.; Yadav, D. Magnitude and Determinants of Patients at Risk of Developing Obstructive Sleep Apnea in a Non-Communicable Disease Clinic. Medicina 2019, 55, 391. https://doi.org/10.3390/medicina55070391
Mathiyalagen P, Govindasamy V, Rajagopal A, Vasudevan K, Gunasekaran K, Yadav D. Magnitude and Determinants of Patients at Risk of Developing Obstructive Sleep Apnea in a Non-Communicable Disease Clinic. Medicina. 2019; 55(7):391. https://doi.org/10.3390/medicina55070391
Chicago/Turabian StyleMathiyalagen, Prakash, Venkatesh Govindasamy, Anandaraj Rajagopal, Kavita Vasudevan, Kalaipriya Gunasekaran, and Dhananjay Yadav. 2019. "Magnitude and Determinants of Patients at Risk of Developing Obstructive Sleep Apnea in a Non-Communicable Disease Clinic" Medicina 55, no. 7: 391. https://doi.org/10.3390/medicina55070391
APA StyleMathiyalagen, P., Govindasamy, V., Rajagopal, A., Vasudevan, K., Gunasekaran, K., & Yadav, D. (2019). Magnitude and Determinants of Patients at Risk of Developing Obstructive Sleep Apnea in a Non-Communicable Disease Clinic. Medicina, 55(7), 391. https://doi.org/10.3390/medicina55070391






