Is Scientific Medical Literature Related to Endometriosis Treatment Evidence-Based? A Systematic Review on Methodological Quality of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Methods
2.3. Data Analysis
3. Results
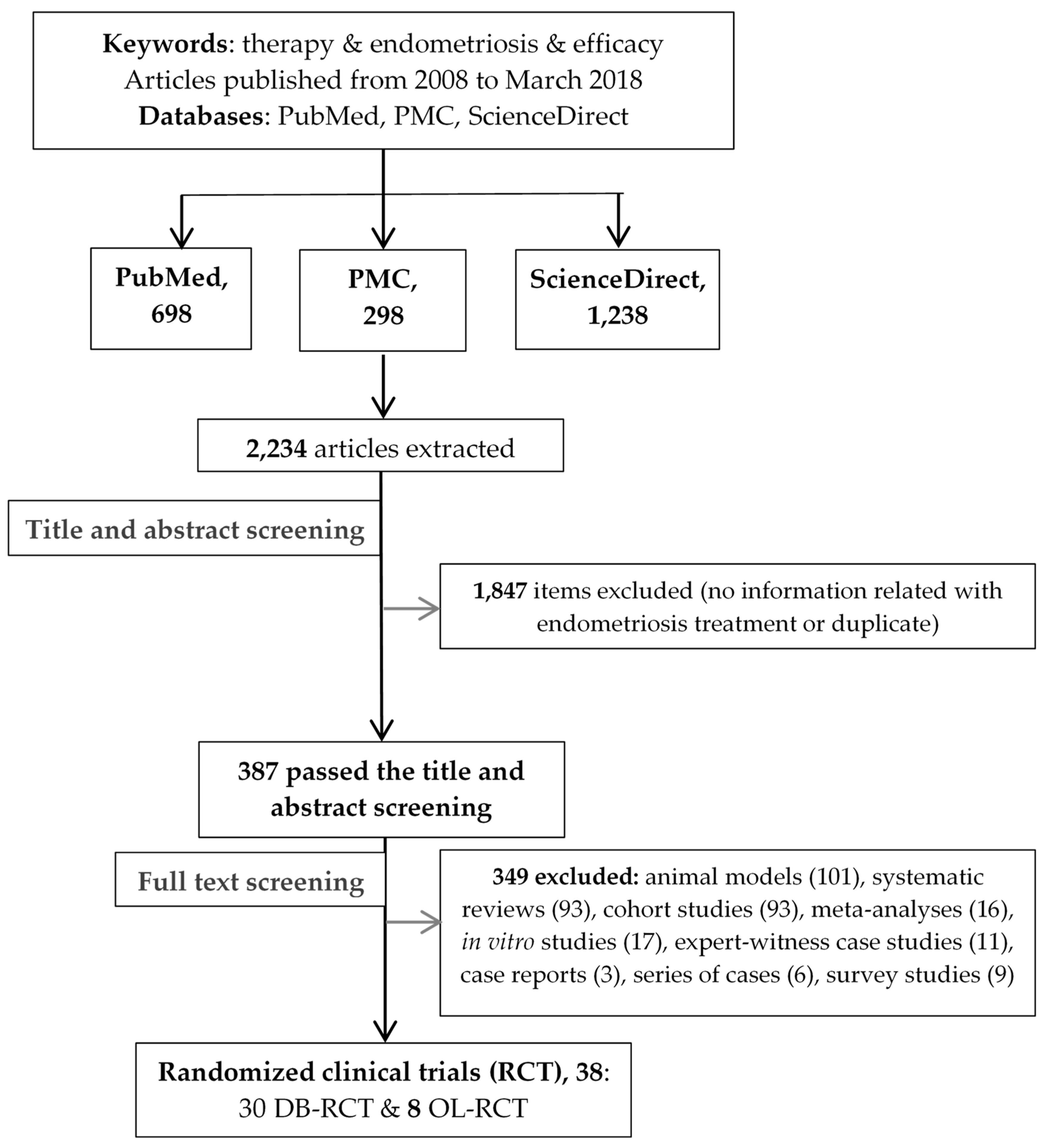
3.1. Description of Study Retrieval
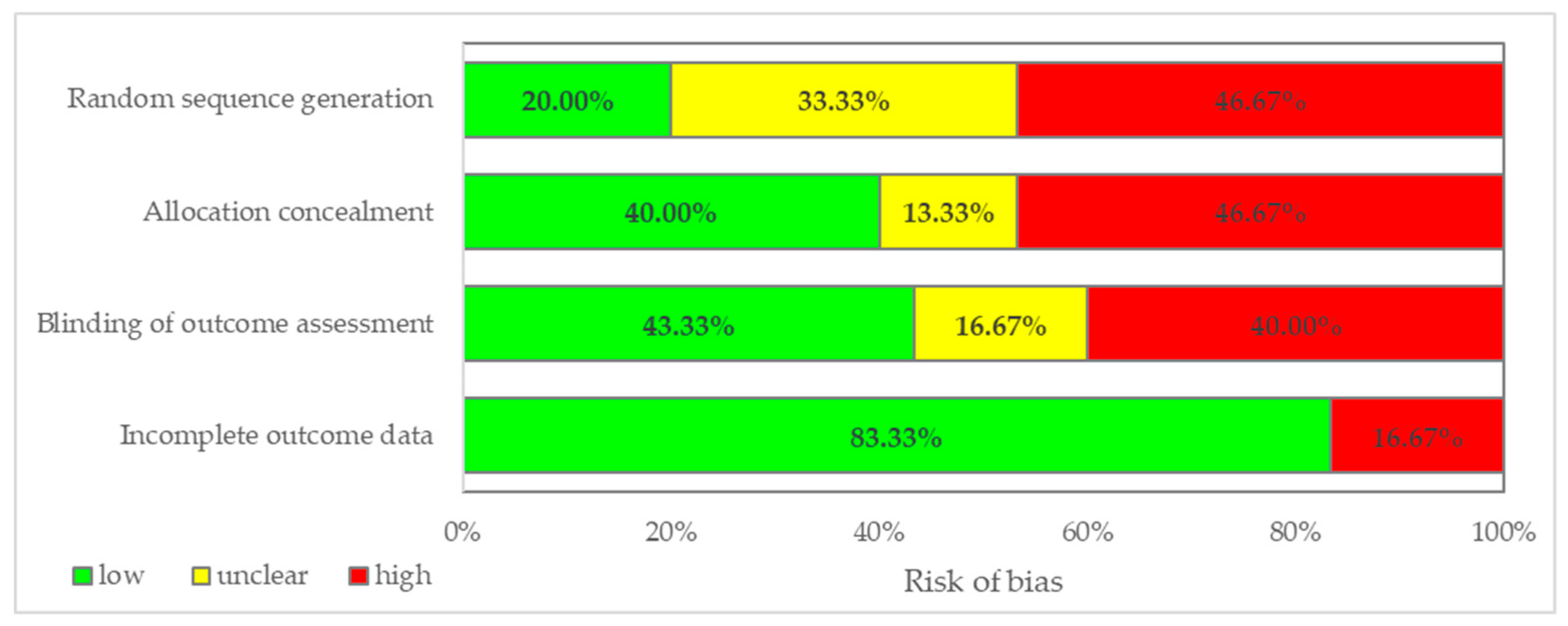
3.2. Analysis of Double-Blinded Randomized Clinical Trials
3.3. Analysis of Open-Label Randomized Clinical Trials
4. Discussion
Study Limitations and Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heneghan, C.; Mahtani, K.; Goldacre, B.; Godlee, F.; Macdonald, H.; Jarvies, D. Evidence based medicine manifesto for better healthcare. Evid. Based Med. 2017, 22, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Bekelman, J.E.; Li, Y.; Gross, C.P. Scope and impact of financial conflicts of interest in biomedical research: A systematic review. JAMA 2003, 289, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Susanna, D.; Ian, W.; Paula, W. Departure from treatment protocol in published randomised controlled trials: A review. Trials 2011, 12, A129. [Google Scholar] [CrossRef][Green Version]
- Montori, V.M.; Devereaux, P.J.; Adhikari, N.K.; Burns, K.E.; Eggert, C.H.; Briel, M.; Lacchetti, C.; Leung, T.W.; Darling, E.; Bryant, D.M.; et al. Randomized trials stopped early for benefit: A systematic review. JAMA 2005, 294, 2203–2209. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Hróbjartsson, A.; Johansen, H.K.; Haahr, M.T.; Altman, D.G.; Chan, A.W. Ghost authorship in industry-initiated randomised trials. PLoS Med. 2007, 4, e19. [Google Scholar] [CrossRef]
- Song, F.; Parekh, S.; Hooper, L.; Loke, Y.K.; Ryder, J.; Sutton, A.J.; Hing, C.; Kwok, C.S.; Pang, C.; Harvey, I. Dissemination and publication of research findings: An updated review of related biases. Health Technol. Assess. 2010, 14. [Google Scholar] [CrossRef]
- Kessel, M.; Mark, K. Restoring the pharmaceutical industry’s reputation. Nat. Biotechnol. 2014, 32, 983–990. [Google Scholar] [CrossRef]
- Light, D.W.; Lexchin, J.; Darrow, J.J. Institutional corruption of pharmaceuticals and the myth of safe and effective drugs. J. Law Med. Ethics 2013, 41, 590–600. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L. Bad pharma: How drug companies mislead doctors and harm patients. Aust. Prescr. 2013, 36, 55. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding randomised controlled trials. Arch. Dis. Child 2005, 90, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Cooper, B.; Shin, S.; Kondziolka, D. Randomized controlled trials and neurosurgery: The ideal fit or should alternative methodologies be considered? JNS 2016, 124, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C. The need for large-scale randomized evidence. Br. J. Clin. Pharmacol. 1997, 43, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Collins, R.; Peto, R. Why do we need some large, simple randomized trials? Stat. Med. 1984, 3, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.; Xu, W.; Sahgal, A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 2012, 118, 2486–2493. [Google Scholar] [CrossRef]
- Tsao, M.N.; Lloyd, N.; Wong, R.K.; Chow, E.; Rakovitch, E.; Laperriere, N.; Xu, W.; Sahgal, A. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2012, 4, CD003869. [Google Scholar] [CrossRef]
- Tsao, M.N.; Lloyd, N.S.; Wong, R.K. Supportive Care Guidelines Group of Cancer Care Ontario’s Program in Evidence-based C. Clinical practice guideline on the optimal radiotherapeutic management of brain metastases. BMC Cancer 2005, 5, 34. [Google Scholar] [CrossRef]
- Mansouri, A.; Shin, S.; Cooper, B.; Srivastava, A.; Bhandari, M.; Kondziolka, D. Randomized controlled trials and neuro-oncology: Should alternative designs be considered? J. Neurooncol. 2015, 124, 345–356. [Google Scholar] [CrossRef]
- Moher, D.; Jones, A.; Lepage, L.; Group, C. Use of the CONSORT statement and quality of reports of randomized trials: A comparative before-and-after evaluation. JAMA 2001, 285, 1992–1995. [Google Scholar] [CrossRef]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Gray Valbrun, T.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing Research Gaps and Unmet Needs in Endometriosis. Am. J. Obstet. Gynecol. 2019. [Google Scholar] [CrossRef]
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J. Med. Life 2014, 7, 349–357. [Google Scholar] [PubMed]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—The gold standard for effectiveness research. BJOG 2018, 125, 1716. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Critical Appraisal Notes and Checklists. Methodology Checklist 2: Randomised Controlled Trials. Available online: https://www.sign.ac.uk/checklists-and-notes.html (accessed on 10 April 2018).
- Almassinokiani, F.; Mehdizadeh, A.; Sariri, E.; Rezaei, M.; Almasi, A.; Akbari, H.; Pazouki, A.; Solaymani-Dodaran, M.; Asadollah, S.; Amirkhani, J.; et al. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med. Sci. Monit. 2013, 19, 534–539. [Google Scholar] [CrossRef] [PubMed]
- DiVasta, A.D.; Feldman, H.A.; Gallagher, J.S.; Stokes, N.A.; Laufer, M.R.; Hornstein, M.D.; Gordon, C.M. Hormonal Add-Back Therapy for Females Treated With Gonadotropin-Releasing Hormone Agonist for Endometriosis: A Randomized Controlled Trial. Obstet. Gynecol. 2015, 126, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Bayoglu Tekin, Y.; Dilbaz, B.; Altinbas, S.K.; Dilbaz, S. Postoperative medical treatment of chronic pelvic pain related to severe endometriosis: Levonorgestrel-releasing intrauterine system versus gonadotropin-releasing hormone analogue. Fertil. Steril. 2011, 95, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.L.; Riverón, A.M.; Leonard, Y.; González, J.; Heredia, B.; Sánchez, C. Mifepristone 2.5, 5, 10 mg versus placebo in the treatment of endometriosis. J. Reprod. Health Med. 2016, 2, 17–25. [Google Scholar] [CrossRef]
- Carr, B.; Dmowski, W.P.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: Effects on bone mineral density. Reprod. Sci. 2014, 21, 1341–1351. [Google Scholar] [CrossRef]
- Chen, J.M.; Gao, H.Y.; Ding, Y.; Yuan, X.; Wang, Q.; Li, Q.; Jiang, G.H. Efficacy and safety investigation of Kuntai capsule for the add-back therapy of gonadotropin releasing hormone agonist administration to endometriosis patients: A randomized, double-blind, blank- and tibolone-controlled study. Chin. Med. J. 2015, 128, 427–432. [Google Scholar] [CrossRef]
- Chen, J.; Gao, H.; Li, Q.; Cong, J.; Wu, J.; Pu, D.; Jiang, G. Efficacy and Safety of Remifemin on Peri-Menopausal Symptoms Induced by Post-Operative GnRH-a Therapy for Endometriosis: A Randomized Study versus Tibolone. Med. Sci. Monit. 2014, 20, 1950–1957. [Google Scholar] [CrossRef][Green Version]
- Cobellis, L.; Castaldi, M.A.; Giordano, V.; Trabucco, E.; De Franciscis, P.; Torella, M.; Colacurci, N. Effectiveness of the association micronized N-Palmitoylethanolamine (PEA)–transpolydatin in the treatment of chronic pelvic pain related to endometriosis after laparoscopic assessment: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Creus, M.; Fábregues, F.; Carmona, F.; del Pino, M.; Manau, D.; Balasch, J. Combined laparoscopic surgery and pentoxifylline therapy for treatment of endometriosis-associated infertility: A preliminary trial. Hum. Reprod. 2008, 23, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Carr, B.; Dmowski, W.P.; Koltun, W.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix treatment for endometriosis-associated pain: Results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod. Sci. 2014, 21, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Granese, R.; Perino, A.; Calagna, G.; Saitta, S.; De Franciscis, P.; Colacurci, N.; Triolo, O.; Cucinella, G. Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: A multi-center randomized trial. Acta. Obstet. Gynecol. Scand. 2015, 94, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Guzick, D.S.; Huang, L.-S.; Broadman, B.A.; Nealon, M.; Hornstein, M.D. Randomized trial of leuprolide versus continuous oral contraceptives in the treatment of endometriosis-associated pelvic pain. Fertil. Steril. 2011, 95, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kosaka, S.; Elliesen, J.; Yasuda, M.; Ito, M.; Momoeda, M. Ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: A randomized controlled trial. Fertil. Steril. 2017, 108, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Momoeda, M.; Taketani, Y.; Aso, T.; Fukunaga, M.; Hagino, H.; Terakawa, N. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis—A randomized, double-blind, multicenter, controlled trial. Fertil. Steril. 2009, 91, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Momoeda, M.; Taketani, Y.; Hoshiai, H.; Terakawa, N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: A placebo-controlled, double-blind, randomized trial. Fertil. Steril. 2008, 90, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Uchida, M.; Sashihara, T.; Ji, Z.-S.; Li, J.; Tang, Q.; Ni, S.; Song, L.; Kaminogawa, S. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: Randomized, double-blind, placebo-controlled study. Cytotechnology 2011, 63, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Craessaerts, M.; Timmerman, D.; Cornillie, F.; Kennedy, S. Anti-TNF-α treatment for deep endometriosis-associated pain: A randomized placebo-controlled trial. Hum. Reprod. 2008, 23, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Yu, Q.; Zhang, S.; Li, H.; Gude, K.; von Ludwig, C.; Ren, X.; Dong, L. Dienogest for Treatment of Endometriosis in Chinese Women: A Placebo-Controlled, Randomized, Double-Blind Phase 3 Study. J. Womens Health 2018, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, H.Y.; Zhu, Y.J.; Hu, Y.J.; Qu, P.P. A randomized study comparing the side effects and hormonal status of triptorelin and leuprorelin following conservative laparoscopic surgery for ovarian endometriosis in Chinese women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 183, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Mendes da Silva, D.; Azevedo Gross, L.; Guedes Neto, E.P.; Lessey, B.A.; Savaris, R.F. The Use of Resveratrol as an Adjuvant Treatment of Pain in Endometriosis: A Randomized Clinical Trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Remorgida, V.; Venturini, P.L.; Ferrero, S. Progestogen-only contraceptive pill compared with combined oral contraceptive in the treatment of pain symptoms caused by endometriosis in patients with migraine without aura. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, A.; Conceição Dos Santos, C.C.; Costa, G.D.; Deitos, A.; de Souza, A.; de Souza, I.C.; Torres, I.L.; da Cunha Filho, J.S.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase II, randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef]
- Shokeir, T.; Mousa, S. A randomized, placebo-controlled, double-blind study of hysteroscopic-guided pertubal diluted bupivacaine infusion for endometriosis-associated chronic pelvic pain. Int. J. Gynaecol. Obstet. 2015, 130, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, T.; Marr, J.; Gerlinger, C.; Faustmann, T.; Seitz, C. Detailed analysis of a randomized, multicenter, comparative trial of dienogest versus leuprolide acetate in endometriosis. Int. J. Gynaecol. Obstet. 2012, 117, 228–233. [Google Scholar] [CrossRef]
- Strowitzki, T.; Faustmann, T.; Gerlinger, C.; Seitz, C. Dienogest in the treatment of endometriosis-associated pelvic pain: A 12-week, randomized, double-blind, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 193–198. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Teixeira, M.Z.; Podgaec, S.; Baracat, E.C. Potentized estrogen in homeopathic treatment of endometriosis-associated pelvic pain: A 24-week, randomized, double-blind, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 211, 48–55. [Google Scholar] [CrossRef]
- Wayne, P.M.; Kerr, C.E.; Schnyer, R.N.; Legedza, A.T.R.; Savetsky-German, J.; Shields, M.H.; Buring, J.E.; Davis, R.B.; Conboy, L.A.; Highfield, E.; et al. Japanese-Style Acupuncture for Endometriosis-Related Pelvic Pain in Adolescents and Young Women: Results of a Randomized Sham-Controlled Trial. J. Pediatr. Adolesc. Gynecol. 2008, 21, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.; Tang, L.C.; Chin, R.K. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: A randomised controlled trial. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Long, Q.; Zhang, S.; Han, Y.; Zhang, W. Oral continuous combined 0.5 mg estradiol valerate and 5 mg dydrogesterone as daily add-back therapy during post-operative GnRH agonist treatment for endometriosis in Chinese women. Int. J. Clin. Exp. Med. 2013, 6, 67–73. [Google Scholar] [PubMed]
- Ghahiri, A.; Najafian, A.; Ghasemi, M.; Najafian, A. Comparison study on effectiveness of pentoxifyllin with LD to prevent recurrent endometriosis. Iran. J. Reprod. Med. 2012, 10, 219–222. [Google Scholar] [PubMed]
- Cheewadhanaraks, S.; Choksuchat, C.; Dhanaworavibul, K.; Liabsuetrakul, T. Postoperative depot medroxyprogesterone acetate versus continuous oral contraceptive pills in the treatment of endometriosis-associated pain: A randomized comparative trial. Gynecol. Obstet. Investig. 2012, 74, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Venturini, P.L.; Gillott, D.J.; Remorgida, V. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: A randomized controlled trial. Reprod. Biol. Endocrinol. 2011, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, S.; Han, Y.; Long, Q.; Zou, S.; Cao, Y. Initiation of GnRH agonist treatment on 3–5 days postoperatively in endometriosis patients: A randomized controlled trial. J. Clin. Pharmacol. 2015, 55, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Kamencic, H.; Thiel, J.A. Pentoxifylline after conservative surgery for endometriosis: A randomized, controlled trial. J. Minim. Invasive Gynecol. 2008, 15, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Faustmann, T.A.; Gerlinger, C.; Seitz, C.; Mueck, A.O. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4 mg of dienogest daily for endometriosis. Int. J. Gynaecol. Obstet. 2010, 108, 21–25. [Google Scholar] [CrossRef]
- Strowitzki, T.; Marr, J.; Gerlinger, C.; Faustmann, T.; Seitz, C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: A 24-week, randomized, multicentre, open-label trial. Hum. Reprod. 2010, 25, 633–641. [Google Scholar] [CrossRef]
- Walch, K.; Unfried, G.; Huber, J.; Kurz, C.; van Trotsenburg, M.; Pernicka, E.; Wenzl, R. Implanon versus medroxyprogesterone acetate: Effects on pain scores in patients with symptomatic endometriosis—A pilot study. Contraception 2009, 79, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Omer, R. International Scientific Publication in ISI Journals: Chances and Obstacles. World J. Edu. 2015, 5, 81–90. [Google Scholar] [CrossRef]
- Benson, K.; Hartz, A.J. A comparison of observational studies and randomised controlled trials. N. Engl. J. Med. 2000, 342, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Redwine, D.; Mann, C.H.; Wright, J.T. Evidence on endometriosis. Elitism about randomised controlled trials is inappropriate. BMJ 2000, 321, 1077. [Google Scholar] [PubMed]
- Lorscheitter, J.; Stein, C.; Plentz, R. Methodological Quality of Randomized Clinical Trials of Respiratory Physiotherapy in Coronary Artery Bypass Grafting Patients in the Intensive Care Unit: A Systematic Review. Braz. J. Cardiovasc. Surg. 2017, 32, 318–337. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Tian, J.; Ren, D.; Wei, H.; Zhang, L.; Wang, Q.; Yang, K. Methodological Reporting of Randomized Trials in Five Leading Chinese Nursing Journals. PLoS ONE 2014, 9, e113002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Huang, W.X.; Li, X.L.; Jiang, X.L.; Wang, S.P. Investigation on randomized controlled trials of nursing care field in China. J. Pract. Nurs. 2003, 19, 60–61. [Google Scholar]
- Mei, L.J.; Zheng, G.H.; Chen, Q.Y.; Lin, R.; Yan, Y.; Yang, Z.-H. Methodological Evaluation on Domestic Clinical Trials on Traditional Chinese Medicine Nursing from 2006 to 2011. Chin. J. Evid-Based Med. 2012, 12, 735–739. [Google Scholar]
- Marti-Carvajal, A. Chapter 2. Proper Randomization Reduces the Chance of Wasted Biomedical Research. In Randomization, Masking, and Allocation Concealment; Berger, V.W., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 9–28. [Google Scholar]
- Bougie, O.; Yap, M.I.; Sikora, L.; Flaxman, T.; Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: A systematic review and meta-analysis. BJOG 2019. [Google Scholar] [CrossRef]
- Farland, L.V.; Horne, A.W. Disparity in endometriosis diagnoses between racial/ethnic groups. BJOG 2019. [Google Scholar] [CrossRef]
- Williams, C.; Long, A.J.; Noga, H.; Allaire, C.; Bedaiwy, M.A.; Lisonkova, S.; Yong, P.J. East and South East Asian Ethnicity and Moderate-to-Severe Endometriosis. J. Minim Invasive Gynecol. 2019, 26, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Park, M.; Lee, G.W.; Lee, K.H.; Chang, H.K.; Kwak, S.M.; Yoo, H.J. Efficacy of levonorgestrel releasing intrauterine system as a postoperative maintenance therapy of endometriosis: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Indraccolo, U.; Indraccolo, S.R.; Mignini, F. Micronized palmitoylethanolamide/trans-polydatin treatment of endometriosis-related pain: A meta-analysis. Ann. Ist. Super. Sanita 2017, 53, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, C.; Faustmann, T.; Hassall, J.J.; Seitz, C. Treatment of endometriosis in different ethnic populations: A meta-analysis of two clinical trials. BMC Women’s Health 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Fourquet, J.; Sinaii, N.; Stratton, P.; Khayel, F.; Alvarez-Garriga, C.; Bayona, M.; Ballweg, M.L.; Flores, I. Characteristics of women with endometriosis from the USA and Puerto Rico. J. Endometr. Pelvic Pain Disord. 2015, 7, 129–135. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Reserch 2019, 8. [Google Scholar] [CrossRef]
- Patel, B.G.; Lenk, E.E.; Lebovic, D.I.; Shu, Y.; Yu, J.; Taylor, R.N. Pathogenesis of endometriosis: Interaction between Endocrine and inflammatory pathways. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 50–60. [Google Scholar] [CrossRef]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Forman, J.P.; Missmer, S.A. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. Endometriosis and Risk of Coronary Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 257–264. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Brozek, J.L.; Akl, E.A.; Alonso-Coello, P.; Lang, D.; Jaeschke, R.; Williams, J.W.; Phillips, B.; Lelgemann, M.; Lethaby, A.; Bousquet, J.; et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 2009, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.T.; Tsertsvadze, A.; Moher, D. Grading Quality of Evidence and Strength of Recommendations: A Perspective. PLoS Med. 2009, 6, e1000151. [Google Scholar] [CrossRef]

| Section | Checklist Item (Yes = 1 vs. No/Cannot Say = 0) |
|---|---|
| Title |
|
| Abstract |
|
| Background and objectives |
|
| Methods |
|
| Randomization |
|
| Results |
|
| Author, Year [Ref] | TABO | Methods | Randomization | Results | Total Score |
|---|---|---|---|---|---|
| Almassinokiani et al., 2013 [25] | 3 | 10 | 0 | 1 | 14 |
| DiVasta et al., 2015 [26] | 4 | 15 | 4 | 1 | 24 |
| Bayoglu Tekin et al., 2011 [27] | 4 | 12 | 1 | 1 | 18 |
| Carbonell et al., 2016 [28] | 4 | 12 | 3 | 1 | 20 |
| Carr et al., 2014 [29] | 3 | 16 | 2 | 1 | 22 |
| Chen et al., 2015 [30] | 4 | 12 | 0 | 1 | 17 |
| Chen et al., 2014 [31] | 4 | 10 | 0 | 1 | 15 |
| Cobellis et al., 2011 [32] | 3 | 12 | 0 | 1 | 16 |
| Creus et al., 2008 [33] | 4 | 15 | 2 | 1 | 22 |
| Diamond et al., 2014 [34] | 3 | 16 | 3 | 1 | 23 |
| Granese et al., 2015 [35] | 4 | 13 | 1 | 1 | 19 |
| Guzick et al., 2011 [36] | 4 | 12 | 0 | 1 | 17 |
| Harada et al., 2017 [37] | 4 | 16 | 3 | 0 | 23 |
| Harada et al., 2009 [38] | 4 | 14 | 0 | 1 | 19 |
| Harada et al., 2008 [39] | 4 | 16 | 4 | 1 | 25 |
| Itoh et al., 2011 [40] | 3 | 16 | 2 | 1 | 22 |
| Koninckx et al., 2008 [41] | 4 | 16 | 4 | 1 | 25 |
| Lang et al., 2018 [42] | 4 | 15 | 1 | 1 | 21 |
| Li et al., 2014 [43] | 4 | 15 | 1 | 1 | 21 |
| Mendes da Silva et al., 2017 [44] | 4 | 14 | 4 | 1 | 23 |
| Morotti et al., 2014 [45] | 3 | 12 | 0 | 1 | 16 |
| Schwertner et al., 2013 [46] | 3 | 15 | 1 | 1 | 20 |
| Shokeir and Mousa, 2015 [47] | 4 | 15 | 2 | 1 | 22 |
| Strowitzki et al., 2012 [48] | 4 | 15 | 1 | 1 | 21 |
| Strowitzki et al., 2010 [49] | 4 | 15 | 0 | 1 | 20 |
| Taylor et al., 2017 [50] | 4 | 14 | 1 | 1 | 20 |
| Teixeira et al., 2017 [51] | 4 | 15 | 3 | 1 | 23 |
| Wayne et al., 2008 [52] | 4 | 14 | 1 | 0 | 19 |
| Wong et al., 2010 [53] | 4 | 13 | 1 | 1 | 19 |
| Zou et al., 2013 [54] | 2 | 11 | 0 | 1 | 14 |
| Author, Year [Ref] | TABO | Methods | Randomization | Results | Total Score |
|---|---|---|---|---|---|
| Ghahiri et al., 2012 [55] | 3 | 11 | 0 | 1 | 15 |
| Cheewadhanaraks et al., 2012 [56] | 4 | 11 | 2 | 1 | 18 |
| Ferrero et al., 2011 [57] | 4 | 11 | 2 | 1 | 18 |
| Gong et al., 2015 [58] | 4 | 11 | 1 | 1 | 17 |
| Kamencic and Thiel, 2008 [59] | 4 | 13 | 0 | 1 | 18 |
| Köhler et al., 2010 [60] | 4 | 13 | 1 | 1 | 19 |
| Strowitzki et al., 2010 [61] | 4 | 15 | 1 | 1 | 21 |
| Walch et al., 2009 [62] | 3 | 13 | 1 | 1 | 18 |
| Medical Field [Ref] | TABO | Random Sequence Generation | Allocation Concealment | Blinding Methods |
|---|---|---|---|---|
| Neurosurgery [12] | Good quality: most of the objectives | Poorly reported: 65.8% | ||
| Neuro-oncology [18] | Poorly reported: 70% | |||
| Respiratory physiotherapy post coronary bypass grafting [66] | Inadequate titles | Correctly reported: 51.28% | Insufficient details | Both patients and investigators: 7.69% |
| Nursing [67] | The type applied: 1.8% | Described: 0.3% | Specified: 5.9% | |
| Nursing [68] | Good description: 3.8% | |||
| Traditional Chinese nursing [69] | Described: 7.8% | Described: 1.4% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capraş, R.-D.; Urda-Cîmpean, A.E.; Bolboacă, S.D. Is Scientific Medical Literature Related to Endometriosis Treatment Evidence-Based? A Systematic Review on Methodological Quality of Randomized Clinical Trials. Medicina 2019, 55, 372. https://doi.org/10.3390/medicina55070372
Capraş R-D, Urda-Cîmpean AE, Bolboacă SD. Is Scientific Medical Literature Related to Endometriosis Treatment Evidence-Based? A Systematic Review on Methodological Quality of Randomized Clinical Trials. Medicina. 2019; 55(7):372. https://doi.org/10.3390/medicina55070372
Chicago/Turabian StyleCapraş, Roxana-Denisa, Andrada Elena Urda-Cîmpean, and Sorana D. Bolboacă. 2019. "Is Scientific Medical Literature Related to Endometriosis Treatment Evidence-Based? A Systematic Review on Methodological Quality of Randomized Clinical Trials" Medicina 55, no. 7: 372. https://doi.org/10.3390/medicina55070372
APA StyleCapraş, R.-D., Urda-Cîmpean, A. E., & Bolboacă, S. D. (2019). Is Scientific Medical Literature Related to Endometriosis Treatment Evidence-Based? A Systematic Review on Methodological Quality of Randomized Clinical Trials. Medicina, 55(7), 372. https://doi.org/10.3390/medicina55070372







