Association between Circle of Willis Configuration and Rupture of Cerebral Aneurysms
Abstract
1. Introduction
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asaithambi, G.; Adil, M.M.; Chaudhry, S.A.; Qureshi, A.I. Incidences of unruptured intracranial aneurysms and subarachnoid hemorrhage: Results of a statewide study. J. Vascinterv. Neurol. 2014, 7, 14–17. [Google Scholar]
- D’Souza, S.; MBBS; FRCA; FCARCS. Aneurysmal subarachnoid hemorrhage. J. Neurosurg. Anesth. 2015, 27, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Rinkel, G.J.; Djibuti, M.; Algra, A.; van Gijn, J. Prevalence and risk of rupture of intracranial aneurysms: A systematic review. Stroke 1998, 29, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wiebers, D.O. The international study of unruptured intracranial aneurysms investigators: Unruptured intracranial aneurysms—Risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [Google Scholar]
- Wiebers, D.O.; Piepgras, D.G.; Meyer, F.B.; Kallmes, D.F.; Meissner, I.; Atkinson, J.L.; Link, M.J.; Brown, R.D. Pathogenesis, natural history, and treatment of unruptured intracranial aneurysms. Mayo Clin. Proc. 2004, 79, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Wiebers, D.O.; Torner, J.C.; Meissner, I. Impact of unruptured intracranial aneurysms on public health in the United States. Stroke 1992, 23, 1416–1419. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Iqbal, S. A comprehensive study of the anatomical variations of the circle of willis in adult human brains. J.Clin. Diagn. Res. 2013, 7, 2423–2427. [Google Scholar] [CrossRef]
- Ujiie, H.; Dieter, W.; Goetz, M.; Yamaguchi, R.; Yonetani, H.; Takakura, K. Hemodynamic study of the anterior communicating artery. Stroke 1996, 27, 2086–2094. [Google Scholar] [CrossRef]
- Ferrandez, A.; David, T.; Bamford, J.; Scott, J.; Guthrie, A. Coputational models of blood flow in the circle of Willis. Comput. Methods Biomech. Biomed. Eng. 2000, 4, 1–26. [Google Scholar] [CrossRef]
- Vrselja, Z.; Brkic, H.; Mrdenovic, S.; Radic, R.; Curic, G. Function of circle of Willis. J. Cereb. Blood Flow Metab. 2014, 34, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Barkeij Wolf, J.J.H.; Foster-Dingley, J.C.; Moonen, J.E.F.; van Osch, M.J.P.; de Craen, A.J.M.; de Ruijter, W.; van der Mast, R.C.; van der Grond, J. Unilateral fetal-type circle of Willis anatomy causes right-left asymmetry in cerebral blood flow with pseudo-continuous arterial spin labeling: A limitation of arterial spin labeling-based cerebral blood flow measurements? Sendto. J. Cereb.Blood Flow Metab. 2016, 36, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Aydin, I.H.; Takci, E.; Kadioglu, H.H.; Tuzun, Y.; Kayaoglu, C.R.; Barlas, E. Vascular variotions associated with anterior communicating artery aneurysms—An intraoperative study. Minim. Invasive Neurosusrg. 1997, 40, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Krzyżewski, R.M.; Tomaszewski, K.A.; Kochana, M.; Kopeć, M.; Klimek-Piotrowska, W.; Walocha, J.A. Anatomical variations of the anterior communicating artery complex: Gender relationship. Surg. Radiol. Anat. 2015, 37, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, L.; Li, W.; Zhang, G.; He, X.; Wang, G.; Li, M.; Qi, S. Relationship between the morphology of A-1 segment of anterior cerebral artery and anterior communicating artery aneurysms. Afr. Health Sci. 2014, 14, 83–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, G.; Yuan, Q.; Yang, J.; Yeo, J.H. Experimental study of hemodynamics in the circle of Willis. Biomed. Eng. Online 2015, 14, S10. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kikuchi, H.; Hashimoto, N.; Kojima, M.; Kang, Y.; Hazama, F. Involvement of internal elastic lamina in development of induced cerebral aneurysms in rats. Stroke 1988, 19, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.M.; Chang, W.C.; Liao, C.C. Experimental study of steady and pulsatile flows in cerebral aneurysm model of various sizes at branching site. ASME J. Biomech. Eng. 1997, 119, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, T.; Morgan, M.K.; Faulder, K.; Ingebrigtsen, L.; Sparr, T.; Schirmer, H. Bifurcation geometry and the presence of cerebral artery aneurysms. J. Neurosurg. 2004, 101, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, J.; van Raamt, A.F.; van der Graaf, Y.; Mali, W.P.; van der Ground, J. Distribution of cerebral blood flow in the circle of Willis. Radiology 2005, 235, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Dong, W.; Cai, X.; Zhang, Z.; Zhang, L.; Gao, F.; Kang, X.; Li, J.; Wang, H.; Gao, N.; et al. CTA Characteristics of the Circle of Willis and Intracranial Aneurysm in a Chinese Crowd with Family History of Stroke. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Hosein, M.; Karimi, A.; Ghojazadeh, M.; Teymouri, A. Evaluating the circle of Willis aneurysms location and relationship with its variations by multi detector CT Angiography. J. Am. Sci. 2013, 9, 86–90. [Google Scholar]
- Ersin, E.; Alper, B.; Onder, O.; Yusuf, I.; Erdener, T. Haemodynamic effect on the growth of experimentally indiced saccular aneurysms in rats. Ann. Neurosurg. 2002, 2, 1–6. [Google Scholar]
- Ren, Y.; Chen, Q.; Li, Z.Y. A 3D numerical study of the collateral capacity of the circle of Willis with anatomical variation in the posterior circulation. Biomed. Eng. Online 2015, 14, S11. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, N.K.; Velthuis, B.K.; Algra, A.; Rinkel, G.J. Configuration of the circle of Willis, direction of flow, and shape of the aneurysm as risk factors for rupture of intracranial aneurysms. J. Neurol. 2009, 256, 45–50. [Google Scholar] [CrossRef] [PubMed]
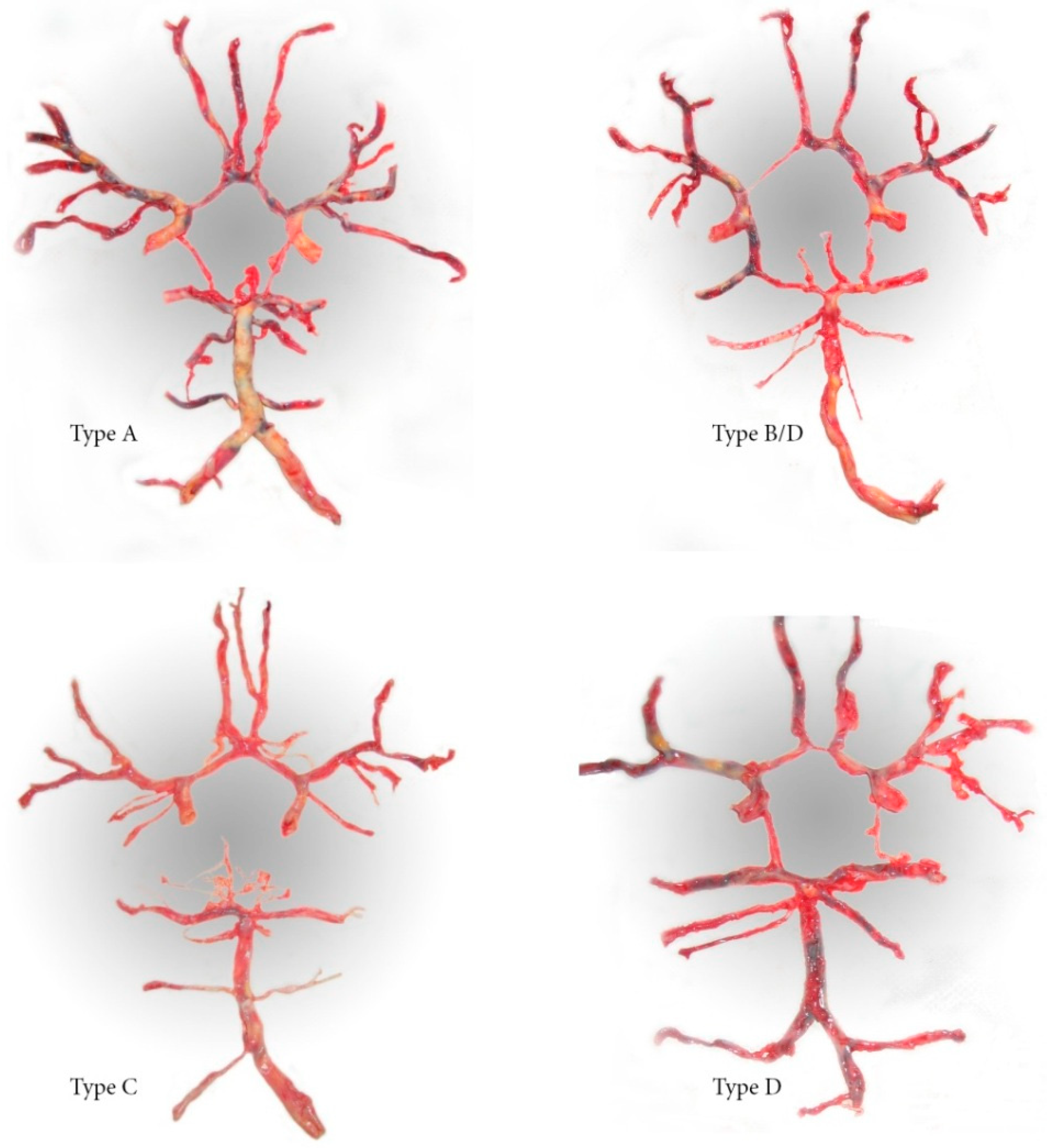
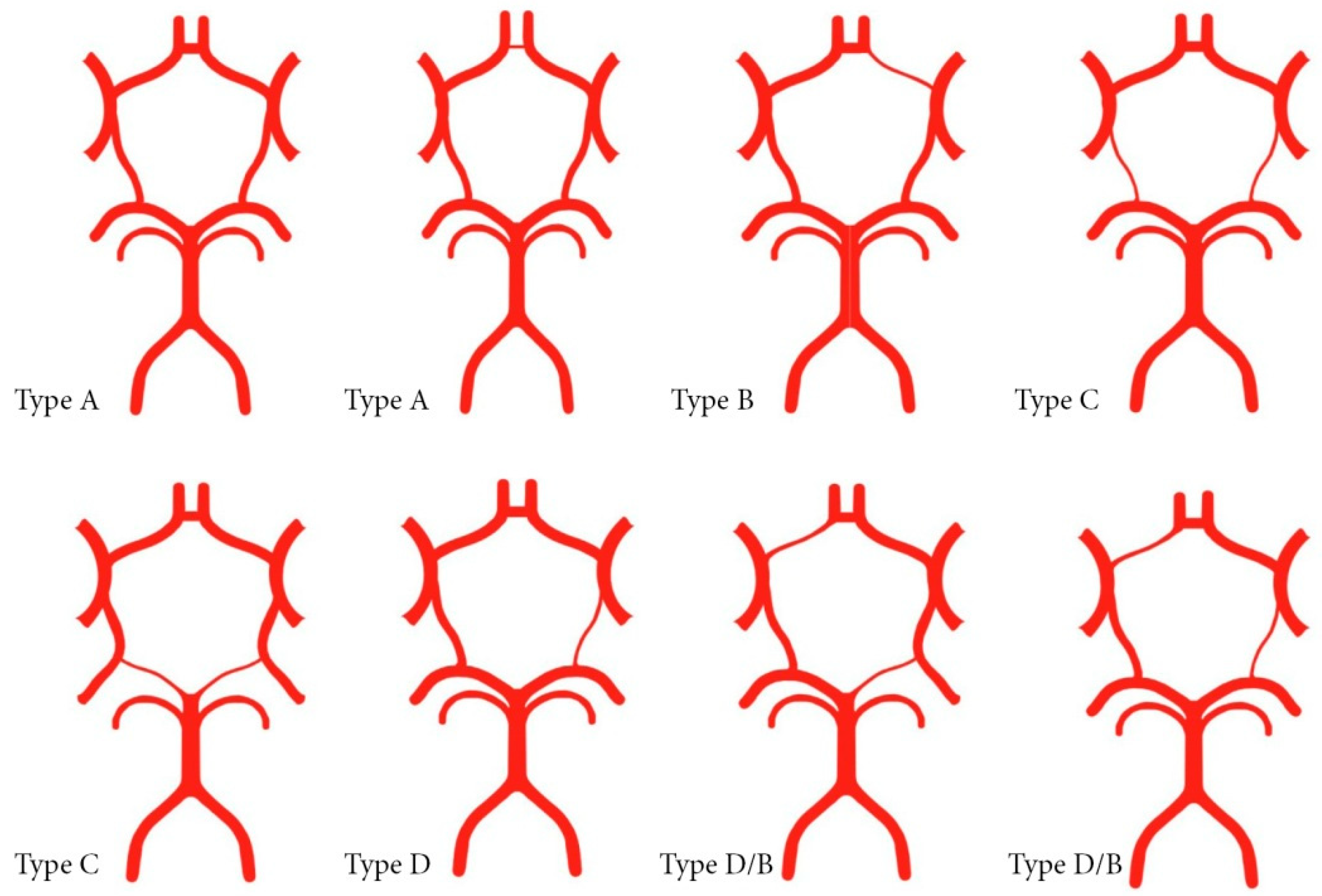
| Configuration of the Circle of Willis | Autopsied Patients n = 56 | Surgically Treated Patients n = 114 | p-Values 1 | |
|---|---|---|---|---|
| Symmetric Willis—Type A and Type C | 41(73.2%) | 41 (36.0%) | <0.001 | |
| Asymmetric Willis—Type B and Type D | 15 (26.8%) | 73 (64.0%) | ||
| Asymmetric Willis—Type B | Changes in the A1 segment | 9(16.0%) | 50 (43.9 %) | 0.737 |
| Asymmetric Willis—Type D | Changes in the posterior segments | 6(10.8%) | 23 (20.2%) | |
| Type Circle of Willis | Autopsied Patients | Surgically Treated Patients | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| Solitary Aneurysm | Multiple Aneurysms | ||||||
| Type A | 27 | (48.2%) | 20 | (24.7%) 2 | 5 | (15.2%) 2 | 0.001 |
| Type B | 4 | (16.0%) | 32 | (42.0%) 2 | 15 | (51.5%) 2 | <0.001 |
| subtype B/D | 5 | 2 | 2 | ||||
| Type C | 14 | (25.0%) | 13 | (16.0%) | 3 | (9%) | 0.143 |
| Type D | 6 | (10.8%) | 14 | (17.3%) | 8 | (24.3%) | 0.242 |
| Σ | 56 | (100%) | 81 | (100.0%) | 33 | (100%) | |
| Types Willis | Multiple Aneurysms Locations of Ruptured Aneurysms | Σ | |||||
| ACoA | MCA | ICA | PCoA | perA | VBA | ||
| Type A | 0 | 3 | 1 | 1 | 0 | 0 | 5 (15.2%) |
| Type B | 12 | 0 | 3 | 0 | 0 | 0 | 17 (51.5%) |
| Sub type B/D | 0 | 0 | 0 | 2 | 0 | 0 | |
| Type C | 0 | 1 | 2 | 0 | 0 | 0 | 3 (9.0%) |
| Type D | 0 | 6 | 0 | 2 | 0 | 0 | 8 (24.3%) |
| Σ | 12 | 10 | 6 | 5 | 0 | 0 | 33 (100.0%) |
| Type Willis | Locations of Solitary Ruptured Aneurysms | Σ | |||||
| ACoA | MCA | ICA | PCoA | perA | VBA | ||
| Type A | 9 | 5 | 2 | 2 | 1 | 1 | 20 (24.7%) |
| Type B | 24 | 3 | 2 | 0 | 3 | 0 | 34 (42.0%) |
| subtype B/D | 0 | 0 | 0 | 2 | 0 | 0 | |
| Type C | 0 | 8 | 2 | 2 | 0 | 1 | 13 (16.0%) |
| Type D | 0 | 4 | 24 | 6 | 0 | 0 | 14 (17.3%) |
| Σ | 33 | 20 | 10 | 12 | 4 | 2 | 81 (100.0%) |
| Locations Ruptured Aneurysms | Ruptured Aneurysm in the Surgically Treated Group | Hypoplasia A1 Right | Hypoplasia A1 Left | p-Value 1 |
|---|---|---|---|---|
| ACoA | 36 (72%) | 25 (80.6%) | 11 (57.9%) | 0.157 |
| MCA | 3 (6%) | 1 (3.2%) | 2 (10.5%) | 0.549 2 |
| ICA | 5 (10%) | 1 (3.2%) | 4 (21.1%) | 0.062 2 |
| PCoA | 3 (6%) | 2 (6.5%) | 1 (5.3%) | 1.000 2 |
| perA | 3 (6%) | 2 (6.5%) | 1 (5.3%) | 1.000 2 |
| Surgically treated group | 50 (43.8%) 3 | 31 (62%) | 19 (38%) | <0.001 1,4 |
| Autopsied group | 9 (16.1%) 3 | 5 (55.6%) | 4 (44.4%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, N.N.; Kostić, A.; Mitić, R.; Berilažić, L.; Radisavljević, M. Association between Circle of Willis Configuration and Rupture of Cerebral Aneurysms. Medicina 2019, 55, 338. https://doi.org/10.3390/medicina55070338
Stojanović NN, Kostić A, Mitić R, Berilažić L, Radisavljević M. Association between Circle of Willis Configuration and Rupture of Cerebral Aneurysms. Medicina. 2019; 55(7):338. https://doi.org/10.3390/medicina55070338
Chicago/Turabian StyleStojanović, Nebojša N., Aleksandar Kostić, Radisav Mitić, Luka Berilažić, and Miša Radisavljević. 2019. "Association between Circle of Willis Configuration and Rupture of Cerebral Aneurysms" Medicina 55, no. 7: 338. https://doi.org/10.3390/medicina55070338
APA StyleStojanović, N. N., Kostić, A., Mitić, R., Berilažić, L., & Radisavljević, M. (2019). Association between Circle of Willis Configuration and Rupture of Cerebral Aneurysms. Medicina, 55(7), 338. https://doi.org/10.3390/medicina55070338





