The Antiproliferative and Apoptotic Effects of Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin, ORL-48
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Capsaicin Compound Preparation
2.3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.4. Fluorescent Microscopy
2.5. Flow Cytometric Analysis of Apoptosis by FITC-Annexin V/PI
2.6. Determination of Caspase Activities
2.7. Measurement of Mitochondrial Membrane Potential (Δψm) Assay
2.8. Cell Cycle Analysis Using Flow Cytometry
2.9. Statistical Analysis
3. Results
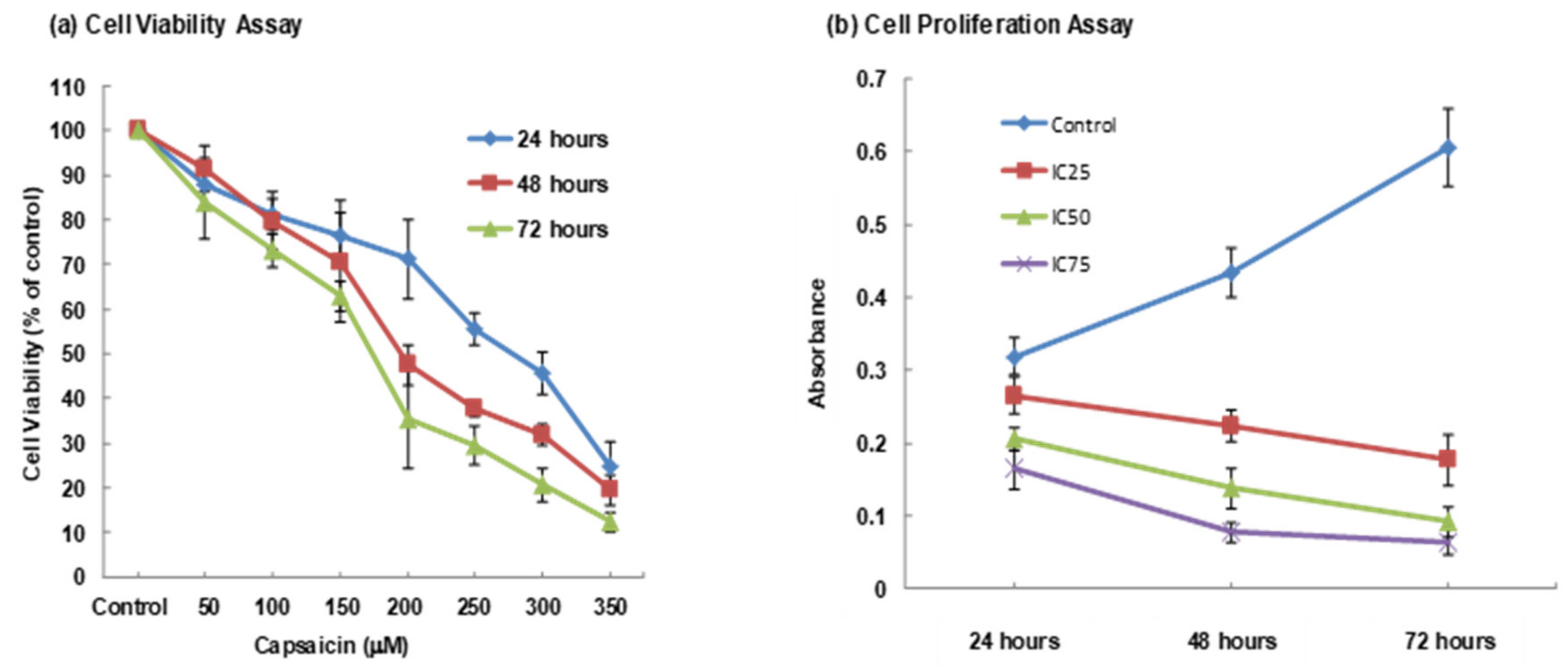
3.1. MTT Assay
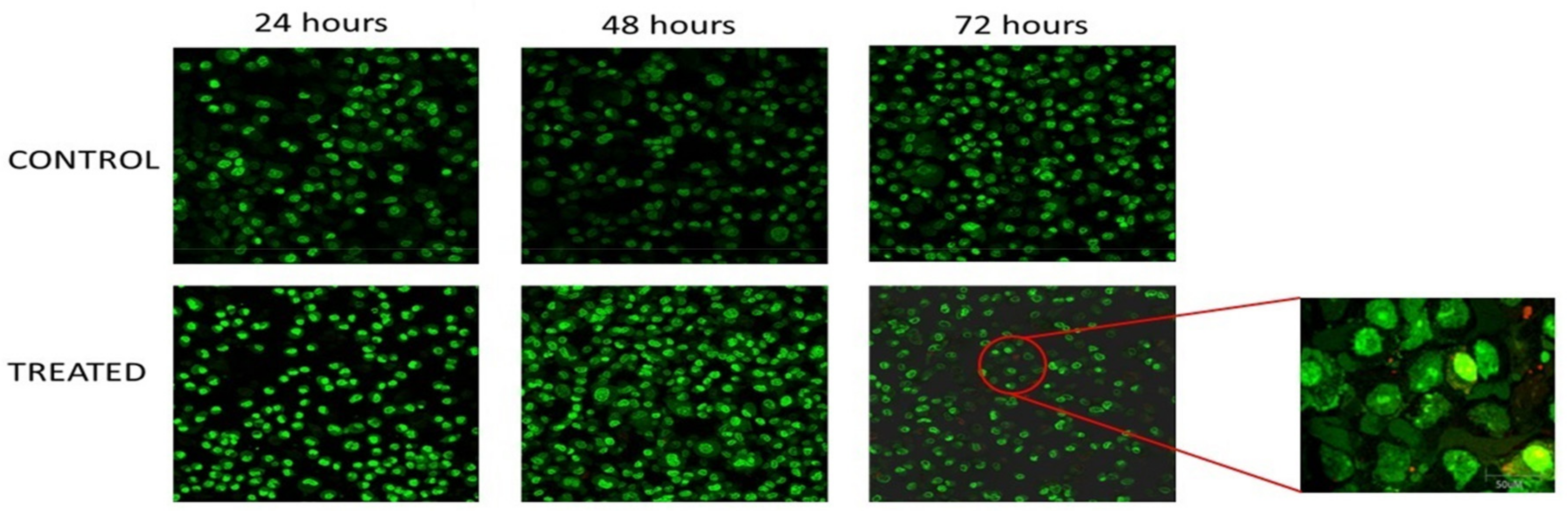
3.2. Morphological Analysis
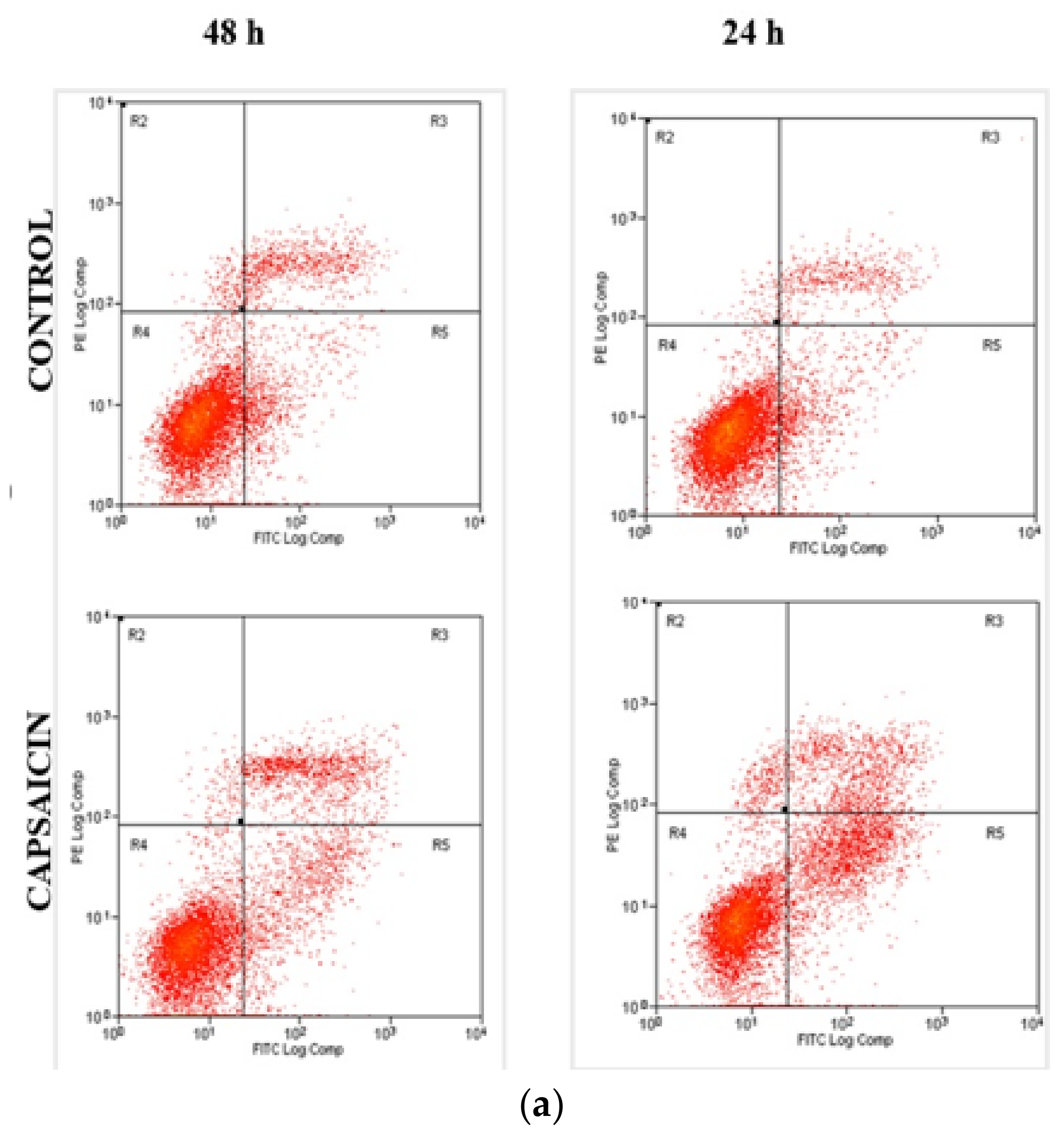
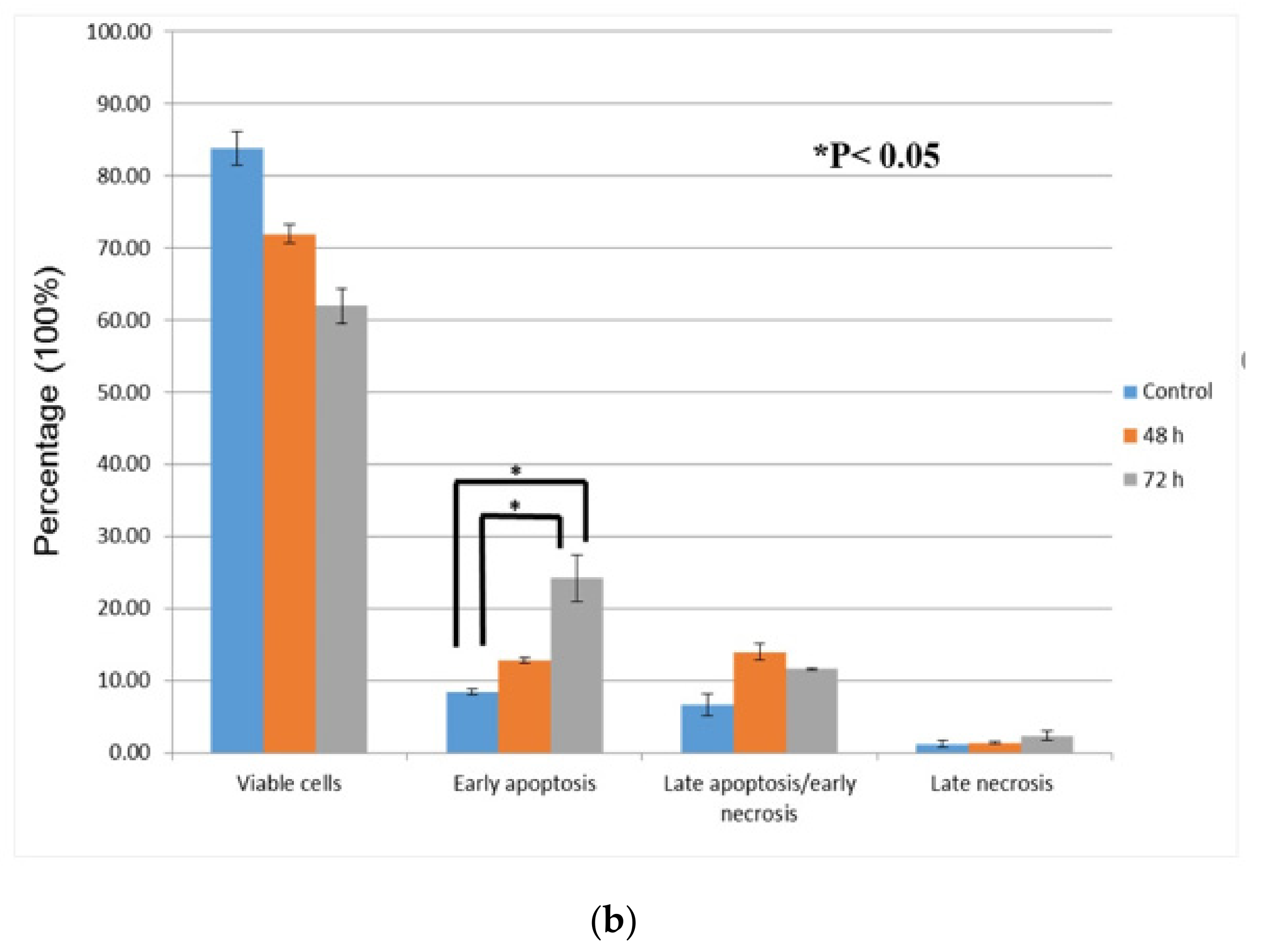
3.3. Quantification of Apoptotic/Necrotic Cells by FITC-Annexin V/PI
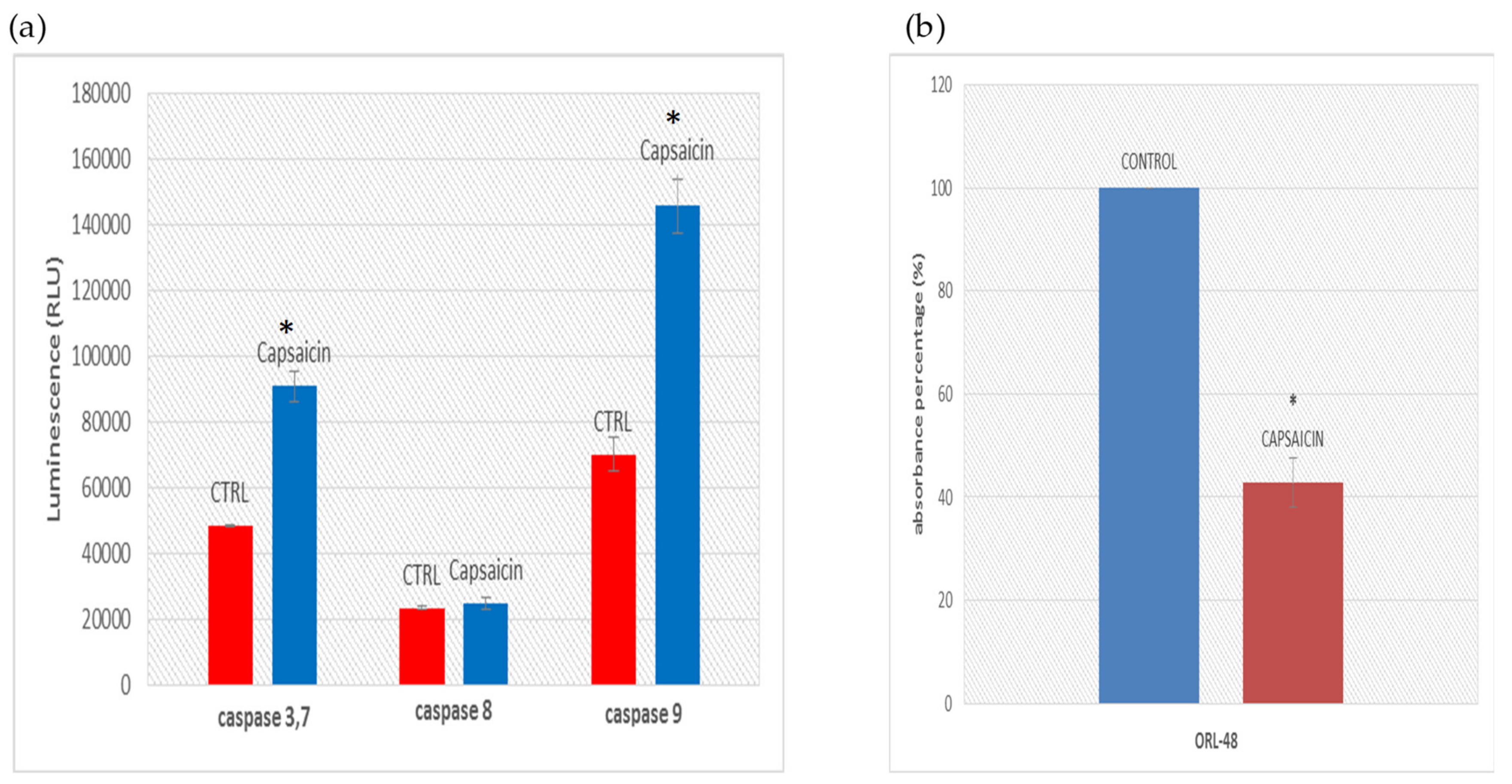
3.4. Determination of Caspase Activities and Disruption of Mitochondrial Membrane Potential
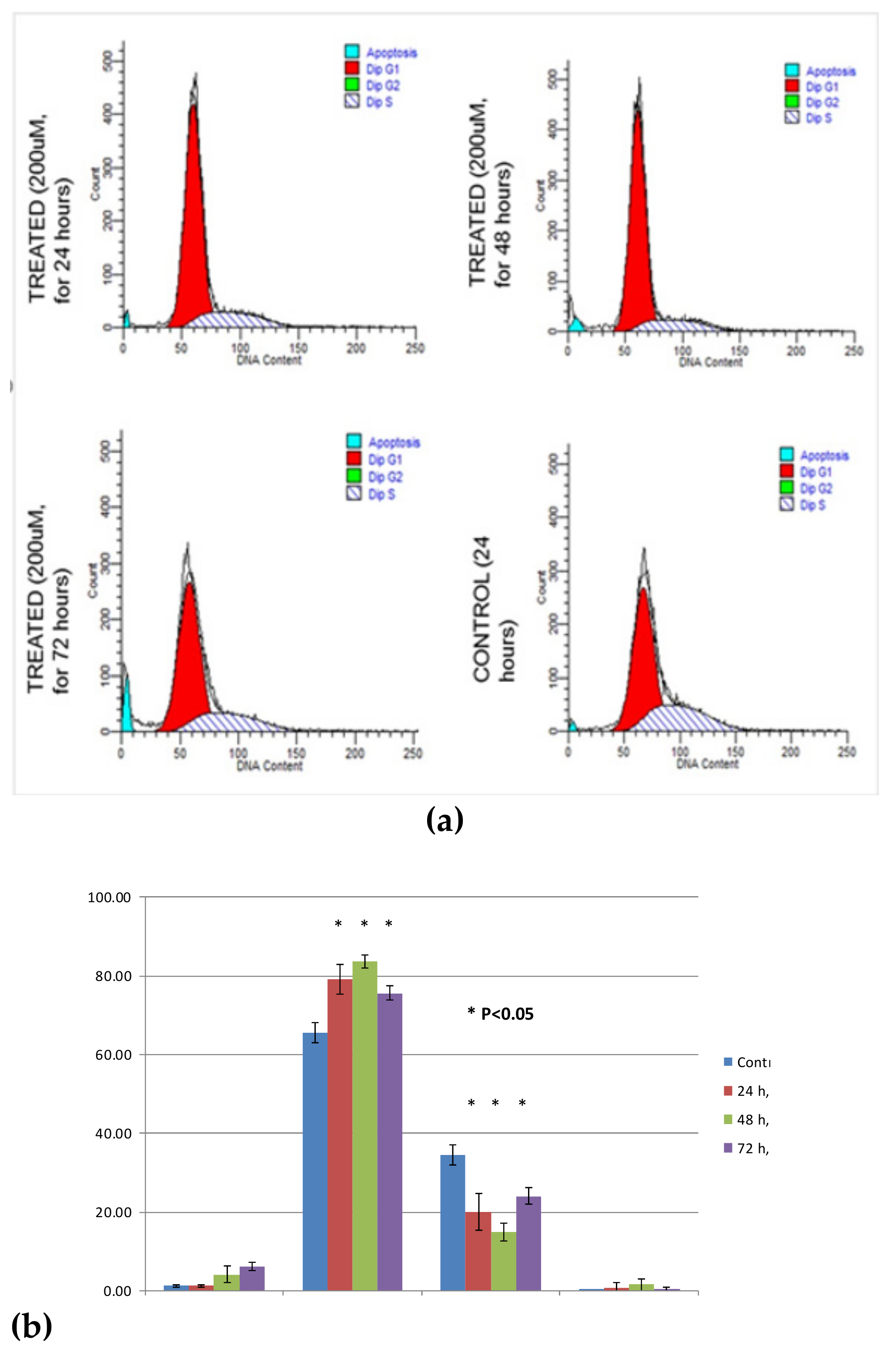
3.5. Cell Cycle Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, S.K.; Rao, P.S. Alteration in the Radiosensitivity of HeLa Cells by Dichloromethane Extract of Guduchi (Tinospora cordifolia). Integr. Cancer Ther. 2010, 9, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N. Tobacco use and oral cancer: A global perspective. J. Dent. Educ. 2001, 65, 328–339. [Google Scholar] [PubMed]
- Seitz, H.K.; Stickel, F. Acetaldehyde as an underestimated risk factor for cancer development: Role of genetics in ethanol metabolism. Genes Nutr. 2009, 5, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bakri, M.M.; Cannon, R.; Holmes, A.R.; Rich, A.M. Detection of Candida albicans ADH1 and ADH2 mRNAs in human archival oral biopsy samples. J. Oral Pathol. Med. 2014, 43, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Enquist, I.B.; Good, Z.; Jubb, A.M.; Fuh, G.; Wang, X.; Junttila, M.R.; Jackson, E.L.; Leong, K.G. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nat. Commun. 2014, 5, 3530. [Google Scholar] [CrossRef] [PubMed]
- Warnakulsuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Ferlay, J.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M.; Shin, H.-R.; Shin, H. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Lee, S.H.; Tang, Y.Q.; Jaganath, I.B.; Sekaran, S. Phyllanthus, a broad spectrum anticancer agent: Properties and mechanisms of activities. J. Health Transl. Med. 2013, 16, 26. [Google Scholar]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting Apoptosis Pathways in Cancer and Perspectives with Natural Compounds from Mother Nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef]
- Lodi, A.; Saha, A.; Lu, X.; Wang, B.; Sentandreu, E.; Collins, M.; Kolonin, M.G.; DiGiovanni, J.; Tiziani, S. Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. NPJ Precis. Oncol. 2017, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H.; Dolphin, A.C. Unravelling the Mystery of Capsaicin: A Tool to Understand and Treat Pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.; Simkhovich, B.Z.; Hale, S.L.; Kay, G.; Kloner, R.A. Capsaicin-induced cardioprotection. Is hypothermia or the salvage kinase pathway involved? Cardiovasc. Drug Ther. 2014, 28, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Ando, H.; Unno, S.; Masuda, Y.; Kitagawa, J. Activation of TRPV1 and TRPM8 Channels in the Larynx and Associated Laryngopharyngeal Regions Facilitates the Swallowing Reflex. Int. J. Mol. Sci. 2018, 19, 4113. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Bakri, M.M.; Yahya, F.; Ando, H.; Unno, S.; Kitagawa, J. The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain. Int. J. Mol. Sci. 2019, 20, 526. [Google Scholar] [CrossRef] [PubMed]
- Zakir, H.M.; Mostafeezur, R.M.; Suzuki, A.; Hitomi, S.; Suzuki, I.; Maeda, T.; Seo, K.; Yamada, Y.; Yamamura, K.; Lev, S.; et al. Expression of TRPV1 Channels after Nerve Injury Provides an Essential Delivery Tool for Neuropathic Pain Attenuation. PLoS ONE 2012, 7, e44023. [Google Scholar] [CrossRef] [PubMed]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A Comprehensive Review of the Carcinogenic and Anticarcinogenic Potential of Capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lu, W.-C.; Wang, C.-W.; Chan, Y.-C.; Chen, M.-K. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement. Altern. Med. 2013, 13, 46. [Google Scholar] [CrossRef]
- Lee, S.H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of β-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef]
- Chou, C.-C.; Wu, Y.-C.; Wang, Y.-F.; Chou, M.-J.; Kuo, S.-J.; Chen, D.-R. Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol. Rep. 2009, 21, 665–671. [Google Scholar]
- Kim, C.-S.; Park, W.-H.; Park, J.-Y.; Kang, J.-H.; Kim, M.-O.; Kawada, T.; Yoo, H.; Han, I.-S.; Yu, R. Capsaicin, a Spicy Component of Hot Pepper, Induces Apoptosis by Activation of the Peroxisome Proliferator-Activated Receptor γ in HT-29 Human Colon Cancer Cells. J. Med. Food 2004, 7, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Kang, H.J.; Moon, A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2011, 165, 139–145. [Google Scholar] [CrossRef]
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin Displays Anti-Proliferative Activity against Human Small Cell Lung Cancer in Cell Culture and Nude Mice Models via the E2F Pathway. PLoS ONE 2010, 5, e10243. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Lim, K.; Zain, R.; Ismail, S.; Lau, S.; Mustafa, W.; Abraham, M.; Nam, N.; Teo, S.-H.; Cheong, S. Establishment and characterization of Asian oral cancer cell lines as in vitro models to study a disease prevalent in Asia. Int. J. Mol. Med. 2007, 19, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Lee, S. Capsaicin in hot chili pepper: Carcinogen, co-carcinogen or anticarcinogen? Food Chem. Toxicol. 1996, 34, 313–316. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Chiloeches, A.; Díaz-Laviada, I.; Vara-Ciruelos, D. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis 2007, 12, 2013–2024. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic of biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef]
- Fan, T.-J.; Han, L.-H.; Cong, R.-S.; Liang, J. Caspase Family Proteases and Apoptosis. Acta Biochim. et Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Vitale, M.; Zamai, L.; Mazzotti, G.; Cataldi, A.; Falcieri, E. Differential kinetics of propidium iodide uptake in apoptotic and necrotic thymocytes. Histochem. Cell Boil. 1993, 100, 223–229. [Google Scholar] [CrossRef]
- Fadok, V.; Voelker, D.R.; A Campbell, P.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207. [Google Scholar] [PubMed]
- Demchenko, A.P. Beyond annexin V: Fluorescence response of cellular membranes to apoptosis. Cytotechnology 2013, 65, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: the executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A Basic Physiologic Process in Wound Healing. Int. J. Low. Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψm) apoptosis; An update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Sun, W.L. Ambra1 in autophagy and apoptosis: Implications for cell survival and chemotherapy resistance (Review). Oncology Lett. 2016, 12, 367–374. [Google Scholar] [CrossRef]
- Fried, J.; Perez, A.G.; Clarkson, B.D. Flow cytofluorometric of cell cycle distributions using propidium iodide. Properties of the method and mathematical anaslyis of the data. J. Cell Biol. 1976, 7, 172. [Google Scholar] [CrossRef]
- Nunez, R. DNA measurement and cell cycle analysis by flow cytometry. Curr. Issues Mol. Biol. 2001, 3, 67–70. [Google Scholar]
- Bertoli, C.; Skotheim, J.M.; De Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Jin, J.; Lin, G.; Huang, H.; Xu, D.; Yu, H.; Ma, X.; Zhu, L.; Ma, D.; Jiang, H. Capsaicin Mediates Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells via Stabilizing and Activating p53. Int. J. Biol. Sci. 2014, 10, 285–295. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaruddin, M.F.; Hossain, M.Z.; Mohamed Alabsi, A.; Mohd Bakri, M. The Antiproliferative and Apoptotic Effects of Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin, ORL-48. Medicina 2019, 55, 322. https://doi.org/10.3390/medicina55070322
Kamaruddin MF, Hossain MZ, Mohamed Alabsi A, Mohd Bakri M. The Antiproliferative and Apoptotic Effects of Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin, ORL-48. Medicina. 2019; 55(7):322. https://doi.org/10.3390/medicina55070322
Chicago/Turabian StyleKamaruddin, Mohammad Firdaus, Mohammad Zakir Hossain, Aied Mohamed Alabsi, and Marina Mohd Bakri. 2019. "The Antiproliferative and Apoptotic Effects of Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin, ORL-48" Medicina 55, no. 7: 322. https://doi.org/10.3390/medicina55070322
APA StyleKamaruddin, M. F., Hossain, M. Z., Mohamed Alabsi, A., & Mohd Bakri, M. (2019). The Antiproliferative and Apoptotic Effects of Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin, ORL-48. Medicina, 55(7), 322. https://doi.org/10.3390/medicina55070322





