Association between P2X7 Polymorphisms and Susceptibility to Tuberculosis: An Updated Meta-Analysis of Case-Control Studies
Abstract
1. Introduction
2. Methods
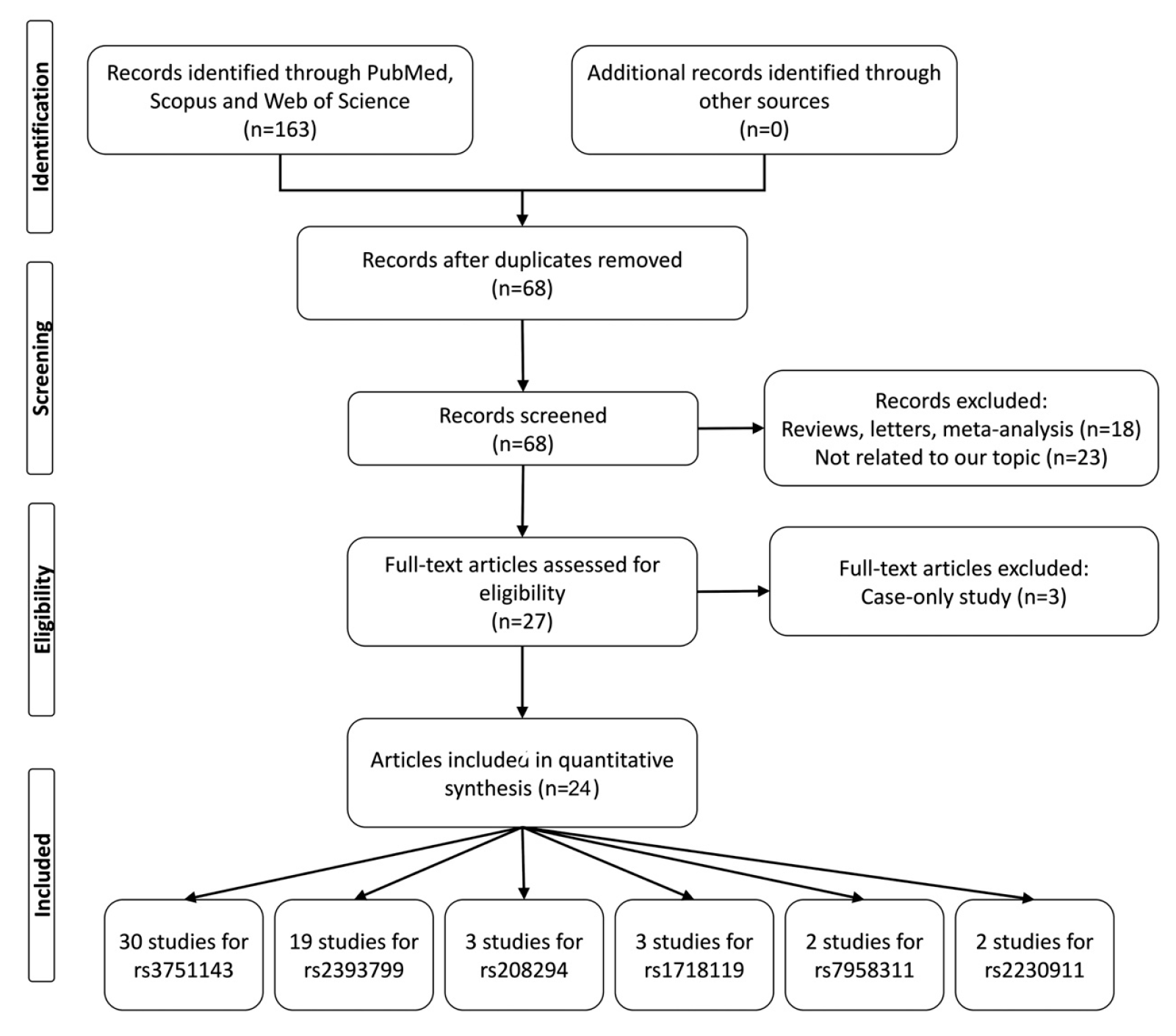
2.1. Literature Search
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Study Characteristics
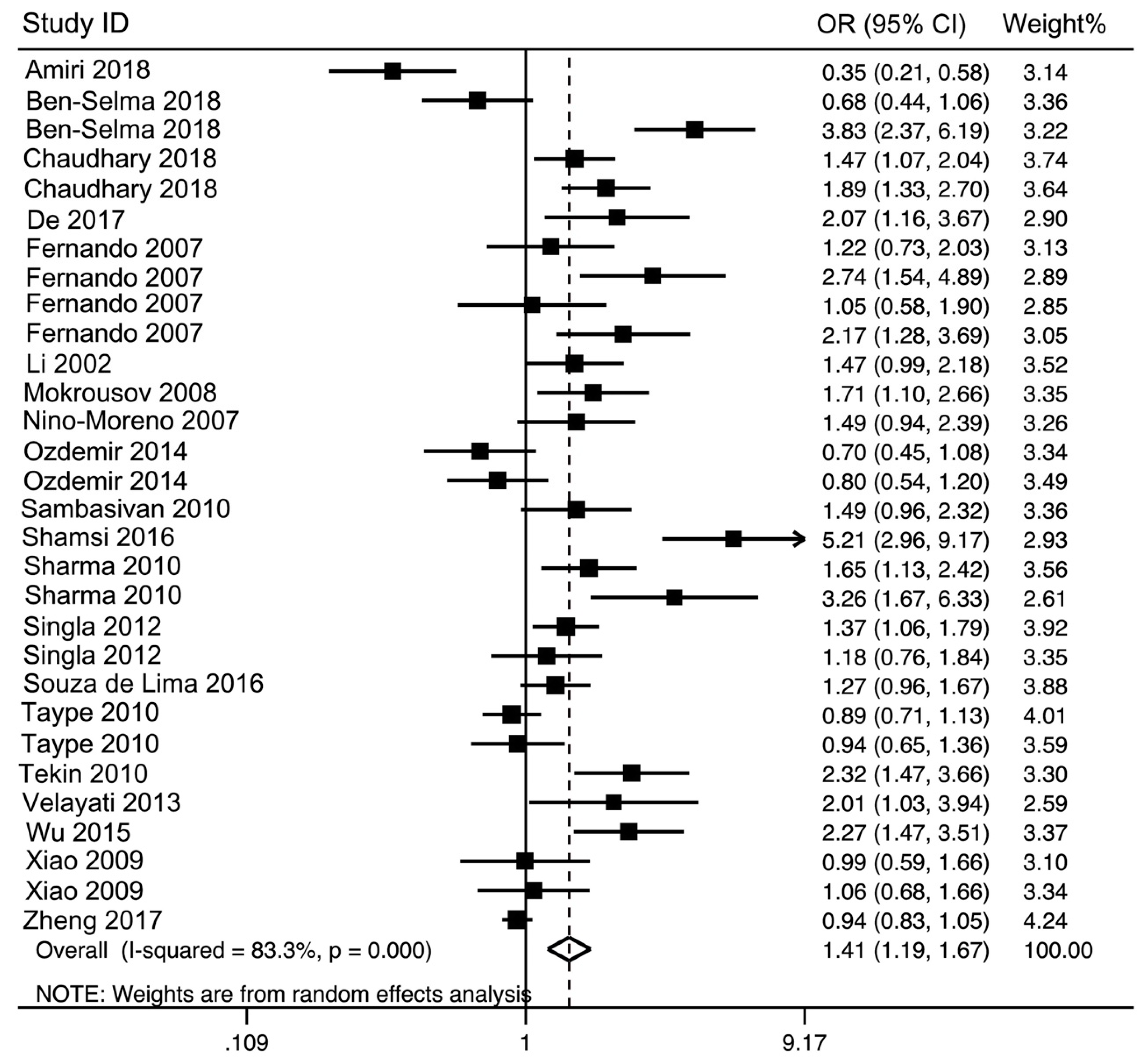
3.2. Main Analysis Results
3.3. Subgroup Analysis Results
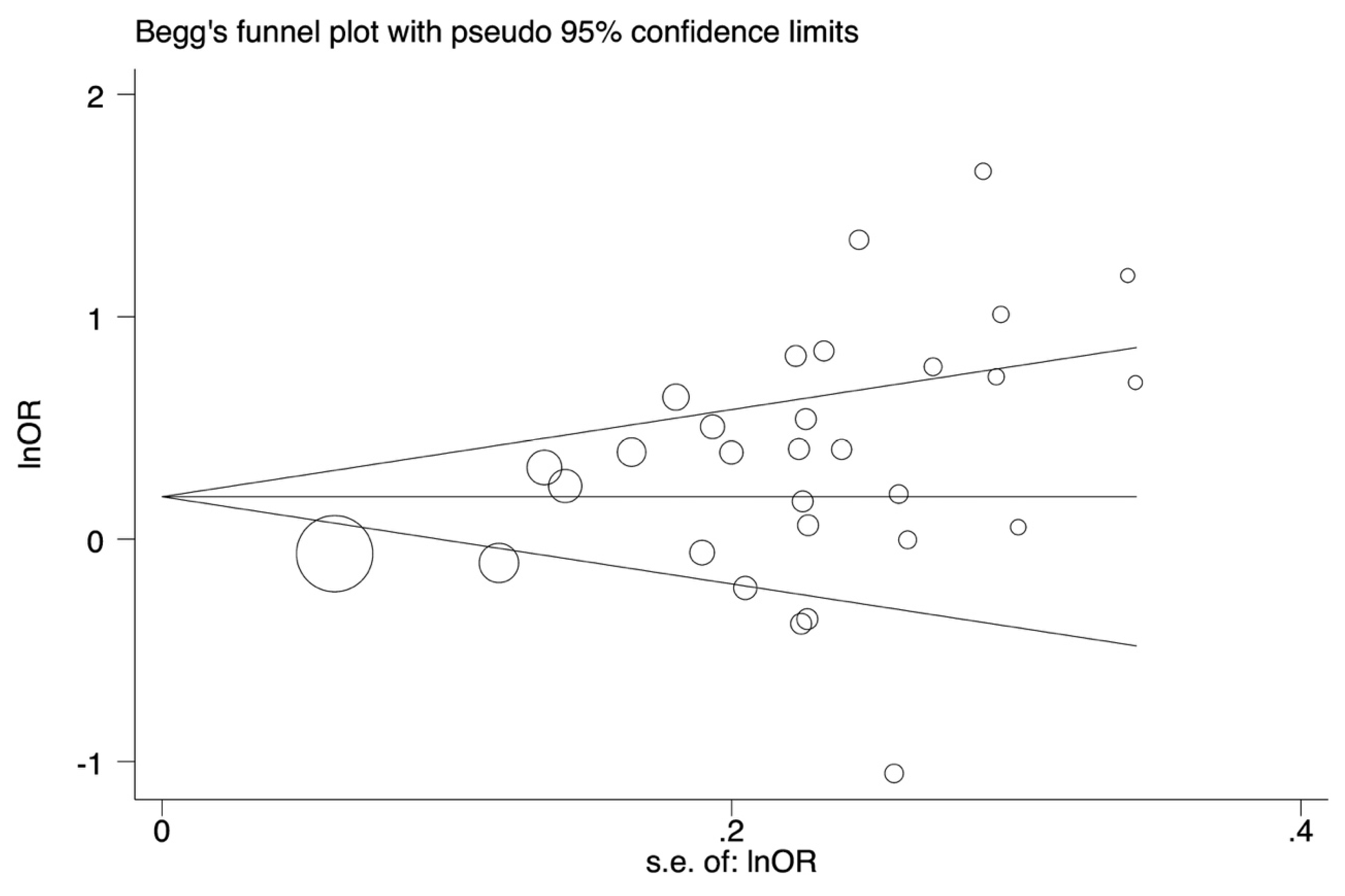
3.4. Heterogeneity and Publication Bias
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Trebucq, A.; Schwoebel, V. Numbers of tuberculosis cases: Dreams and reality. Int. J. Tuberc. Lung Dis. 2016, 20, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Raviglione, M.; Hafner, R.; von Reyn, C.F. Tuberculosis. N. Engl. J. Med. 2013, 368, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Hashemi, M.; Ansari, H. Macrophage migration inhibitory factor −173 G > C polymorphism and risk of tuberculosis: A meta-analysis. EXCLI J. 2017, 16, 313–320. [Google Scholar] [PubMed]
- Naderi, M.; Hashemi, M.; Mirshekari, H.; Bahari, G.; Taheri, M. Toll-like Receptor 1 Polymorphisms Increased the Risk of Pulmonary Tuberculosis in an Iranian Population Sample. Biomed. Environ. Sci. 2016, 29, 825–828. [Google Scholar] [PubMed]
- Qiu, Y.; Cao, S.; Gou, C.; Yue, Y.; Jiang, S.; Ma, T.; Xue, X. Associations of tumor necrosis factor-alpha polymorphisms with the risk of tuberculosis: A meta-analysis. Scand. J. Immunol. 2018, e12719. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Miller, C.M.; Boulter, N.R.; Fuller, S.J.; Zakrzewski, A.M.; Lees, M.P.; Saunders, B.M.; Wiley, J.S.; Smith, N.C. The role of the P2X7 receptor in infectious diseases. PLoS Pathog. 2011, 7, e1002212. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; North, R.A. P2X receptors as cell-surface ATP sensors in health and disease. Nature 2006, 442, 527–532. [Google Scholar] [CrossRef]
- Placido, R.; Auricchio, G.; Falzoni, S.; Battistini, L.; Colizzi, V.; Brunetti, E.; Di Virgilio, F.; Mancino, G. P2X7 purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cell. Immunol. 2006, 244, 10–18. [Google Scholar] [CrossRef]
- Fairbairn, I.P.; Stober, C.B.; Kumararatne, D.S.; Lammas, D.A. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X7-dependent process inducing bacterial death by phagosome-lysosome fusion. J. Immunol. 2001, 167, 3300–3307. [Google Scholar] [CrossRef]
- Amiri, A.; Sabooteh, T.; Ahmadi, S.A.Y.; Azargoon, A.; Shahsavar, F. Association of P2X7 gene common polymorphisms with pulmonary tuberculosis in Lur population of Iran. Egypt. J. Med Hum. Genet. 2018, 19, 231–234. [Google Scholar] [CrossRef]
- Ben-Selma, W.; Ben-Kahla, I.; Boukadida, J.; Harizi, H. Contribution of the P2X7 1513A/C loss-of-function polymorphism to extrapulmonary tuberculosis susceptibility in Tunisian populations. FEMS Immunol. Med. Microbiol. 2011, 63, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Singh, J.P.; Sehajpal, P.K.; Sarin, B.C. P2X7 receptor polymorphisms and susceptibility to tuberculosis in a North Indian Punjabi population. Int. J. Tuberc. Lung Dis. 2018, 22, 884–889. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Kundu, J.K. Tuberculosis risk in P2X7 1513A/C polymorphism of the tribes of Jhargram, West Bengal. Int. J. Zool. Stud. 2017, 2, 189–193. [Google Scholar]
- Fernando, S.L.; Saunders, B.M.; Sluyter, R.; Skarratt, K.K.; Goldberg, H.; Marks, G.B.; Wiley, J.S.; Britton, W.J. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 2007, 175, 360–366. [Google Scholar] [CrossRef]
- Li, C.M.; Campbell, S.J.; Kumararatne, D.S.; Bellamy, R.; Ruwende, C.; McAdam, K.P.; Hill, A.V.; Lammas, D.A. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J. Infect. Dis. 2002, 186, 1458–1462. [Google Scholar] [CrossRef]
- Mokrousov, I.; Sapozhnikova, N.; Narvskaya, O. Mycobacterium tuberculosis co-existence with humans: Making an imprint on the macrophage P2X7 receptor gene? J. Med. Microbiol. 2008, 57, 581–584. [Google Scholar] [CrossRef]
- Nino-Moreno, P.; Portales-Perez, D.; Hernandez-Castro, B.; Portales-Cervantes, L.; Flores-Meraz, V.; Baranda, L.; Gómez-Gómez, A.; Acuña-Alonzo, V.; Granados, J.; González-Amaro, R. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clin. Exp. Immunol. 2007, 148, 469–477. [Google Scholar] [CrossRef]
- Ozdemir, F.A.; Erol, D.; Konar, V.; Yuce, H.; Kara Senli, E.; Bulut, F.; Deveci, F. Lack of association of 1513 A/C polymorphism in P2X7 gene with susceptibility to pulmonary and extrapulmonary tuberculosis. Tuberkuloz ve toraks 2014, 62, 7–11. [Google Scholar] [CrossRef]
- Sambasivan, V.; Murthy, K.J.; Reddy, R.; Vijayalakshimi, V.; Hasan, Q. P2X7 gene polymorphisms and risk assessment for pulmonary tuberculosis in Asian Indians. Dis. Markers 2010, 28, 43–48. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, V.; Khosla, R.; Kajal, N.; Sarin, B.; Sehajpal, P. Association of P2X7 receptor +1513 (A-->C) polymorphism with tuberculosis in a Punjabi population. Int. J. Tuberc. Lung Dis. 2010, 14, 1159–1163. [Google Scholar] [PubMed]
- Singla, N.; Gupta, D.; Joshi, A.; Batra, N.; Singh, J. Genetic polymorphisms in the P2X7 gene and its association with susceptibility to tuberculosis. Int. J. Tuberc. Lung Dis. 2012, 16, 224–229. [Google Scholar] [CrossRef] [PubMed]
- De Lima, D.S.; Ogusku, M.M.; Sadahiro, A.; Pontillo, A. Inflammasome genetics contributes to the development and control of active pulmonary tuberculosis. Infect. Genet. Evol. 2016, 41, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Taype, C.A.; Shamsuzzaman, S.; Accinelli, R.A.; Espinoza, J.R.; Shaw, M.A. Genetic susceptibility to different clinical forms of tuberculosis in the Peruvian population. Infect. Genet. Evol. 2010, 10, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Tekin, D.; Kayaalti, Z.; Dalgic, N.; Cakir, E.; Soylemezoglu, T.; Kutlubay, B.I.; Kilic, B.A. Polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis in Turkish children. Pediatr. Infect. Dis. J. 2010, 29, 779–782. [Google Scholar] [CrossRef]
- Velayati, A.A.; Farnia, P.; Farahbod, A.M.; Karahrudi, M.A.; Derakhshaninezhad, Z.; Kazampour, M.; Sheikhghomi, S.; Saeif, S. Association of receptors, purinergic P2X7 and tumor necrosis factor-alpha gene polymorphisms in susceptibility to tuberculosis among Iranian patients. Arch. Clin. Infect. Dis. 2013. [Google Scholar] [CrossRef]
- Wu, J.; Lu, L.; Zhang, L.; Ding, Y.; Wu, F.; Zuo, W.; Zhang, W. Single Nucleotide Polymorphisms in P2X7 Gene Are Associated with Serum Immunoglobulin G Responses to Mycobacterium tuberculosis in Tuberculosis Patients. Dis. Markers 2015, 2015, 671272. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Sun, L.; Jiao, W.; Li, Z.; Zhao, S.; Li, H.; Jin, J.; Jiao, A.; Guo, Y.; Jiang, Z.; et al. Lack of association between polymorphisms in the P2X7 gene and tuberculosis in a Chinese Han population. FEMS Immunol. Med. Microbiol. 2009, 55, 107–111. [Google Scholar] [CrossRef]
- Zheng, X.; Li, T.; Chen, Y.; Pan, H.; Zhang, Z.; Dai, Y.; Wang, J. Genetic polymorphisms of the P2X7 gene associated with susceptibility to and prognosis of pulmonary tuberculosis. Infect. Genet. Evol. 2017, 53, 24–29. [Google Scholar] [CrossRef]
- Bahari, G.; Hashemi, M.; Taheri, M.; Naderi, M.; Moazeni-Roodi, A.; Kouhpayeh, H.R.; Eskandari-Nasab, E. Association of P2X7 gene polymorphisms with susceptibility to pulmonary tuberculosis in Zahedan, Southeast Iran. Genet. Mol. Res. GMR 2013, 12, 160–166. [Google Scholar] [CrossRef]
- Shamsi, M.; Zolfaghari, M.R.; Farnia, P. Association of IFN-gamma and P2X7 Receptor Gene Polymorphisms in Susceptibility to Tuberculosis among Iranian Patients. Acta Microbiol. Immunol. Hung. 2016, 63, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Songane, M.; Kleinnijenhuis, J.; Alisjahbana, B.; Sahiratmadja, E.; Parwati, I.; Oosting, M.; Plantinga, T.S.; Joosten, L.A.; Netea, M.G.; Ottenhoff, T.H.; et al. Polymorphisms in autophagy genes and susceptibility to tuberculosis. PLoS ONE 2012, 7, e41618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tan, C.Y.; Mo, Z.J.; Gao, Q.L.; He, D.; Li, J.; Huang, R.F.; Li, Y.B.; Guo, C.F.; Guo, Q.; et al. P2X7 receptor in spinal tuberculosis: Gene polymorphisms and protein levels in Chinese Han population. Infect. Genet. Evol. 2018, 57, 138–144. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, W.; Ren, G.; He, X.; Hu, Q.; Zhang, Y.; Kang, L.; Yuan, D.; Jin, T. P2X7R Gene Polymorphisms are Associated with Increased Risk of Pulmonary Tuberculosis in the Tibetan Chinese Population. Am. J. Trop. Med. Hyg. 2016, 95, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.B.; Chen, S. A meta-analysis of P2X7 gene-1513A/C polymorphism and pulmonary tuberculosis susceptibility. Hum. Immunol. 2016, 77, 126–130. [Google Scholar] [CrossRef]
- Alshammari, E.M.; Mandal, R.K.; Wahid, M.; Dar, S.A.; Jawed, A.; Areeshi, M.Y.; Khan, S.; Khan, M.E.A.; Panda, A.K.; Haque, S. Genetic association study of P2X7 A1513C (rs 3751143) polymorphism and susceptibility to pulmonary tuberculosis: A meta-analysis based on the findings of 11 case-control studies. Asian Pac. J. Trop. Med. 2016, 9, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Areeshi, M.Y.; Mandal, R.K.; Dar, S.; Wahid, M.; Khan, M.E.; Panda, A.K.; Jawed, A.; Haque, S. P2X7 1513 A>C Polymorphism Confers Increased Risk of Extrapulmonary Tuberculosis: A Meta-analysis of Case-Control Studies. Curr. Genom. 2015, 17, 450–458. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, M.; Gu, X.; Yao, Y.; Liu, H.; Song, Y. The effect of P2X7 receptor 1513 polymorphism on susceptibility to tuberculosis: A meta-analysis. Infect. Genet. Evol. 2014, 24, 82–91. [Google Scholar] [CrossRef]
- Yi, L.; Cheng, D.; Shi, H.; Huo, X.; Zhang, K.; Zhen, G. A meta-analysis of P2X7 gene-762T/C polymorphism and pulmonary tuberculosis susceptibility. PLoS ONE 2014, 9, e96359. [Google Scholar] [CrossRef]
- Wesselius, A.; Bours, M.J.; Arts, I.C.; Theunisz, E.H.; Geusens, P.; Dagnelie, P.C. The P2X7 loss-of-function Glu496Ala polymorphism affects ex vivo cytokine release and protects against the cytotoxic effects of high ATP-levels. BMC Immunol. 2012, 13, 64. [Google Scholar] [CrossRef]
- Cabrini, G.; Falzoni, S.; Forchap, S.L.; Pellegatti, P.; Balboni, A.; Agostini, P.; Cuneo, A.; Castoldi, G.; Baricordi, O.R.; Di Virgilio, F. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J. Immunol. 2005, 175, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Koduri, H.; Singh, R.; Koul, A.; Ghildiyal, M.; Sharma, K.; Tyagi, A.K.; Singh, Y. Nucleoside diphosphate kinase of Mycobacterium tuberculosis acts as GTPase-activating protein for Rho-GTPases. FEBS Lett. 2004, 571, 212–216. [Google Scholar] [CrossRef] [PubMed]

| First Author | Year | Country | Ethnicity | TB | Source of Control | Genotyping Method | Case/Control | Cases | Controls | HWE (p) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs3751143 A > C | AA | AC | CC | A | C | AA | AC | CC | A | C | ||||||||
| Amiri | 2018 | Iran | Asian | PTB | PB | PCR-RFLP | 100/100 | 76 | 21 | 3 | 173 | 27 | 40 | 58 | 2 | 138 | 62 | <0.001 |
| Ben-Selma | 2011 | Tunisia | African | PTB | HB | PCR-RFLP | 168/150 | 130 | 34 | 4 | 294 | 42 | 104 | 40 | 6 | 248 | 52 | 0.395 |
| Ben-Selma | 2011 | Tunisia | African | EPTB | HB | PCR-RFLP | 55/150 | 19 | 23 | 13 | 61 | 49 | 104 | 40 | 6 | 248 | 52 | 0.395 |
| Chaudhary | 2018 | India | Asian | PTB | PB | ARMS-PCR | 145/247 | 63 | 73 | 9 | 199 | 91 | 141 | 95 | 11 | 377 | 117 | 0.315 |
| Chaudhary | 2018 | India | Asian | EPTB | PB | ARMS-PCR | 100/247 | 42 | 42 | 16 | 126 | 74 | 141 | 95 | 11 | 377 | 117 | 0.315 |
| De | 2017 | India | Asian | PTB | PB | PCR-RFLP | 56/60 | 26 | 18 | 12 | 70 | 42 | 36 | 21 | 3 | 93 | 27 | 0.978 |
| Fernando | 2007 | Southeast Asia | Asian | PTB | PB | TaqMan | 56/167 | 34 | 17 | 5 | 85 | 27 | 105 | 55 | 7 | 265 | 69 | 0.952 |
| Fernando | 2007 | Southeast Asia | Asian | EPTB | PB | TaqMan | 30/167 | 9 | 17 | 4 | 35 | 25 | 105 | 55 | 7 | 265 | 69 | 0.952 |
| Fernando | 2007 | Australia | Caucasian | PTB | PB | TaqMan | 49/102 | 28 | 21 | 0 | 77 | 21 | 64 | 34 | 4 | 162 | 42 | 0.845 |
| Fernando | 2007 | Australia | Caucasian | EPTB | PB | TaqMan | 50/102 | 18 | 28 | 4 | 64 | 36 | 64 | 34 | 4 | 162 | 42 | 0.845 |
| Li | 2002 | Gambia | African | PTB | HB | PCR-RFLP | 325/297 | 261 | 58 | 6 | 580 | 70 | 256 | 37 | 4 | 549 | 45 | 0.057 |
| Mokrousov | 2008 | Russia | Caucasian | PTB | HB | PCR-RFLP | 188/126 | 120 | 59 | 9 | 299 | 77 | 96 | 27 | 3 | 219 | 33 | 0.511 |
| Nino-Moreno | 2007 | México | Mixed | PTB | HB | PCR-RFLP | 94/110 | 53 | 33 | 8 | 139 | 49 | 70 | 38 | 2 | 178 | 42 | 0.215 |
| Ozdemir | 2014 | Turkey | Asian | PTB | PB | PCR-RFLP | 71/160 | 44 | 18 | 9 | 106 | 36 | 76 | 63 | 21 | 215 | 105 | 0.176 |
| Ozdemir | 2014 | Turkey | Asian | EPTB | PB | PCR-RFLP | 89/160 | 47 | 34 | 8 | 128 | 50 | 76 | 63 | 21 | 215 | 105 | 0.176 |
| Sambasivan | 2010 | India | Asian | PTB | HB | PCR-RFLP | 156/100 | 89 | 55 | 12 | 233 | 79 | 71 | 21 | 8 | 163 | 37 | 0.002 |
| Shamsi | 2016 | Iran | Asian | PTB | HB | PCR-RFLP | 100/100 | 33 | 66 | 1 | 132 | 68 | 83 | 16 | 1 | 182 | 18 | 0.817 |
| Sharma | 2010 | India | Asian | PTB | PB | T-ARMS-PCR | 181/177 | 102 | 75 | 4 | 279 | 83 | 126 | 48 | 3 | 300 | 54 | 0.515 |
| Sharma | 2010 | India | Asian | EPTB | PB | T-ARMS-PCR | 23/177 | 8 | 13 | 2 | 29 | 17 | 126 | 48 | 3 | 300 | 54 | 0.515 |
| Singla | 2012 | India | Asian | PTB | PB | PCR-RFLP | 286/392 | 162 | 112 | 12 | 436 | 136 | 258 | 123 | 11 | 639 | 145 | 0.420 |
| Singla | 2012 | India | Asian | EPTB | PB | PCR-RFLP | 71/392 | 45 | 22 | 4 | 112 | 30 | 258 | 123 | 11 | 639 | 145 | 0.420 |
| Souza de Lima | 2016 | Brazil | South American | PTB | HB | TaqMan | 288/287 | 170 | 95 | 23 | 435 | 141 | 184 | 89 | 14 | 457 | 117 | 0.450 |
| Taype | 2010 | Peru | Caucasian | PTB | HB | PCR-RFLP | 498/513 | 352 | 130 | 16 | 834 | 162 | 347 | 149 | 17 | 843 | 183 | 0.838 |
| Taype | 2010 | Peru | Caucasian | EPTB | HB | PCR-RFLP | 121/513 | 82 | 37 | 2 | 201 | 41 | 347 | 149 | 17 | 843 | 183 | 0.838 |
| Tekin | 2010 | Turkey | Caucasian | EPTB | HB | PCR-RFLP | 74/192 | 39 | 28 | 7 | 106 | 42 | 141 | 46 | 5 | 328 | 56 | 0.595 |
| Velayati | 2013 | Iran | Asian | PTB | HB | PCR- RFLP | 79/50 | 42 | 35 | 2 | 119 | 39 | 37 | 12 | 1 | 86 | 14 | 0.981 |
| Wu | 2015 | China | Asian | PTB | PB | PCR-RFLP | 103/87 | 33 | 49 | 21 | 115 | 91 | 51 | 27 | 9 | 129 | 45 | 0.075 |
| Xiao | 2009 | China | Asian | PTB | HB | PCR-RFLP | 41/384 | 21 | 18 | 2 | 60 | 22 | 221 | 119 | 44 | 561 | 207 | <0.001 |
| Xiao | 2009 | China | Asian | EPTB | HB | PCR-RFLP | 55/384 | 30 | 19 | 6 | 79 | 31 | 221 | 119 | 44 | 561 | 207 | <0.001 |
| Zheng | 2017 | China | Asian | PTB | PB | TaqMan | 1595/1521 | 972 | 551 | 72 | 2495 | 695 | 900 | 544 | 77 | 2344 | 698 | 0.655 |
| rs2393799 C > T | CC | CT | TT | C | T | CC | CT | TT | C | T | ||||||||
| Amiri | 2018 | Iran | Asian | PTB | PB | PCR-RFLP | 100/100 | 8 | 88 | 4 | 104 | 96 | 4 | 95 | 1 | 103 | 97 | <0.001 |
| Bahari | 2013 | Iran | Asian | PTB | PB | ARMS-PCR | 150/150 | 71 | 54 | 25 | 196 | 104 | 104 | 40 | 6 | 248 | 52 | 0.395 |
| Ben-Selma | 2011 | Tunisia | African | PTB | HB | ARMS-PCR | 168/150 | 16 | 57 | 95 | 89 | 247 | 14 | 51 | 85 | 79 | 221 | 0.130 |
| Ben-Selma | 2011 | Tunisia | African | EPTB | HB | ARMS-PCR | 55/150 | 4 | 15 | 36 | 23 | 87 | 14 | 51 | 85 | 79 | 221 | 0.130 |
| Chaudhary | 2018 | India | Asian | PTB | PB | ARMS-PCR | 145/247 | 62 | 67 | 16 | 191 | 99 | 101 | 111 | 35 | 313 | 181 | 0.614 |
| Chaudhary | 2018 | India | Asian | EPTB | PB | ARMS-PCR | 100/247 | 44 | 48 | 8 | 136 | 64 | 101 | 111 | 35 | 313 | 181 | 0.614 |
| Li | 2002 | Gambia | African | PTB | HB | PCR-RFLP | 323/347 | 23 | 118 | 182 | 164 | 482 | 44 | 140 | 163 | 228 | 466 | 0.111 |
| Mokrousov | 2008 | Russia | Caucasian | PTB | HB | ARMS | 190/127 | 86 | 87 | 17 | 259 | 121 | 65 | 46 | 16 | 176 | 78 | 0.093 |
| Nino-Moreno | 2007 | México | Mixed | PTB | HB | ARMS | 92/110 | 8 | 32 | 52 | 48 | 136 | 15 | 44 | 51 | 74 | 146 | 0.275 |
| Sambasivan | 2010 | India | Asian | PTB | HB | PCR-RFLP | 156/100 | 38 | 88 | 30 | 164 | 148 | 15 | 49 | 36 | 79 | 121 | 0.801 |
| Shamsi | 2016 | Iran | Asian | PTB | HB | PCR-RFLP | 100/100 | 1 | 99 | 0 | 101 | 99 | 6 | 93 | 1 | 105 | 95 | <0.001 |
| Singla | 2012 | India | Asian | PTB | PB | ARMS | 286/392 | 143 | 115 | 28 | 401 | 171 | 231 | 143 | 18 | 605 | 179 | 0.485 |
| Singla | 2012 | India | Asian | EPTB | PB | ARMS | 71/392 | 40 | 25 | 6 | 105 | 37 | 231 | 143 | 18 | 605 | 179 | 0.485 |
| Songane | 2012 | Indonesia | Asian | PTB | PB | MassARRAY | 842/844 | 181 | 413 | 248 | 775 | 909 | 177 | 412 | 255 | 766 | 922 | <0.001 |
| Velayati | 2013 | Iran | Asian | PTB | HB | ARMS | 79/50 | 10 | 67 | 2 | 87 | 71 | 3 | 47 | 0 | 53 | 47 | <0.001 |
| Wu | 2015 | China | Asian | PTB | PB | PCR-RFLP | 103/87 | 35 | 47 | 21 | 117 | 89 | 9 | 30 | 48 | 48 | 126 | 0.202 |
| Xiao | 2009 | China | Asian | PTB | HB | ARMS | 38/384 | 23 | 11 | 4 | 57 | 19 | 208 | 135 | 41 | 551 | 217 | 0.009 |
| Xiao | 2009 | China | Asian | EPTB | HB | ARMS | 58/384 | 40 | 12 | 6 | 92 | 24 | 208 | 135 | 41 | 551 | 217 | 0.009 |
| Zhou | 2018 | China | Asain | EPTB | HB | Mass Spectrometry | 179/324 | 81 | 77 | 21 | 239 | 119 | 122 | 143 | 59 | 387 | 261 | 0.137 |
| rs1718119 G > A | GG | AG | AA | G | A | GG | AG | AA | G | A | ||||||||
| Bahari | 2013 | Iran | Asian | PTB | PB | T-ARMS-PCR | 150/150 | 63 | 72 | 15 | 198 | 102 | 66 | 69 | 15 | 201 | 99 | 0.622 |
| Zheng | 2017 | China | Asian | PTB | PB | TaqMan | 1568/1454 | 1090 | 440 | 38 | 2620 | 516 | 978 | 417 | 59 | 2373 | 535 | 0.087 |
| Zhu | 2016 | China | Asian | PTB | HB | MassARRAY | 467/503 | 372 | 91 | 4 | 835 | 99 | 412 | 89 | 2 | 913 | 93 | 0.222 |
| rs208294 G > A | GG | AG | AA | G | A | GG | AG | AA | G | A | ||||||||
| Chaudhary | 2018 | India | Asian | Mixed | PB | PCR-RFLP | 245/246 | 56 | 147 | 42 | 259 | 231 | 49 | 143 | 54 | 241 | 251 | 0.011 |
| Zheng | 2017 | China | Asian | PTB | PB | TaqMan | 1570/1467 | 597 | 732 | 241 | 1926 | 1214 | 578 | 679 | 210 | 1835 | 1099 | 0.642 |
| Zhou | 2018 | China | Asian | EPTB | HB | Mass Spectrometry | 179/324 | 22 | 80 | 77 | 124 | 234 | 70 | 145 | 109 | 285 | 363 | 0.099 |
| rs7958311 G > A | GG | AG | AA | G | A | GG | AG | AA | G | A | ||||||||
| Zheng | 2017 | China | Asian | PTB | PB | TaqMan | 1533/1503 | 402 | 797 | 334 | 1601 | 1465 | 396 | 775 | 332 | 1567 | 1439 | 0.199 |
| Zhu | 2016 | China | Asian | PTB | HB | MassARRAY | 467/503 | 114 | 215 | 138 | 443 | 491 | 137 | 262 | 104 | 536 | 470 | 0.300 |
| rs2230911 C > G | CC | CG | GG | C | G | CC | CG | GG | C | G | ||||||||
| Souza de Lima | 2016 | Brazil | South American | PTB | HB | TaqMan | 288/288 | 170 | 95 | 23 | 435 | 141 | 193 | 89 | 6 | 475 | 101 | 0.245 |
| Zheng | 2017 | China | Asian | PTB | PB | TaqMan | 1565/1509 | 1029 | 482 | 54 | 2540 | 590 | 997 | 467 | 45 | 2461 | 557 | 0.274 |
| Polymorphism | No. | Genetic Model | Association Test | Heterogeneity | Publication Bias Tests | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | Z | P | χ2 | I2 (%) | P | Egger’s Test p-Value | Begg’s Test p-Value | |||
| rs3751143 | 30 | AC vs. AA | 1.44 (1.17–1.78) | 3.42 | 0.0006 | 158.86 | 81.7 | 0.000 | 0.016 | 0.016 |
| CC vs. AA | 1.87 (1.40–2.49) | 4.26 | 0.0004 | 61.79 | 54.7 | 0.000 | 0.002 | 0.047 | ||
| AC + CC vs. AA | 1.50 (1.22–1.85) | 3.78 | 0.0002 | 178.85 | 83.8 | 0.000 | 0.007 | 0.018 | ||
| CC vs. AC + AA | 1.61 (1.25–2.07) | 3.65 | 0.001 | 50.79 | 44.9 | 0.005 | 0.006 | 0.051 | ||
| C vs. A | 1.41 (1.19–1.67) | 3.97 | <0.0001 | 173.41 | 83.3 | 0.000 | 0.006 | 0.066 | ||
| rs2393799 | 19 | CT v CC | 1.00 (0.83–1.20) | 0.01 | 0.989 | 32.34 | 44.3 | 0.020 | 0.460 | 0.753 |
| TT vs. CC | 0.99 (0.68–1.44) | 0.04 | 0.965 | 73.55 | 75.5 | 0.000 | 0.935 | 0.510 | ||
| CT + TT vs. CC | 0.97 (0.77–1.23) | 0.22 | 0.825 | 58.41 | 69.2 | 0.000 | 0.557 | 0.649 | ||
| TT vs. CT + CC | 0.99 (0.74–1.32) | 0.08 | 0.938 | 71.34 | 74.8 | 0.000 | 0.962 | 0.680 | ||
| T vs. C | 0.98 (0.83–1.17) | 0.20 | 0.844 | 95.15 | 81.1 | 0.000 | 0.657 | 0.763 | ||
| rs1718119 | 3 | AG vs. GG | 0.99 (0.86–1.13) | 0.16 | 0.88 | 1.13 | 0 | 0.57 | 0.308 | 0.602 |
| AA vs. GG | 0.70 (0.49–0.99) | 1.99 | 0.05 | 3.56 | 44 | 0.17 | 0.136 | 0.117 | ||
| AG + AA vs. GG | 0.96 (0.84–1.09) | 0.68 | 0.50 | 2.22 | 10 | 0.33 | 0.312 | 0.602 | ||
| AA vs. AG + GG | 0.70 (0.49–1.00) | 1.98 | 0.05 | 3.24 | 38 | 0.20 | 0.141 | 0.117 | ||
| G vs. A | 0.93 (0.83–1.04) | 1.24 | 0.21 | 3.49 | 43 | 0.17 | 0.242 | 0.602 | ||
| rs208294 | 3 | AG vs. GG | 1.03 (0.93–1.23) | 0.89 | 0.37 | 3.77 | 47 | 0.15 | 0.694 | 0.602 |
| AA vs. GG | 1.18 (0.69–2.02) | 0.61 | 0.54 | 8.99 | 78 | 0.010 | 0.900 | 0.602 | ||
| AG + AA vs. GG | 1.16 (0.80–1.68) | 0.76 | 0.45 | 6.50 | 69 | 0.04 | 0.751 | 0.602 | ||
| AA vs. AG + GG | 1.08 (0.78–1.49) | 0.47 | 0.64 | 5.60 | 64 | 0.06 | 0.904 | 0.602 | ||
| A vs. G | 1.09 (0.85–1.40) | 0.70 | 0.49 | 8.82 | 77 | 0.01 | 0.860 | 0.602 | ||
| rs7958311 | 2 | AG vs. GG | 1.01 (0.87–1.17) | 0.09 | 0.93 | 0.02 | 0 | 0.88 | ||
| AA vs. GG | 1.23 (0.77–1.95) | 0.87 | 0.38 | 5.15 | 81 | 0.02 | ||||
| AG + AA vs. GG | 0.81 (0.50–1.31) | 0.87 | 0.38 | 8.09 | 88 | 0.004 | ||||
| AA vs. AG + GG | 1.24 (0.76–2.01) | 0.87 | 0.38 | 8.09 | 88 | 0.004 | ||||
| A vs. G | 1.11 (0.88–1.40) | 0.87 | 0.38 | 5.18 | 81 | 0.02 | ||||
| rs2230911 | 2 | CG vs. CC | 1.03 (0.90–1.19) | 0.42 | 0.67 | 0.95 | 0 | 0.33 | ||
| GG vs. CC | 2.10 (0.58–7.66) | 1.13 | 0.26 | 6.65 | 85 | 0.010 | ||||
| CG + GG vs. CC | 1.01 (0.88–1.16) | 0.13 | 0.89 | 0.28 | 0 | 0.60 | ||||
| GG vs. CG + CC | 2.03 (0.60–6.94) | 1.13 | 0.26 | 6.12 | 84 | 0.01 | ||||
| G vs. C | 1.22 (0.83–1.80) | 1.03 | 0.30 | 6.09 | 84 | 0.01 | ||||
| Parameters | No. | AC vs. AA | CC vs. AA | AC + CC vs. AA | CC vs. AC + AA | C vs. A | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | ||
| rs3751143 | |||||||||||
| Tuberculosis | |||||||||||
| PTB | 21 | 1.35 (1.05–1.74) | 0.020 | 1.50 (1.10–2.04) | 0.010 | 1.39 (1.09–1.78) | 0.009 | 1.34 (1.04–1.73) | 0.020 | 1.31 (1.09–1.58) | 0.004 |
| EPTB | 9 | 1.68 (1.17–2.42) | 0.005 | 2.62 (1.19–5.78) | 0.020 | 1.84 (1.21–2.79) | 0.004 | 2.05 (1.07–3.93) | 0.030 | 1.67 (1.16–2.42) | 0.006 |
| Ethnicities | |||||||||||
| Asian | 19 | 1.48 (1.09–2.00) | 0.010 | 1.70 (1.17–2.48) | 0.006 | 1.53 (1.13–2.06) | 0.006 | 1.47 (1.07–2.00) | 0.020 | 1.57 (1.22–2.02) | 0.0005 |
| Caucasian | 6 | 1.47 (0.99–2.17) | 0.05 | 1.56 (0.70 − 3.51) | 0.28 | 1.49 (0.98–2.26) | 0.06 | 1.36 (0.70–2.66) | 0.37 | 1.37 (0.96–1.97) | 0.09 |
| African | 3 | 1.45 (0.66–3.19) | 0.36 | 2.16 (0.33–13.95) | 0.42 | 1.60 (0.62–4.13) | 0.33 | 1.89 (0.41–8.81) | 0.42 | 1.56 (0.62–3.94) | 0.35 |
| rs2393799 | CT vs. CC | TT vs. CC | CT + TT vs. CC | TT vs. CT + CC | C vs. T | ||||||
| Asian | 14 | 0.92 (0.74–1.14) | 0.44 | 0.87 (0.54–1.41) | 0.58 | 0.86 (0.66–1.14) | 0.30 | 0.92 (0.61–1.40) | 0.70 | 0.98 (0.83–1.17) | 0.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, M.; Sarani, H.; Moazeni-Roodi, A.; Naderi, M.; Hashemi, M. Association between P2X7 Polymorphisms and Susceptibility to Tuberculosis: An Updated Meta-Analysis of Case-Control Studies. Medicina 2019, 55, 298. https://doi.org/10.3390/medicina55060298
Taheri M, Sarani H, Moazeni-Roodi A, Naderi M, Hashemi M. Association between P2X7 Polymorphisms and Susceptibility to Tuberculosis: An Updated Meta-Analysis of Case-Control Studies. Medicina. 2019; 55(6):298. https://doi.org/10.3390/medicina55060298
Chicago/Turabian StyleTaheri, Mohsen, Hosna Sarani, Abdolkarim Moazeni-Roodi, Mohammad Naderi, and Mohammad Hashemi. 2019. "Association between P2X7 Polymorphisms and Susceptibility to Tuberculosis: An Updated Meta-Analysis of Case-Control Studies" Medicina 55, no. 6: 298. https://doi.org/10.3390/medicina55060298
APA StyleTaheri, M., Sarani, H., Moazeni-Roodi, A., Naderi, M., & Hashemi, M. (2019). Association between P2X7 Polymorphisms and Susceptibility to Tuberculosis: An Updated Meta-Analysis of Case-Control Studies. Medicina, 55(6), 298. https://doi.org/10.3390/medicina55060298





