Comparison of the Therapeutic Efficacies of Topical Rivoceranib and Topical Bevacizumab in a Murine Model of Corneal Neovascularization
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Drug
2.2. Mouse Model of Corneal Neovascularization
2.3. Clinical Measurement of CNV
2.4. Immunohistochemical Measurement of CNV
2.5. Statistical Analysis
3. Results
3.1. CNV Area
3.2. CNV Index
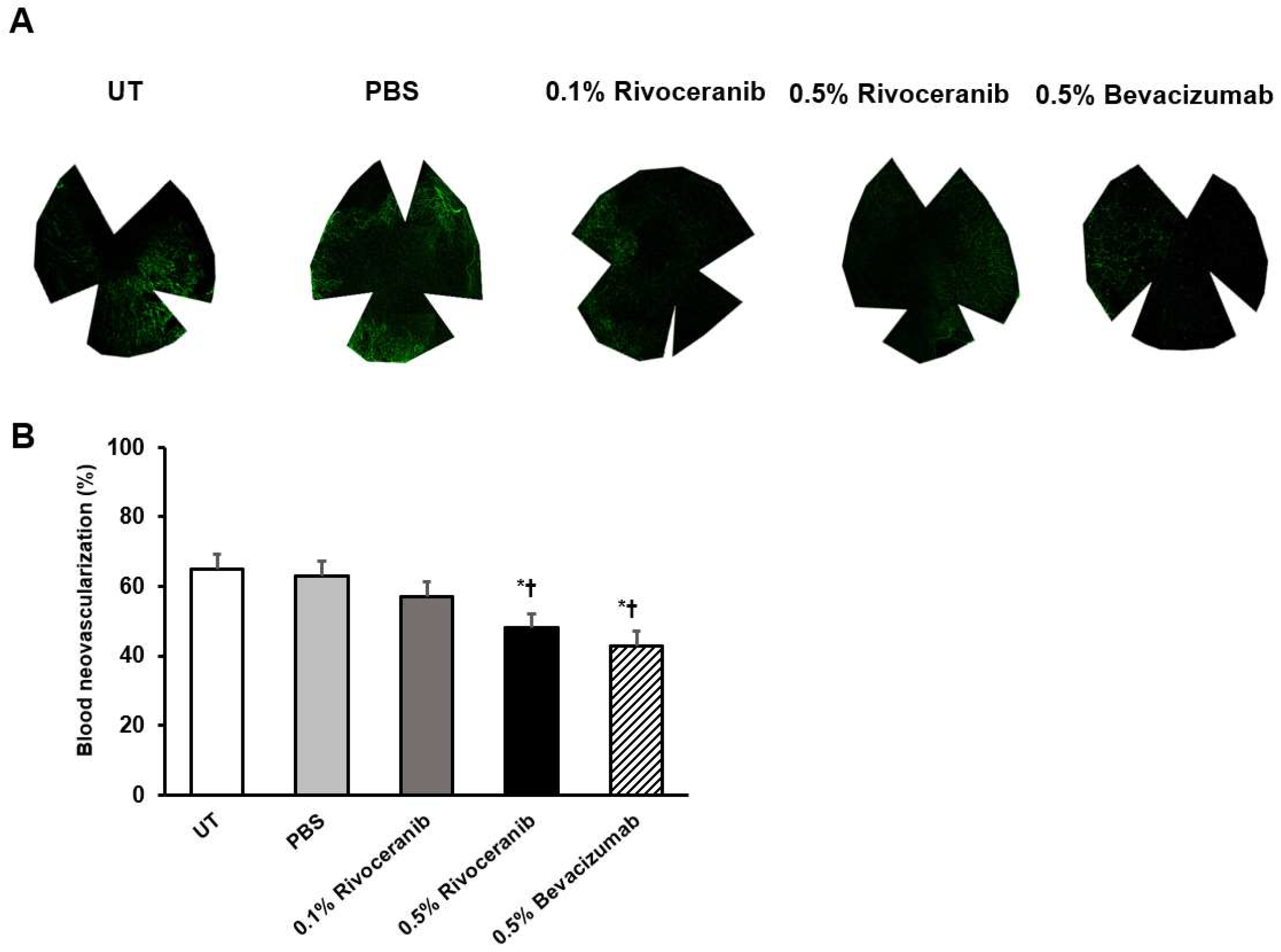
3.3. Immunofluorescent Staining of Blood Vessels
3.4. Immunofluorescent Staining of Lymphatic Vessels
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roshandel, D.; Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.; Jabbehdari, S.; Maiz, A.; Jalali, S.; Djalilian, A.R.; Holland, E.J. Current and emerging therapies for corneal neovascularization. Ocul. Surf. 2018, 16, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Z.; Sharif, W. Corneal neovascularization: Updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [PubMed]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A.; Hussein, H.; Abd El-Baky, N. Molecular underpinnings of corneal angiogenesis: Advances over the past decade. Int. J. Ophthalmol. 2016, 18, 768–779. [Google Scholar]
- Chang, J.H.; Garg, N.K.; Lunde, E.; Han, K.Y.; Jain, S.; Azar, D.T. Corneal Neovascularization: An Anti-VEGF Therapy Review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- You, I.-C.; Kang, I.-S.; Lee, S.-H.; Yoon, K.-C. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009, 87, 653–658. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. VEGF receptor signal transduction—A brief update. Vasc. Pharmacol. 2016, 86, 14–17. [Google Scholar] [CrossRef]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef]
- Gacche, R.N. Compensatory angiogenesis and tumor refractoriness. Oncogenesis 2015, 4, e153. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, M.K.; Seo, K.Y.; Ueta, M.; Yoon, K.C. Effectiveness of photodynamic therapy with verteporfin combined with intrastromal bevacizumab for corneal neovascularization in Stevens–Johnson syndrome. Int. Ophthalmol. 2019, 39, 55–62. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2019. (ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Asena, L.; Akova, Y.A.; Cetinkaya, A.; Kucukerdonmez, C. The effect of topical bevacizumab as an adjunctive therapy for corneal neovascularization. Acta Ophthalmol. 2013, 91, e246–e248. [Google Scholar] [CrossRef] [PubMed]
- Eiger-Moscovich, M.; Livny, E.; Sella, R.; Gal-Or, O.; Nisgav, Y.; Livnat, T.; Bahar, I. Comparison of Subconjunctival Aflibercept and Betamethasone for the Treatment of Formed Corneal Neovascularization in a Rabbit Model. Ophthalmic Res. 2019, 62, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Perez-Santonja, J.J.; Campos-Mollo, E.; Lledó-Riquelme, M.; Javaloy, J.; Alio, J.L. Inhibition of Corneal Neovascularization by Topical Bevacizumab (Anti-VEGF) and Sunitinib (Anti-VEGF and Anti-PDGF) in an Animal Model. Am. J. Ophthalmol. 2010, 150, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Petsoglou, C.; Balaggan, K.S.; Dart, J.K.; Bunce, C.; Xing, W.; Ali, R.R.; Tuft, S.J. Subconjunctival bevacizumab induces regression of corneal neovascularisation: A pilot randomised placebo-controlled double-masked trial. Br. J. Ophthalmol. 2013, 97, 28–32. [Google Scholar] [CrossRef]
- Al-Debasi, T.; Al-Bekairy, A.; Al-Katheri, A.; Al Harbi, S.; Mansour, M. Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization. Saudi J. Ophthalmol. 2017, 31, 99–105. [Google Scholar] [CrossRef]
- Zheng, N.; Wei, W.; Wang, Z. Emerging roles of FGF signaling in hepatocellular carcinoma. Transl. Cancer Res. 2016, 5, 1–6. [Google Scholar]
- Scott, L.J. Apatinib: A Review in Advanced Gastric Cancer and Other Advanced Cancers. Drugs 2018, 78, 747–758. [Google Scholar] [CrossRef]
- Zhao, D.; Hou, H.; Zhang, X. Progress in the treatment of solid tumors with apatinib: A systematic review. OncoTargets Ther. 2018, 11, 4137–4147. [Google Scholar] [CrossRef]
- Leng, J.; Li, D.-R.; Huang, L.-M.; Ji, X.-H.; Wang, D.-L. Apatinib is effective as third-line and more treatment of advanced metastatic non-small-cell lung cancer: A retrospective analysis in a real-world setting. Medicine 2019, 98, e16967. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Q.; Li, J.; Zhang, N.; Hua, Y.; Xu, L.; Deng, Y.; Lai, J.; Peng, Z.; Peng, B.; et al. Apatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinoma. Oncotarget 2016, 7, 17220–17229. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Polom, K.; Roviello, F.; Marrelli, D.; Multari, A.G.; Paganini, G.; Pacifico, C.; Generali, D. Targeting VEGFR-2 in Metastatic Gastric Cancer: Results from a Literature-Based Meta-Analysis. Cancer Investig. 2017, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, K.L.; Kim, D.; Yeo, Y.; Han, H.; Kim, M.G.; Kim, S.H.; Kim, H.; Jeong, J.H.; Suh, W. Apatinib-loaded nanoparticles suppress vascular endothelial growth factor-induced angiogenesis and experimental corneal neovascularization. Int. J. Nanomed. 2017, 12, 4813–4822. [Google Scholar] [CrossRef] [PubMed]
- Morabito, A.; De Maio, E.; Di Maio, M.; Normanno, N.; Perrone, F. Tyrosine Kinase Inhibitors of Vascular Endothelial Growth Factor Receptors in Clinical Trials: Current Status and Future Directions. Oncologist 2006, 11, 753–764. [Google Scholar] [CrossRef]
- Sahan, B.; Ciftci, F.; Eyuboglu, S.; Yaba, A.; Yilmaz, B.; Yalvac, B.I.; Ucar, A.Y. Comparison of the Effects of Dovitinib and Bevacizumab on Reducing Neovascularization in an Experimental Rat Corneal Neovascularization Model. Cornea 2019, 38, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Ekim, Y.; Kara, S.; Gencer, B.; Karaca, T. Efficacy of Sunitinib, Sunitinib-Hesperetin, and Sunitinib-Doxycycline Combinations on Experimentally-Induced Corneal Neovascularization. Curr. Eye Res. 2019, 44, 590–598. [Google Scholar] [CrossRef]
- Lledó Riquelme, M.; Campos-Mollo, E.; Fernández-Sánchez, L. Topical axitinib is a potent inhibitor of corneal neovascularization. Clin. Exp. Ophthalmol. 2018, 46, 1063–1074. [Google Scholar] [CrossRef]
- Amparo, F.; Sadrai, Z.; Jin, Y.; Alfonso-Bartolozzi, B.; Wang, H.; Shikari, H.; Ciolino, J.B.; Chodosh, J.; Jurkunas, U.; Schaumberg, D.A.; et al. Safety and Efficacy of the Multitargeted Receptor Kinase Inhibitor Pazopanib in the Treatment of Corneal Neovascularization. Investig. Opthalmology Vis. Sci. 2013, 54, 537–544. [Google Scholar] [CrossRef]
- Yoon, K.C.; A Bae, J.; Park, H.J.; Im, S.K.; Oh, H.J.; Lin, X.H.; Kim, M.Y.; Lee, J.H.; E Lee, S.; Ahn, K.Y.; et al. Subconjunctival gene delivery of the transcription factor GA-binding protein delays corneal neovascularization in a mouse model. Gene Ther. 2009, 16, 973–981. [Google Scholar] [CrossRef]
- Barbariga, M.; Fonteyne, P.; Ostadreza, M.; Bignami, F.; Rama, P.; Ferrari, G. Substance P Modulation of Human and Murine Corneal Neovascularization. Investig. Opthalmology Vis. Sci. 2018, 59, 1305–1312. [Google Scholar] [CrossRef]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar]
- Shen, M.; Zhou, X.; Ye, L.; Yuan, Q.; Shi, C.; Zhu, P.; Jiang, N.; Ma, M.; Yang, Q.; Shao, Y. Xanthatin inhibits corneal neovascularization by inhibiting the VEGFR2-mediated STAT3/PI3K/Akt signaling pathway. Int. J. Mol. Med. 2018, 42, 769–778. [Google Scholar] [CrossRef] [PubMed]
- You, I.-C.; Im, S.-K.; Lee, S.-H.; Yoon, K.-C. Photodynamic Therapy with Verteporfin Combined with Subconjunctival Injection of Bevacizumab for Corneal Neovascularization. Cornea 2011, 30, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Di, G.; Zhang, X.; Zhou, Q.; Shi, W. Subconjunctival Bevacizumab Injection Impairs Corneal Innervations and Epithelial Wound Healing in Mice. Investig. Opthalmology Vis. Sci. 2017, 58, 1469. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.; Onderka, J.; Rummelt, C.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Schlo¨tzer-Schrehardt, U.; Cursiefen, C. Safety Profile of Topical VEGF Neutralization at the Cornea. Investig. Opthalmology Vis. Sci. 2009, 50, 2095–2102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giuliano, S.; Pagès, G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie 2013, 95, 1110–1119. [Google Scholar] [CrossRef]
- Menguy, T.; Briaux, A.; Jeunesse, E.; Giustiniani, J.; Calcei, A.; Guyon, T.; Mizrahi, J.; Haegel, H.; Duong, V.; Soler, V.; et al. Anti-CD160, Alone or in Combination with Bevacizumab, Is a Potent Inhibitor of Ocular Neovascularization in Rabbit and Monkey Models. Investig. Opthalmology Vis. Sci. 2018, 59, 2687–2698. [Google Scholar] [CrossRef]
- Kirat, O.M.; Al-Dhibi, H.A. Regression of aggressive corneal vascularization after photodynamic therapy, subconjunctival Avastin injections and topical cyclosporin-A 1% drops: A case report. Saudi J. Ophthalmol. 2010, 24, 151–154. [Google Scholar] [CrossRef]
- Vergallo, S.; Veritti, D.; Lanzetta, P. Triple Therapy for Corneal Neovascularization: A Case Report. Eur. J. Ophthalmol. 2012, 22, 126–128. [Google Scholar]
- Keskin, U.; Totan, Y.; Karadağ, R.; Erdurmuş, M.; Aydın, B. Inhibitory effects of SU5416, a selective vascular endothelial growth factor receptor tyrosine kinase inhibitor, on experimental corneal neovascularization. Ophthalmic Res. 2012, 47, 13–18. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, M.R.; Jang, J.H.; Na, H.-J.; Lee, S.; Lee, S.; Lee, S. A Review of Anti-Angiogenic Targets for Monoclonal Antibody Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1786. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.J.; Woo, J.M.; Ji, Y.S.; Yoon, K.C. Comparison of the Therapeutic Efficacies of Topical Rivoceranib and Topical Bevacizumab in a Murine Model of Corneal Neovascularization. Medicina 2019, 55, 729. https://doi.org/10.3390/medicina55110729
Yoon HJ, Woo JM, Ji YS, Yoon KC. Comparison of the Therapeutic Efficacies of Topical Rivoceranib and Topical Bevacizumab in a Murine Model of Corneal Neovascularization. Medicina. 2019; 55(11):729. https://doi.org/10.3390/medicina55110729
Chicago/Turabian StyleYoon, Hyeon Jeong, Je Moon Woo, Yong Sok Ji, and Kyung Chul Yoon. 2019. "Comparison of the Therapeutic Efficacies of Topical Rivoceranib and Topical Bevacizumab in a Murine Model of Corneal Neovascularization" Medicina 55, no. 11: 729. https://doi.org/10.3390/medicina55110729
APA StyleYoon, H. J., Woo, J. M., Ji, Y. S., & Yoon, K. C. (2019). Comparison of the Therapeutic Efficacies of Topical Rivoceranib and Topical Bevacizumab in a Murine Model of Corneal Neovascularization. Medicina, 55(11), 729. https://doi.org/10.3390/medicina55110729




