The Impact of Pulmonary Vein Anatomy on the Outcomes of Catheter Ablation for Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Transthoracic Echocardiogram
2.3. Analysis of Anatomy
2.4. The Ablation Procedure
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar]
- Saito, T.; Waki, K.; Becker, A.E. Left atrial myocardial extension onto pulmonary veins in humans: Anatomic observations relevant for atrial arrhythmias. J. Cardiovasc. Electrophysiol. 2000, 11, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Knight, B.P.; Tada, H.; Ozaydin, M.; Chugh, A.; Hassan, S.; Scharf, C.; Lai, S.; Greenstein, R.; Pelosi, F., Jr.; et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002, 105, 1077–1081. [Google Scholar] [CrossRef]
- Balk, E.M.; Garlitski, A.C.; Alsheikh-Ali, A.A.; Terasawa, T.; Chung, M.P.H. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: A systematic review. J. Cardiovasc. Electrophysiol. 2010, 21, 1208–1216. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Cabrera, J.A.; Ho, S.Y.; Climent, V.; Sánchez-Quintana, D. The architecture of the left lateral atrial wall: A particular anatomic region with implications for ablation of atrial fibrillation. Eur. Heart J. 2008, 29, 356–362. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; HS, L.; Sullivan, T.; Kurt, C.; Thomson, S.; Sanders, P. Long-term outcomes of catheter ablation of atrial fibrillation: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004549. [Google Scholar] [CrossRef]
- Brooks, A.G.; Stiles, M.K.; Laborderie, J.; Lau, D.H.; Kuklik, P.; Shipp, N.J.; Hsu, L.-F.; Sanders, P. Outcomes of long-standing persistent atrial fibrillation ablation: A systematic review. Heart Rhythm 2010, 7, 835–846. [Google Scholar] [CrossRef]
- Schwartzman, D.; Bazaz, R.; Nosbisch, J. Common left pulmonary vein: A consistent source of arrhythmogenic atrial ectopy. J. Cardiovasc. Electrophysiol. 2004, 15, 560–566. [Google Scholar] [CrossRef]
- Bittner, A.; Mönnig, G.; Vagt, A.J.; Zellerhoff, S.; Wasmer, K.; Köbe, J.; Pott, C.; Milberg, P.; Sauerland, C.; Wessling, J. Pulmonary vein variants predispose to atrial fibrillation: A case-control study using multislice contrast-enhanced computed tomography. EP Europace 2011, 13, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Skowerski, M.; Wozniak-Skowerska, I.; Hoffmann, A.; Nowak, S.; Skowerski, T.; Sosnowski, M. Pulmonary vein anatomy variants as a biomarker of atrial fibrillation–CT angiography evaluation. BMC Cardiovasc. Disord. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Lickfett, L.; Meininger, G.; Dickfeld, T.; Wu, R.; Juang, G.; Angkeow, P.; LaCorte, J.; Bluemke, D.; Berger, R.; et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: Lessons learned by use of magnetic resonance imaging. Circulation 2003, 107, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Lacomis, J.M.; Goitein, O.; Deible, C.; Schwartzman, D. CT of the pulmonary veins. J. Thorac. Imaging 2007, 22, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Köse, S.; Başarıcı, I.; Kabul, K.H.; Bozlar, U.; Amasyalı, B. Catheter ablation of atrial fibrillation in a patient with unusual pulmonary vein anatomy involving right upper pulmonary vein. Anadolu. Kardiyol. Derg. 2012, 12, 76–77. [Google Scholar] [PubMed]
- Stanford, W.; Breen, J.F. CT evaluation of left atrial pulmonary venous anatomy. Int. J. Cardiovasc. Imaging. 2005, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Porres, D.V.; Morenza, O.P.; Pallisa, E.; Roque, A.; Andreu, J.; Martínez, M. Learning from the pulmonary veins. Radiographics 2013, 33, 999–1022. [Google Scholar] [CrossRef]
- Anselmino, M.; Blandino, A.; Beninati, S.; Rovera, C.; Boffano, C.; Belletti, M.; Domenico, C.; Scaglione, M. Morphologic analysis of left atrial anatomy by magnetic resonance angiography in patients with atrial fibrillation: A large single center experience. J. Cardiovasc. Electrophysiol. 2011, 22, 1–7. [Google Scholar] [CrossRef]
- Mlcochová, H.; Tintera, J.; Porod, V.; Peichl, P.; Cihák, R.; Kautzner, J. Magnetic resonance angiography of pulmonary veins: implications for catheter ablation of atrial fibrillation. Pacing Clin. Electrophysiol. 2005, 28, 1073–1080. [Google Scholar] [CrossRef]
- McLellan, A.J.; Ling, L.-H.; Ruggiero, D.; Wong, M.C.; Walters, T.E.; Nisbet, A.; Shetty, A.K.; Azzopardi, S.; Taylor, A.J.; Morton, J.B.; et al. Pulmonary vein isolation: The impact of pulmonary venous anatomy on long-term outcome of catheter ablation for paroxysmal atrial fibrillation. Hear. Rhythm. 2014, 11, 549–556. [Google Scholar] [CrossRef]
- Kubala, M.J.-S.; Quenum, S.; Kubala, M.; Hermida, J.; Nadji, G.; Traulle, S.; Jarry, G. Normal Pulmonary Veins Anatomy is Associated with Better AF-Free Survival after Cryoablation as Compared to Atypical Anatomy with Common Left Pulmonary Vein. Pacing Clin. Electrophysiol. 2011, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Sohns, J.M.; Bergau, L.; Sossalla, S.; Vollmann, D.; Lüthje, L.; Staab, W.; Dorenkamp, M.; Harrison, J.L.; O’Neill, M.D. Pulmonary vein anatomy predicts freedom from atrial fibrillation using remote magnetic navigation for circumferential pulmonary vein ablation. Europace 2013, 15, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xing, Y.; Xu, C.; Peng, F.; Sun, Y.; Wang, S.; Guo, H. A left common pulmonary vein: Anatomical variant predicting good outcomes of repeat catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Khoueiry, Z.; Albenque, J.-P.; Providencia, R.; Combes, S.; Combes, N.; Jourda, F.; Sousa, P.A.; Cardin, C.; Pasquie, J.-L.; Cung, T.T. Outcomes after cryoablation vs. radiofrequency in patients with paroxysmal atrial fibrillation: Impact of pulmonary veins anatomy. Europace 2016, 18, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Mulder, B.A.; Al-Jazairi, M.I.H.; Arends, B.K.O.; Bax, N.; Dijkshoorn, L.A.; Sheikh, U.; Tan, E.S.; Wiesfeld, A.C.P.; Tieleman, R.G.; Vliegenthart, R.; et al. Pulmonary vein anatomy addressed by computed tomography and relation to success of second-generation cryoballoon ablation in paroxysmal atrial fibrillation. Clin. Cardiol. 2019, 42, 438–443. [Google Scholar] [CrossRef]
- Hunter, R.J.; Ginks, M.; Ang, R.; Diab, I.; Goromonzi, F.C.; Page, S.; Baker, V.; Richmond, L.; Tayebjee, M.; Sporton, S. Impact of variant pulmonary vein anatomy and image integration on long-term outcome after catheter ablation for atrial fibrillation. Europace 2010, 12, 1691–1697. [Google Scholar] [CrossRef]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace. 2018, 20, 33–42. [Google Scholar] [CrossRef]
| Baseline Characteristics | Recurrence N = 30 | Non-Recurrence N = 50 | p | |
|---|---|---|---|---|
| Age (years) | 52.9 ± 11.2 | 54.3 ± 8.7 | 0.5 | |
| Sex | M | 20 (66.7%) | 34 (68%) | 1 |
| F | 10 (33.3%) | 16 (32%) | ||
| Coronary artery disease | 4 (13.3%) | 7 (14%) | 1 | |
| Hypertension | 15 (50%) | 30 (60%) | 0.4 | |
| Dyslipidemia | 9 (30%) | 23 (46%) | 0.2 | |
| Diabetes Mellitus | 2 (6.7%) | 4 (8%) | 1 | |
| Valvular heart disease | 20 (66.7%) | 29 (58%) | 0.4 | |
| AF type | Paroxysmal | 14 (46.7%) | 39 (78%) | 0.007 |
| Persistent | 16 (53.3%) | 11 (22%) | ||
| Additional intervention for persistent AF | CFAE | 3 (18.7%) | 1 (9%) | 0.6 |
| Linear ablation | 4 (25%) | 2 (18.1%) | ||
| No additional intervention | 9 (56.2%) | 8 (72.7%) | ||
| LA diameter (mm) | 41.8 ± 6 | 38.4 ± 6.7 | 0.02 | |
| LA volume index (mL/m2) | 52.7 ± 7.7 | 47.1 ± 8.9 | 0.005 | |
| LA volume index >48.5 (mL/m2) | 20 (66.7%) | 14 (28%) | 0.002 | |
| LVESD (mm) | 32.2 (26; 37.2) | 31.5 (26.5; 37) | 0.7 | |
| LVEDD (mm) | 50 (47.5; 56.2) | 49.5 (44.7; 52.5) | 0.2 | |
| LVEF (%) | 55 (50; 60.75) | 56.5 (50; 60) | 0.8 | |
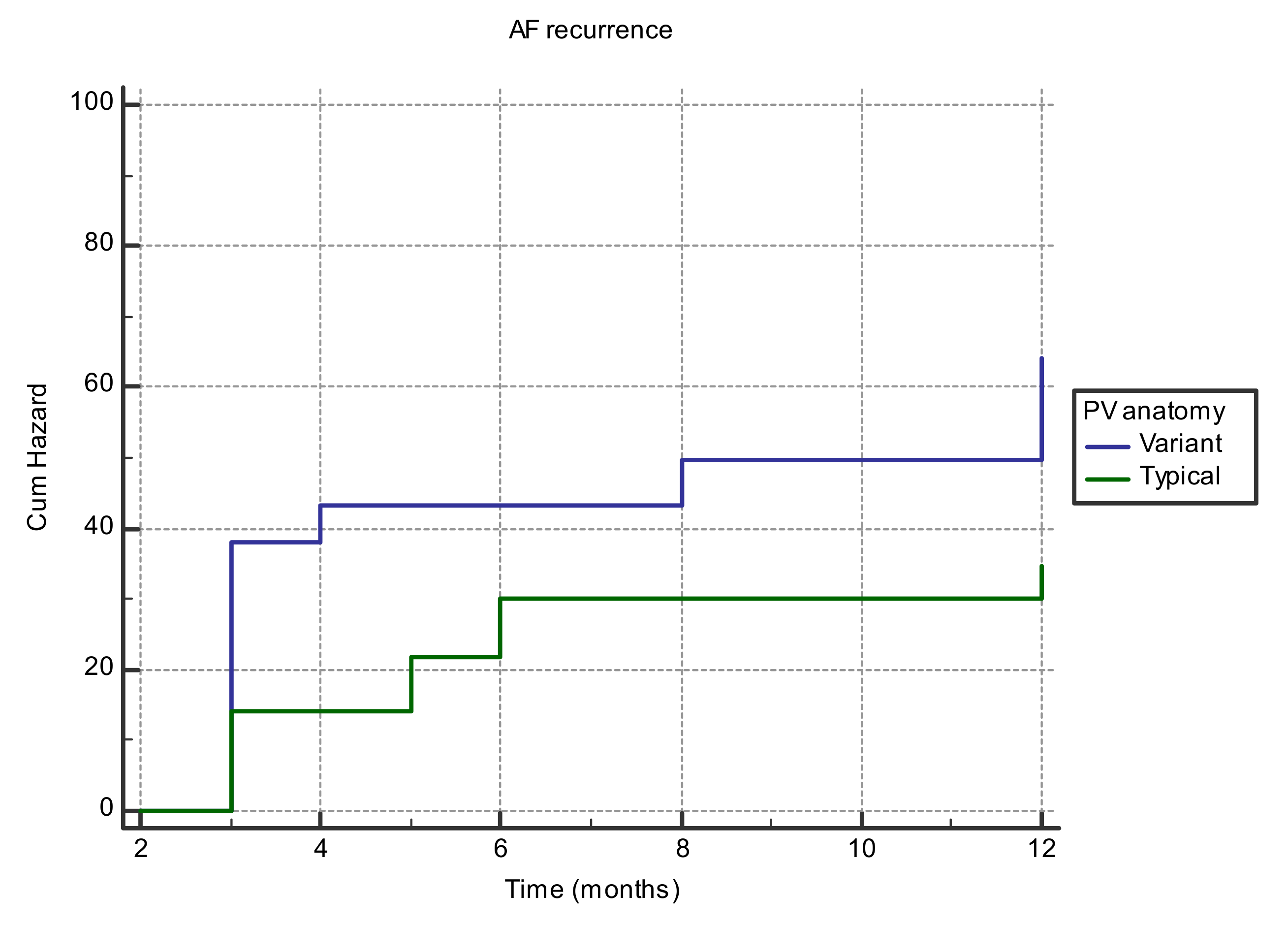
| PV anatomy | Typical PV anatomy | 18 (60%) | 41 (82%) | 0.03 |
| Variant PV anatomy | 12 (40%) | 9 (18%) | ||
| Procedure time (minutes) | 300 (240; 330) | 245 (240; 300) | 0.01 | |
| Procedure time (minutes) | <265 | 9 (30%) | 29 (58%) | 0.02 |
| >265 | 21 (70%) | 21 (42%) | ||
| Antiarrhythmic drug | Propafenone | 11 (36.7%) | 21 (42%) | 0.7 |
| Amiodarone | 15 (50%) | 21 (42%) | ||
| Flecainide | 4 (13.3%) | 8 (16%) | ||
| Baseline Characteristics | Typical PV Anatomy N = 59 | Variant PV Anatomy N = 21 | p | |
|---|---|---|---|---|
| Age (years) | 54.9 ± 9.5 | 50.7 ± 9.7 | 0.08 | |
| Sex | M | 41 (69.5%) | 13 (61.9%) | 0.7 |
| F | 18 (30.5%) | 18 (30.5%) | ||
| Coronary artery disease | 9 (15.3%) | 2 (9.5%) | 0.7 | |
| Hypertension | 34 (57.6%) | 11 (60%) | 0.7 | |
| Dyslipidemia | 27 (45.8%) | 4 (19%) | 0.06 | |
| Diabetes Mellitus | 5 (8.5%) | 1 (4.8%) | 1 | |
| Valvular heart disease | 38 (64.4%) | 11 (52.4%) | 0.4 | |
| AF type | Paroxysmal | 39 (66.1%) | 14 (66.7%) | 1 |
| Persistent | 20 (33.9%) | 7 (33.3%) | ||
| LA diameter (mm) | 39.2 ± 6.5 | 40.9 ± 6.9 | 0.3 | |
| LA volume index (mL/m2) | 48.6 ± 8.7 | 51 ± 9.1 | 0.2 | |
| LVESD (mm) | 31 (25; 37) | 34 (28; 37) | 0.3 | |
| LVEDD (mm) | 50 (46; 54) | 50 (44.7; 55) | 0.8 | |
| LVEF (%) | 60 (50; 60) | 55 (50; 59.5) | 0.09 | |
| Procedure time (minutes) | 270 (240; 330) | 255 (240; 315) | 0.7 | |
| Variables | B | P | HR | 95.0% CI for HR |
|---|---|---|---|---|
| Variant anatomy of PV | 0.68 | 0.05 | 1.9 | 0.95–4.15 |
| LA volume index >48.5 (mL/m2) | 1.11 | 0.004 | 3.04 | 1.41–6.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istratoaie, S.; Roșu, R.; Cismaru, G.; Vesa, Ș.C.; Puiu, M.; Zdrenghea, D.; Pop, D.; Buzoianu, A.D. The Impact of Pulmonary Vein Anatomy on the Outcomes of Catheter Ablation for Atrial Fibrillation. Medicina 2019, 55, 727. https://doi.org/10.3390/medicina55110727
Istratoaie S, Roșu R, Cismaru G, Vesa ȘC, Puiu M, Zdrenghea D, Pop D, Buzoianu AD. The Impact of Pulmonary Vein Anatomy on the Outcomes of Catheter Ablation for Atrial Fibrillation. Medicina. 2019; 55(11):727. https://doi.org/10.3390/medicina55110727
Chicago/Turabian StyleIstratoaie, Sabina, Radu Roșu, Gabriel Cismaru, Ștefan C. Vesa, Mihai Puiu, Dumitru Zdrenghea, Dana Pop, and Anca D. Buzoianu. 2019. "The Impact of Pulmonary Vein Anatomy on the Outcomes of Catheter Ablation for Atrial Fibrillation" Medicina 55, no. 11: 727. https://doi.org/10.3390/medicina55110727
APA StyleIstratoaie, S., Roșu, R., Cismaru, G., Vesa, Ș. C., Puiu, M., Zdrenghea, D., Pop, D., & Buzoianu, A. D. (2019). The Impact of Pulmonary Vein Anatomy on the Outcomes of Catheter Ablation for Atrial Fibrillation. Medicina, 55(11), 727. https://doi.org/10.3390/medicina55110727







