Outcome after Interdisciplinary Treatment for Aneurysmal Subarachnoid Hemorrhage—A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Interdisciplinary Setting
2.3. Statistical Analysis
2.4. Ethical Approval
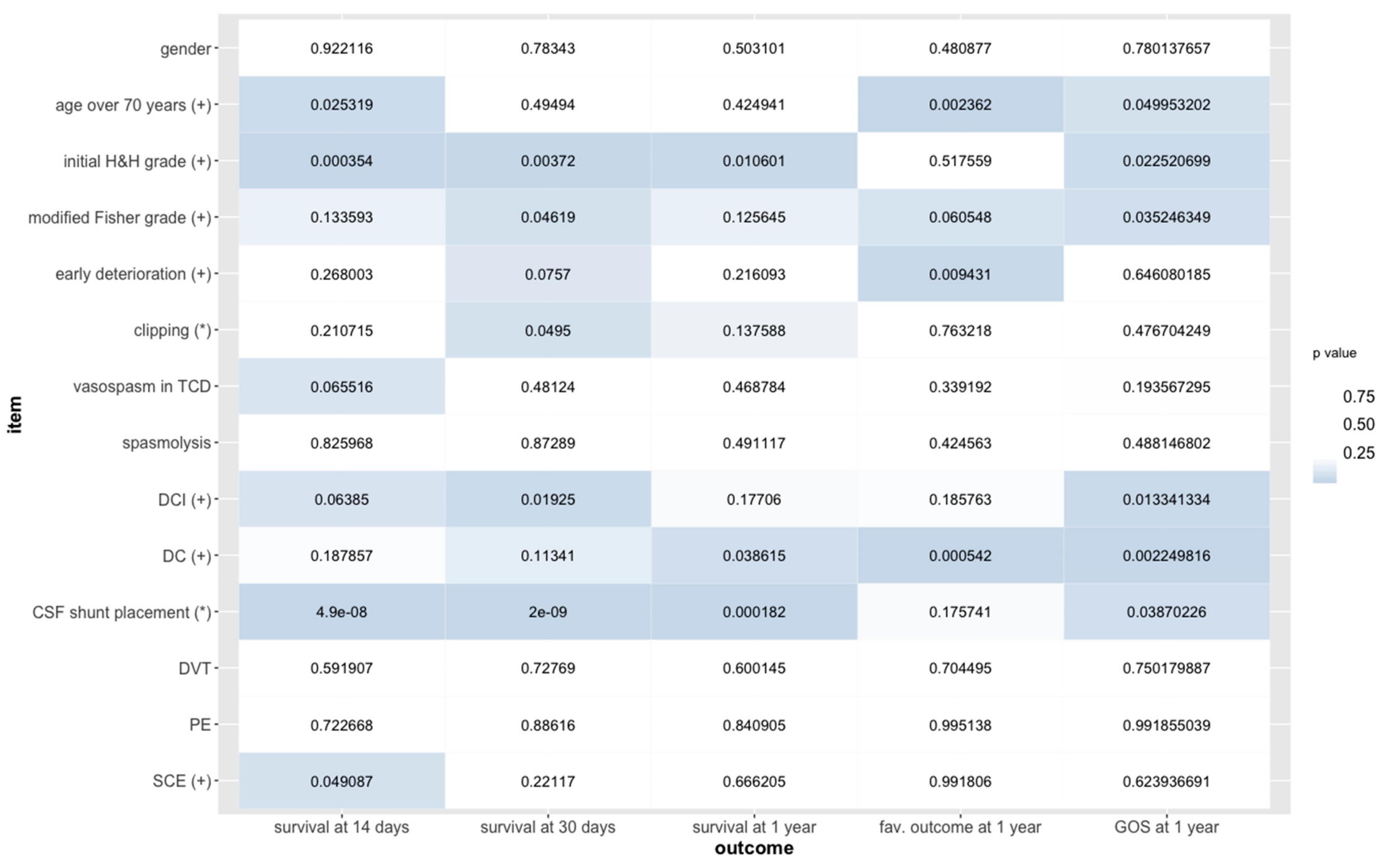
3. Results
4. Discussion
Limitations of Our Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molyneux, A.J.; Kerr, R.S.C.; Yu, L.M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar]
- Guglielmi, G.; Viñuela, F.; Dion, J.; Duckwiler, G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminar clinical experience. J. Neurosurg. 1991, 75, 8–14. [Google Scholar] [CrossRef]
- McDougall, C.G.; Spetzler, R.F.; Zabramski, J.M.; Partovi, S.; Hills, N.K.; Nakaji, P.; Albuquerque, F.C. The Barrow ruptured Aneurysm Trial. J. Neurosurg. 2012, 116, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; McDougall, C.G.; Zabramski, J.M.; Albuquerque, F.C.; Hills, N.K.; Russin, J.J.; Partovi, S.; Nakaji, P.; Wallace, R.C. The Barrow Ruptured Aneurysm Trial: 6-year results. J. Neurosurg. 2015, 123, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; McDougall, C.G.; Zabramski, J.M.; Albuquerque, F.C.; Hills, N.K.; Nakaji, P.; Karis, J.P.; Wallace, R.C. Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J. Neurosurg. 2019, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Güresir, E.; Beck, J.; Vatter, H.; Setzer, M.; Gerlach, R.; Seifert, V.; Raabe, A. Subarachnoid hemorrhage and intracerebral hematoma: Incidence, prognostic factors, and outcome. Neurosurgery 2008, 63, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Proust, F.; Martinaud, O.; Gérardin, E.; Derrey, S.; Lesvèque, S.; Bioux, S.; Tollard, E.; Clavier, E.; Langlois, O.; Godefroy, O.; et al. Quality of life and brain damage after microsurgical clip occlusion or endovascular coil embolization for ruptured anterior communicating artery aneurysms: Neuropsychological assessment. J. Neurosurg. 2009, 110, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Proust, F.; Gérardin, E.; Derrey, S.; Lesvèque, S.; Ramos, S.; Langlois, O.; Tollard, E.; Bénichou, J.; Chassagne, P.; Clavier, E. Interdisciplinary treatment of ruptured cerebral aneurysms in elderly patients. J. Neurosurg. 2010, 112, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Intraobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Schöller, K.; Massmann, M.; Markl, G.; Kunz, M.; Fesl, G.; Brückmann, H.; Pfefferkorn, T.; Tonn, J.C. Aneurysmal subarachnoid hemorrhage in elderly patients: Long-term outcome and prognostic factors in an interdisciplinary treatment approach. J. Neurol. 2013, 260, 1052–1060. [Google Scholar] [CrossRef]
- Sejkorova, A.; Cihlar, F.; Hejcl, A.; Lodin, J.; Vachata, P.; Sames, M. Microsurgery and endovascular treatment of posterior inferior cerebellar artery aneurysms. Neurosurg. Rev. 2016, 39, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.E.; Hess, R.M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J. Neurosurg. 1968, 28, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Pfefferkorn, T.; Ebrahimi, C.; Ottomeyer, C.; Fesl, G.; Bender, A.; Straube, A.; Pfister, H.W.; Heck, S.; Tonn, J.C.; et al. Long-term neurological outcome and quality of life after World Federation of Neurological Societies Grades IV and V aneurysmal subarachnoid hemorrhage in an interdisciplinary treatment concept. Neurosurgery 2017, 80, 967–974. [Google Scholar] [CrossRef] [PubMed]
- AlMatter, M.; Aguilar Pereza, M.; Bhogal, P.; Hellstern, V.; Ganslandt, O.; Henkes, H. Results of interdisciplinary management of 693 patients with aneurysmal subarachnoid hemorrhage: Clinical outcome and relevant prognostic factors. Clin. Neurol. Neurosurg. 2018, 167, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S., Jr.; MacDonald, R.L.; Mayer, S.A. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified Fisher scale. Neurosurgery 2006, 59, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Bond, M. Assessment of Outcome after severe brain damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- Subic, A.; Cermakova, P.; Norrving, B.; Winblad, B.; von Euler, M.; Kramberger, M.B.; Eriksdotter, M.; Garcia-Ptacek, S. Management of acute ischaemic stroke in patients with dementia. J. Intern. Med. 2017, 281, 348–364. [Google Scholar] [CrossRef]
- Claassen, J.; Bernardini, G.L.; Kreiter, K.; Bates, J.; Du, Y.E.; Copeland, D.; Connolly, E.S.; Mayer, S.A. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: The Fisher scale revisited. Stroke 2001, 32, 2012–2020. [Google Scholar] [CrossRef]
- Ransom, E.R.; Mocco, J.; Komotar, R.J.; Sahni, D.; Chang, J.; Hahn, D.K.; Kim, G.H.; Schmidt, J.M.; Sciacca, R.R.; Mayer, S.A.; et al. External ventricular drainage response in poor grade aneurysmal subarachnoid hemorrhage: Effect on preoperative grading and prognosis. Neurocrit. Care 2007, 6, 174–180. [Google Scholar] [CrossRef]
- van der Bilt, I.; Hasan, D.; van den Brink, R.; Cramer, M.J.; van der Jagt, M.; van Kooten, F.; Meertens, J.; van den Berg, M.; Groen, R.; Ten Cate, F.; et al. Cardiac dysfunction after aneurysmal subarachnoid hemorrhage: Relationship with outcome. Neurology 2014, 82, 351–358. [Google Scholar] [CrossRef]
- Norberg, E.; Odenstedt-Herges, H.; Rydenhag, B.; Oras, J. Impact of acute cardiac complications after subarachnoid hemorrhage on long-term mortality and cardiovascular events. Neurocrit. Care 2018, 29, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Zaroff, J.G.; Leong, J.; Kim, H.; Young, W.L.; Cullen, S.P.; Rao, V.A.; Sorel, M.; Quesenberry, C.P., Jr.; Sidney, S. Cardiovascular predictors of long-term outcomes after non-traumatic subarachnoid hemorrhage. Neurocrit. Care 2012, 17, 374–381. [Google Scholar] [CrossRef] [PubMed]
| Initial H&H Grade | Number of Ruptured Aneurysms Clipped (n = 108) | Number of Ruptured Aneurysms Coiled (n = 68) | Statistical Test, p-Value |
|---|---|---|---|
| 1 | 9 | 9 | |
| 2 | 27 | 16 | |
| 3 | 26 | 16 | |
| 4 | 29 | 17 | |
| 5 | 17 | 10 | |
| Wilcoxon rank sum, p = 0.14 |
| Modified Fisher Grade | Number of Ruptured Aneurysms Clipped (n = 108) | Number of Ruptured Aneurysms Coiled (n = 68) |
|---|---|---|
| 0 | 2 | 4 |
| 1 | 9 | 6 |
| 2 | 4 | 7 |
| 3 | 38 | 18 |
| 4 | 55 | 33 |
| Aneurysm Location | Number of Ruptured Aneurysms Clipped (n = 108) | Number of Ruptured Aneurysms Coiled (n = 68) | Statistical Test, p-Value |
|---|---|---|---|
| MCA 1 | 52 | 1 | |
| ACA 1 | 11 | 4 | |
| Acomm 1 | 27 | 26 | |
| ICA paraophthalmic 1 | 3 | 4 | |
| ICA supraophthalmic 1 | 13 | 12 | |
| Pcomm 1 | 2 | 0 | |
| SCA 2 | 0 | 1 | |
| PICA 2 | 0 | 3 | |
| Basilar artery 2 | 0 | 13 | |
| Vertebral artery 2 | 0 | 4 | |
| Ruptured aneurysms of the anterior circulation (subtotal of locations marked with superscript 1) * | 108 | 47 | |
| Ruptured aneurysms of the posterior circulation (subtotal of locations marked with superscript 2) * | 0 | 21 | |
| chi square, p < 0.001 |
| Clinical Event | Number of Ruptured Aneurysms Clipped (n = 108) | Number of Ruptured Aneurysms Coiled (n = 68) | Statistical Test, p-Value |
|---|---|---|---|
| EVD insertion = yes | 102 | 65 | Fisher’s exact, p = 1 |
| SCE = yes | 5 | 7 | Fisher’s exact, p = 0.218 |
| Vasospasm in TCD = yes | 39 | 16 | chi square, p = 0.113 |
| DCI = yes | 49 | 34 | chi square, p = 0.657 |
| Spasmolysis = yes | 5 | 4 | Fisher’s exact, p = 0.736 |
| DC performed = yes | 29 | 13 | chi square, p = 0.322 |
| CSF shunt placed = yes | 48 | 31 | chi square, p = 1 |
| DVT detected = yes * | 8 | 0 | Fisher’s exact, p = 0.024 |
| PE detected = yes | 4 | 1 | Fisher’s exact, p = 0.65 |
| Survival Status | Number of Ruptured Aneurysms Clipped (n = 108) | Number of Ruptured Aneurysms Coiled (n = 68) | Statistical Model, p-Value |
|---|---|---|---|
| Survived at 14 days | 102 | 60 | generalized linear modeling, p = 0.2107 |
| Survived at 30 days * | 101 | 58 | generalized linear modeling, p = 0.0495 |
| GOS | Number of Ruptured Aneurysms Clipped (n = 108) | Number of Ruptured Aneurysms Coiled (n = 68) | Statistical Model, p-Value |
|---|---|---|---|
| 1 at 1 year | 14 | 11 | |
| 2 at 1 year | 6 | 0 | |
| 3 at 1 year | 25 | 11 | |
| 4 at 1 year | 17 | 9 | |
| 5 at 1 year | 24 | 16 | |
| proportional odds logistic regression, p = 0.4767 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voellger, B.; Rupa, R.; Arndt, C.; Carl, B.; Nimsky, C. Outcome after Interdisciplinary Treatment for Aneurysmal Subarachnoid Hemorrhage—A Single Center Experience. Medicina 2019, 55, 724. https://doi.org/10.3390/medicina55110724
Voellger B, Rupa R, Arndt C, Carl B, Nimsky C. Outcome after Interdisciplinary Treatment for Aneurysmal Subarachnoid Hemorrhage—A Single Center Experience. Medicina. 2019; 55(11):724. https://doi.org/10.3390/medicina55110724
Chicago/Turabian StyleVoellger, Benjamin, Rosita Rupa, Christian Arndt, Barbara Carl, and Christopher Nimsky. 2019. "Outcome after Interdisciplinary Treatment for Aneurysmal Subarachnoid Hemorrhage—A Single Center Experience" Medicina 55, no. 11: 724. https://doi.org/10.3390/medicina55110724
APA StyleVoellger, B., Rupa, R., Arndt, C., Carl, B., & Nimsky, C. (2019). Outcome after Interdisciplinary Treatment for Aneurysmal Subarachnoid Hemorrhage—A Single Center Experience. Medicina, 55(11), 724. https://doi.org/10.3390/medicina55110724






