Characterization of Patients with Allergic Rhinitis to Ragweed Pollen in Two Distinct Regions of Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Site and Ethical Approval
2.2. Patient Evaluation
2.3. Statistical Analysis
3. Results
3.1. Sensitization to Ragweed Pollen
3.2. Severity of Allergic Rhinitis
3.3. Association of Other Allergic Manifestations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cecchi, L.; Bonini, S.; Nunes, C.; Behrendt, H.; Liccardi, G.; Popov, T.; Van Cauwenberge, P.; Annesi-Maesano, I.; D’Amato, G. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- Platts-Mills, T.A. The allergy epidemics: 1870–2010. J. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Marusciac, L.; Tamas, P.T.; Valenta, R.B.; Panaitescu, C. Ragweed Pollen Allergy: Burden, Characteristics, and Management of an Imported Allergen Source in Europe. Int. Arch. Allergy Immunol. 2018, 176, 163–180. [Google Scholar] [CrossRef]
- Storkey, J.; Stratonovitch, P.; Chapman, D.S.; Vidotto, F.; Semenov, M.A. A process-based approach to predicting the effect of climate change on the distribution of an invasive allergenic plant in Europe. PLoS ONE 2014, 9, e88156. [Google Scholar] [CrossRef]
- Burbach, G.J.; Heinzerling, L.M.; Röhnelt, C.; Bergmann, K.C.; Behrendt, H.; Zuberbier, T. Ragweed sensitization in Europe–GA2LEN study suggests increasing prevalence. Allergy 2009, 64, 664–665. [Google Scholar] [CrossRef]
- Thibaudon, M.; Hamberger, C.; Guilloux, L.; Massot, R. Ragweed pollen in France: Origin, diffusion, exposure. Eur. Ann. Allergy Clin. Immunol. 2010, 42, 209–215. [Google Scholar]
- Fumanal, B.; Chauvel, B.; Bretagnolle, F. Estimation of pollen and seed production of common ragweed in France. Ann. Agric. Environ. Med. 2007, 14, 233–236. [Google Scholar]
- DellaValle, C.T.; Triche, E.W.; Leaderer, B.P.; Bell, M.L. Effects of ambient pollen concentrations on frequency and severity of asthma symptoms among asthmatic children. Epidemiology 2012, 23, 55–63. [Google Scholar] [CrossRef]
- Salo, P.M.; Arbes, S.J., Jr.; Jaramillo, R.; Calatroni, A.; Weir, C.H.; Sever, M.L.; Hoppin, J.A.; Rose, K.M.; Liu, A.H.; Gergen, P.J.; et al. Prevalence of allergic sensitization in the United States: Results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J. Allergy Clin. Immunol. 2014, 134, 350–359. [Google Scholar] [CrossRef]
- Leru, P.M.; Eftimie, A.M.; Anton, V.F.; Thibaudon, M. Five-Year Data on Pollen Monitoring, Distribution and Health Impact of Allergenic Plants in Bucharest and the Southeastern Region of Romania. Medicina 2019, 55, 140. [Google Scholar] [CrossRef] [PubMed]
- Florincescu-Gheorghe, N.A.; Popescu, F.; Alexandru, D.O.; Popescu, F.D. The Prevalence of Allergic Rhinitis toAmbrosia Elatior in Oltenia Area and the Association with Allergic Conjunctivitis or Asthma. Curr. Health Sci. J. 2019, 45, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Ianovici, N.; Bunu, C.; Marusiac, L. Ambrosia artemisiifolia in Romania. J. Rom. Soc. Allergol. Clin. Immunol. 2009, 6, 146. [Google Scholar]
- Rodinkova, V.; Palamarchuk, O.; Toziuk, O.; Yermishev, O. Modeling hay fever risk factors caused by pollen from Ambrosia spp. using pollen load mapping in Ukraine. Acta Agrobot. 2018, 71, 1742. [Google Scholar] [CrossRef]
- Celenk, S.; Malyer, H. The occurrence of Ambrosia pollen in the atmosphere of Northwest Turkey: Investigation of possible source regions. Int. J. Biometeorol. 2017, 61, 1499–1510. [Google Scholar] [CrossRef]
- Sikoparija, B.; Skjøth, C.A.; Celenk, S.; Testoni, C.; Abramidze, T.; Kübler, K.A.; Belmonte, J.; Berger, U.; Bonini, M.; Charalampopoulos, A.; et al. Spatial and temporal variations in airborne Ambrosia pollen in Europe. Aerobiologia 2017, 33, 181–189. [Google Scholar] [CrossRef]
- Ferus, P.; Sîrbu, C.; Eliáš, P.; Konôpková, J.; Ďurišová, L.; Samuil, C.; Oprea, A. Reciprocal contamination by invasive plants: Analysis of trade exchange between Slovakia and Romania. Biologia 2015, 70, 893–904. [Google Scholar] [CrossRef]
- Matyasovszky, I.; Makra, L.; Tusnády, G.; Csépe, Z.; Nyú, L.G.; Chapman, l.S.; Sümeghy, Z.; Szűcs, G.; Páldy, A.; Magyar, D.; et al. Biogeographical drivers of ragweed pollen concentrations in Europe. Theor. Appl. Climatol. 2018, 133, 277–295. [Google Scholar] [CrossRef]
- Asero, R.; Wopfner, N.; Gruber, P.; Gadermaier, G.; Ferreira, F. Artemisia and Ambrosia hypersensitivity: Co-sensitization or co-recognition? Clin. Exp. Allergy 2006, 36, 658–665. [Google Scholar] [CrossRef]
- Ackermann-Liebrich, U.; Schindler, C.; Frei, P.; Probst-Hensch, N.M.; Imboden, M.; Gemperli, A.; Rochat, T.; Schmid-Grendemeier, P.; Bircher, A.J. Sensitisation to Ambrosia in Switzerland: A public health threat on the wait. Swiss Med. Wkly. 2009, 139, 70–75. [Google Scholar] [PubMed]
- Peternel, R.; Music Milanovic, S.; Srnec, L. Airborne ragweed (Ambrosia artemisiifolia L.) pollen content in the city of Zagreb and implications on pollen allergy. Ann. Agric. Environ. Med. 2008, 15, 125–130. [Google Scholar] [PubMed]
- Jones, N.R.; Agnew, M.; Banic, I.; Grossi, C.M.; Colón-González, F.J.; Plavec, D.; Goodess, C.M.; Epstein, M.M.; Turkalj, M.; Lake, I.R. Ragweed pollen and allergic symptoms in children: Results from a three-year longitudinal study. Sci. Total Environ. 2019, 683, 240–248. [Google Scholar] [CrossRef]
- Mehulić, M.; Mehulić, K.; Vuljanko, I.M.; Kukulj, S.; Grle, S.P.; Vukić, A.D.; Barisić, B.; Plavec, D. Changing pattern of sensitization in Croatia to aeroallergens in adult population referring to allergy clinic during a period of 15 years. Coll. Antropol. 2011, 35, 529–536. [Google Scholar]
- Tosi, A.; Wüthrich, B.; Bonini, M.; Pietragalla-Köhler, B. Time lag between Ambrosia sensitisation and Ambrosia allergy: A 20-year study (1989–2008) in Legnano, northern Italy. Swiss Med. Wkly. 2011, 141, w13253. [Google Scholar] [CrossRef]
- Ellis, A.K.; Ratz, J.D.; Day, A.G.; Day, J.H. Factors that affect the allergic rhinitis response to ragweed allergen exposure. Ann. Allergy Asthma Immunol. 2010, 104, 293–298. [Google Scholar] [CrossRef]
- Gelardi, M.; Bosoni, M.; Morelli, M.; Beretta, S.; Incorvaia, C.; Buttafava, S.; Landi, M.; Masieri, S.; Frati, F.; Quaranta, N.; et al. In children allergic to ragweed pollen, nasal inflammation is not influenced by monosensitization or polysensitization. J. Inflamm. Res. 2016, 9, 21–25. [Google Scholar] [CrossRef]
- Majkowska-Wojciechowska, B.; Pełka, J.; Korzon, L.; Kozłowska, A.; Kaczała, M.; Jarzębska, M.; Gwardys, T.; Kowalski, M.L. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy 2007, 62, 1044–1050. [Google Scholar] [CrossRef]
- Popovic-Grle, S.; Vrbica, Z.; Jankovic, M.; Klaric, I. Different phenotypes of intermittent and persistent respiratory allergy in Zagreb, Croatia. Ann. Agric. Environ. Med. 2009, 16, 137–142. [Google Scholar]
- Márk, Z.; Bikov, A.; Gálffy, G. Characterisctics of ragweed allergy in Hungary. Orv. Hetil. 2016, 157, 1989–1993. [Google Scholar] [CrossRef]
| Patients | NW Center | Central Center | p |
|---|---|---|---|
| Total no. of patients with AR | 405 pts | 706 pts | |
| Patient with AR to ragweed | 74 pts (18.27%) | 29 pts (4.10%) | < 0.001 |
| Parameter | NW Center (n = 74 pts) | Central Center (n = 29 pts) | p | |
|---|---|---|---|---|
| Age (age) * | 39.5 (31–47) | 42.5 (32.5–54) | 0.774 | |
| Gender ^ | M | 47.3% (n = 35) | 44.82% (n = 13) | |
| F | 52.7% (n = 39) | 55.17% (n = 16) | 0.530 | |
| Living area ^ | Urban | 77.02% (n = 57) | 72.41% (n = 21) | |
| Rural | 22.97% (n = 17) | 27.58% (n = 8) | 0.808 | |
| Severity of AR ^ | Mild Moderate/severe | 58.10% (n = 43) 41.90% (n = 31) | 10.3% (n = 3) 89.7% (n = 26) | <0.001 |
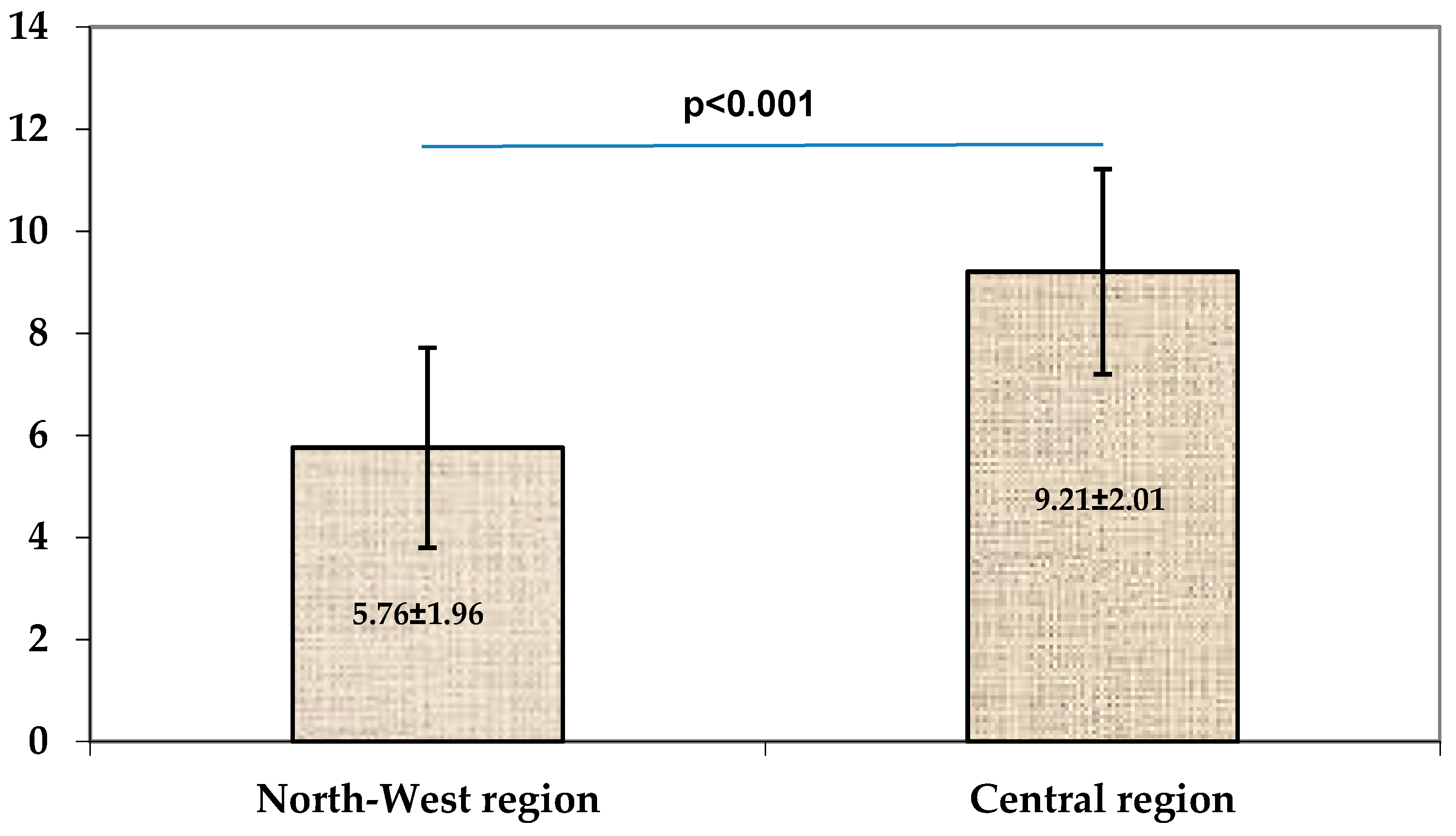
| Disease’s duration * (years) | 4.25 (2–6) | 12 (2–24) | 0.009 | |
| Family history of allergy | 14.90% (11) | 17.25% (5) | 0.344 |
| Parameter | NW Center | Central Center | ||||
|---|---|---|---|---|---|---|
| Monosens. (n = 20 pts) | Polysens. (n = 54 pts) | p | Monosens. (n = 6 pts) | Polysens. (n = 23 pts) | p | |
| AR severity | ||||||
| Mild Moderate- severe | 30% (n = 6) 70% (n = 14) | 68.5% (n = 37) 31.5% (n = 17) | 0.02 | 0% 100% (n = 6) | 13.8% (n = 4) 86.2% (n = 19) | 0.354 |
| TSS | 7.05 ± 1.83 | 5.27 ± 1.09 | <0.001 | 10.33 ± 2.07 | 8.91 ± 2.19 | 0.16 |
| Parameter | NW Center | Central Center | ||||
|---|---|---|---|---|---|---|
| Monosens (n = 20 pts) | Polysens (n = 54 pts) | p | Monosens (n = 6 pts) | Polysens (n = 23 pts) | p | |
| Allergic conjunctivitis | 65% (n = 13) | 33.33% (n = 18) | 0.01 | 100% (n = 6) | 69.56% (n = 16) | 0.12 |
| Bronchial asthma | 15% (n = 3) | 44.44% (n = 24) | 0.02 | 16.7% (n = 1) | 30.43% (n = 7) | 0.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocsan, I.C.; Muntean, I.A.; Ureche, C.; Pop, R.M.; Neag, M.A.; Sabin, O.; Deleanu, D.; Buzoianu, A.D. Characterization of Patients with Allergic Rhinitis to Ragweed Pollen in Two Distinct Regions of Romania. Medicina 2019, 55, 712. https://doi.org/10.3390/medicina55110712
Bocsan IC, Muntean IA, Ureche C, Pop RM, Neag MA, Sabin O, Deleanu D, Buzoianu AD. Characterization of Patients with Allergic Rhinitis to Ragweed Pollen in Two Distinct Regions of Romania. Medicina. 2019; 55(11):712. https://doi.org/10.3390/medicina55110712
Chicago/Turabian StyleBocsan, Ioana Corina, Ioana Adriana Muntean, Corina Ureche, Raluca Maria Pop, Maria Adriana Neag, Octavia Sabin, Diana Deleanu, and Anca Dana Buzoianu. 2019. "Characterization of Patients with Allergic Rhinitis to Ragweed Pollen in Two Distinct Regions of Romania" Medicina 55, no. 11: 712. https://doi.org/10.3390/medicina55110712
APA StyleBocsan, I. C., Muntean, I. A., Ureche, C., Pop, R. M., Neag, M. A., Sabin, O., Deleanu, D., & Buzoianu, A. D. (2019). Characterization of Patients with Allergic Rhinitis to Ragweed Pollen in Two Distinct Regions of Romania. Medicina, 55(11), 712. https://doi.org/10.3390/medicina55110712







