A Meta-Analysis on Randomised Controlled Clinical Trials Evaluating the Effect of the Dietary Supplement Chitosan on Weight Loss, Lipid Parameters and Blood Pressure
Abstract
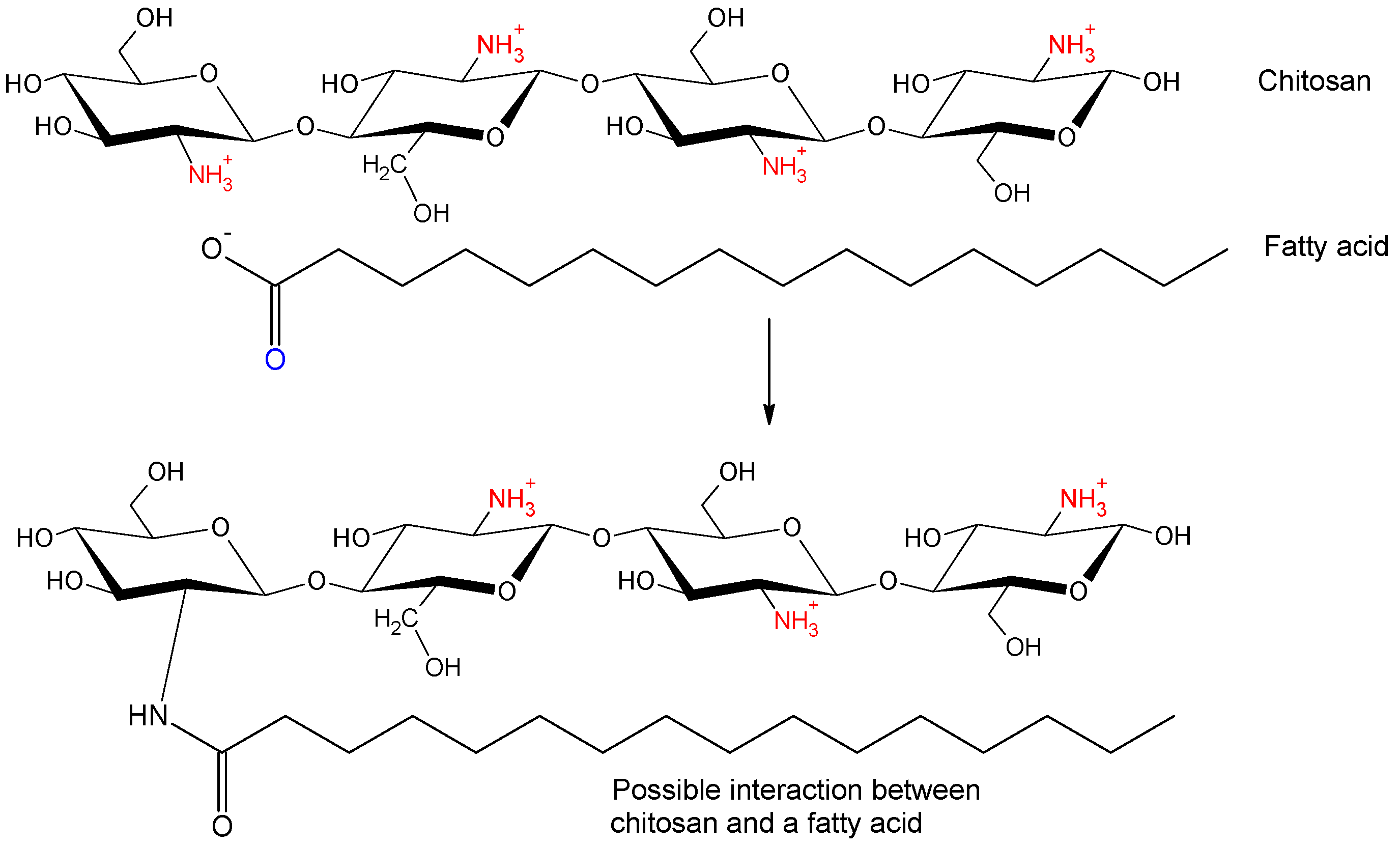
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quantitative Data Synthesis and Analysis
3. Results
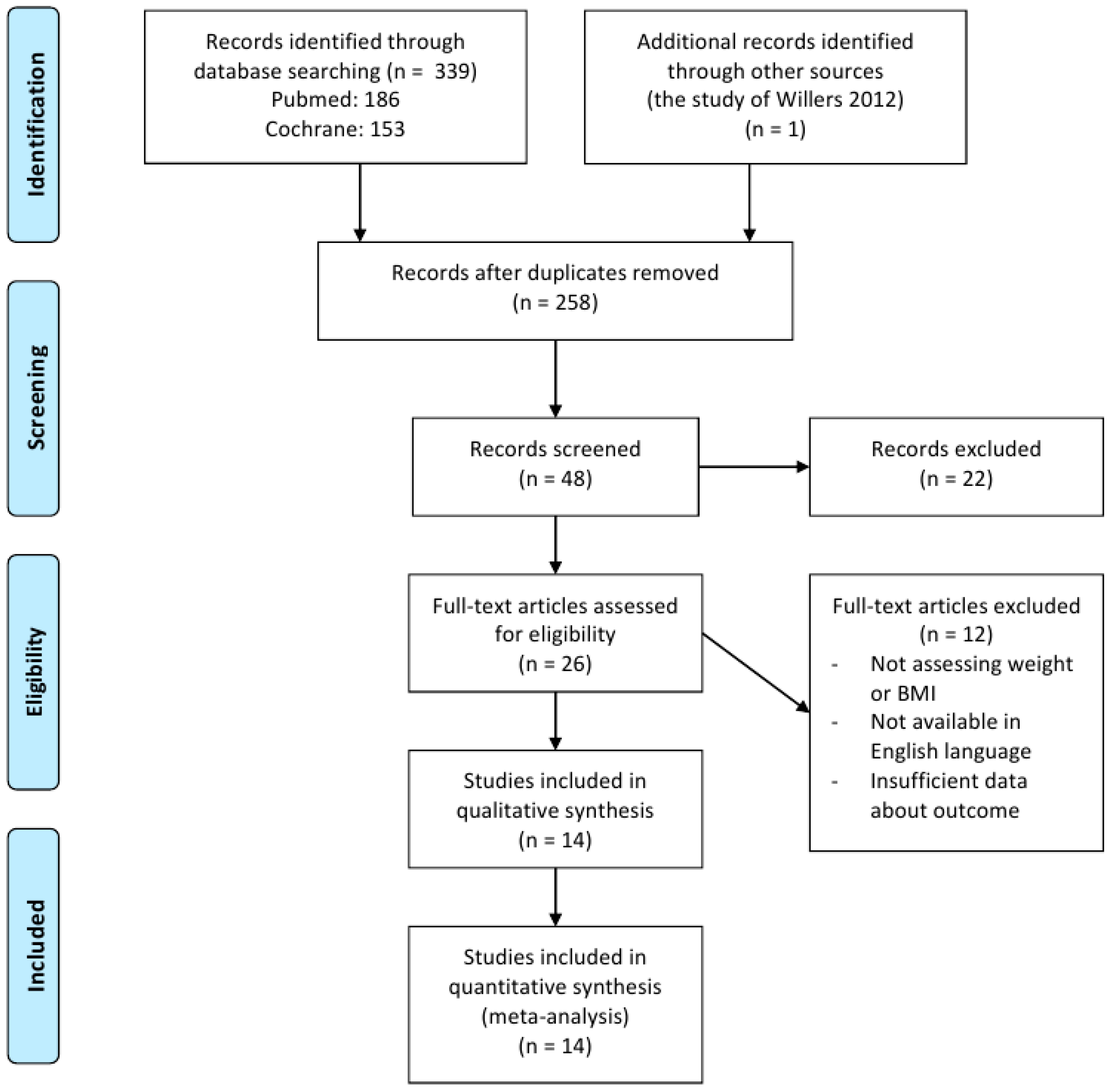
3.1. Retrieved Data and Characteristics of the Trials
3.2. Types of Outcome Measures
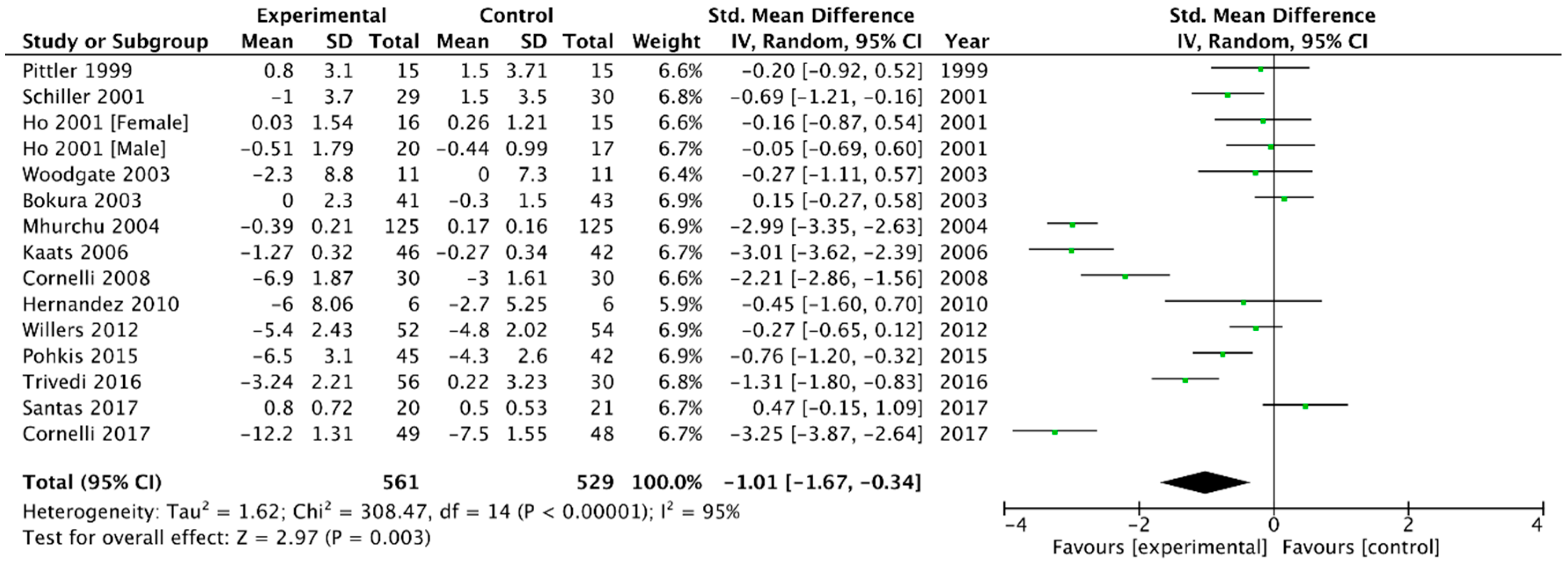
3.2.1. Body Weight
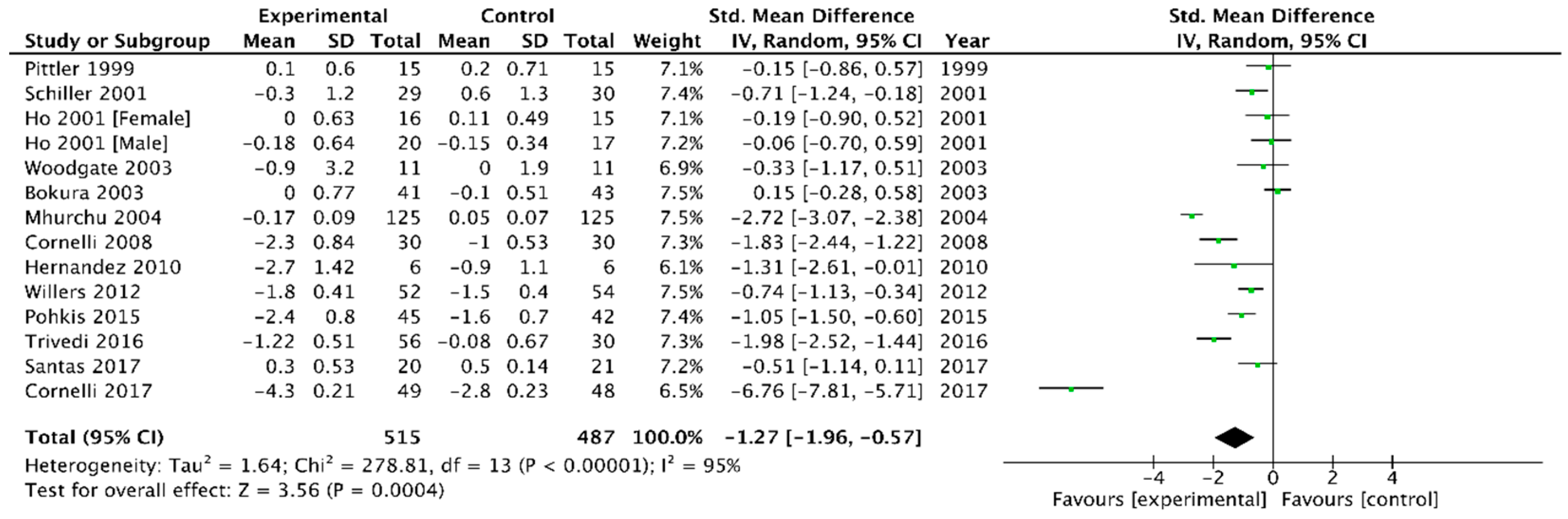
3.2.2. Body Mass Index (BMI)
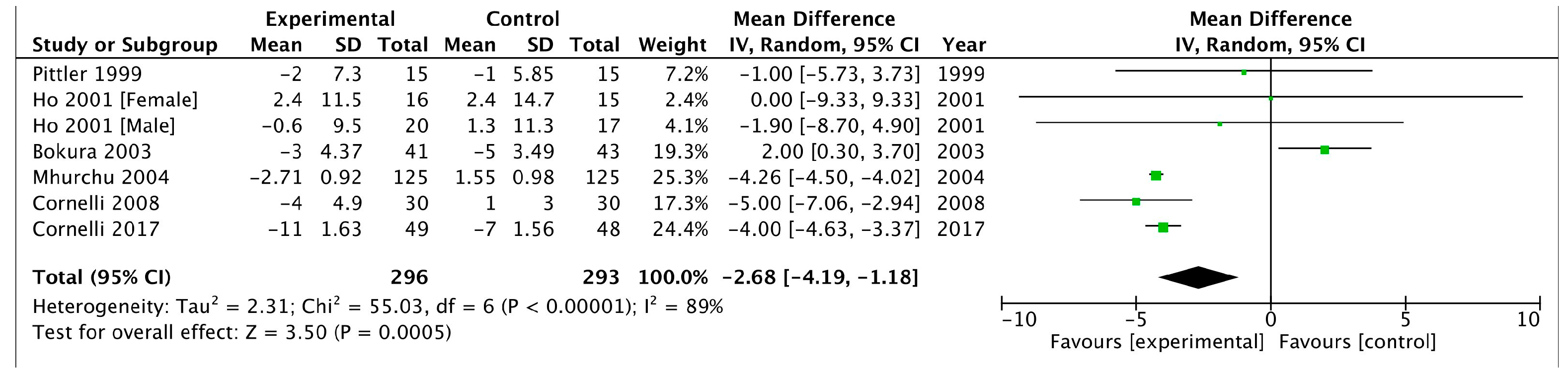
3.2.3. Blood Pressure
Systolic Blood Pressure
Diastolic Blood Pressure
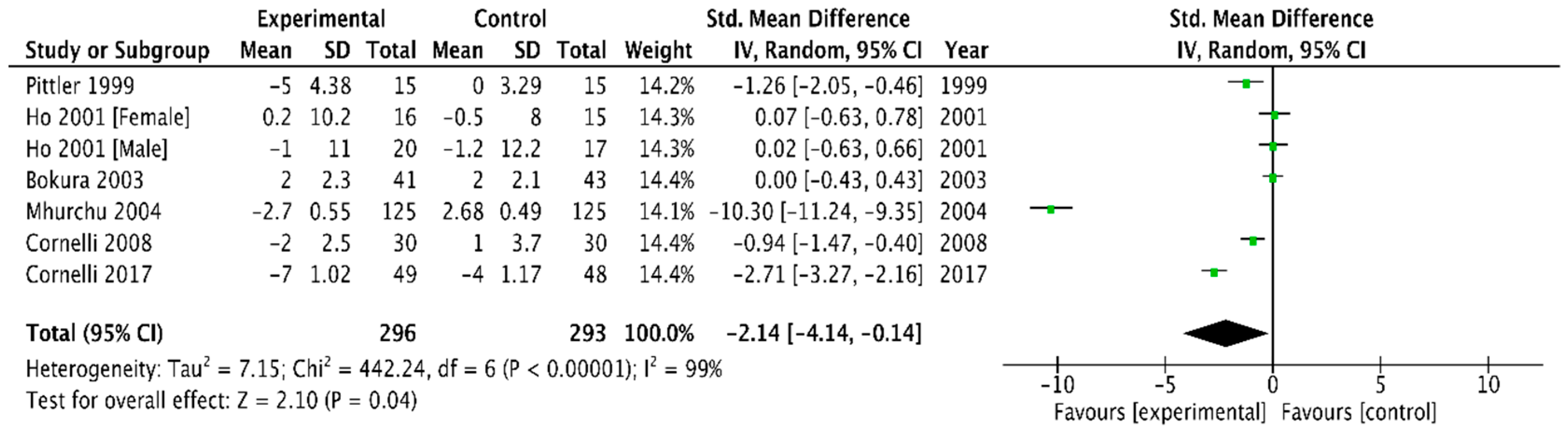
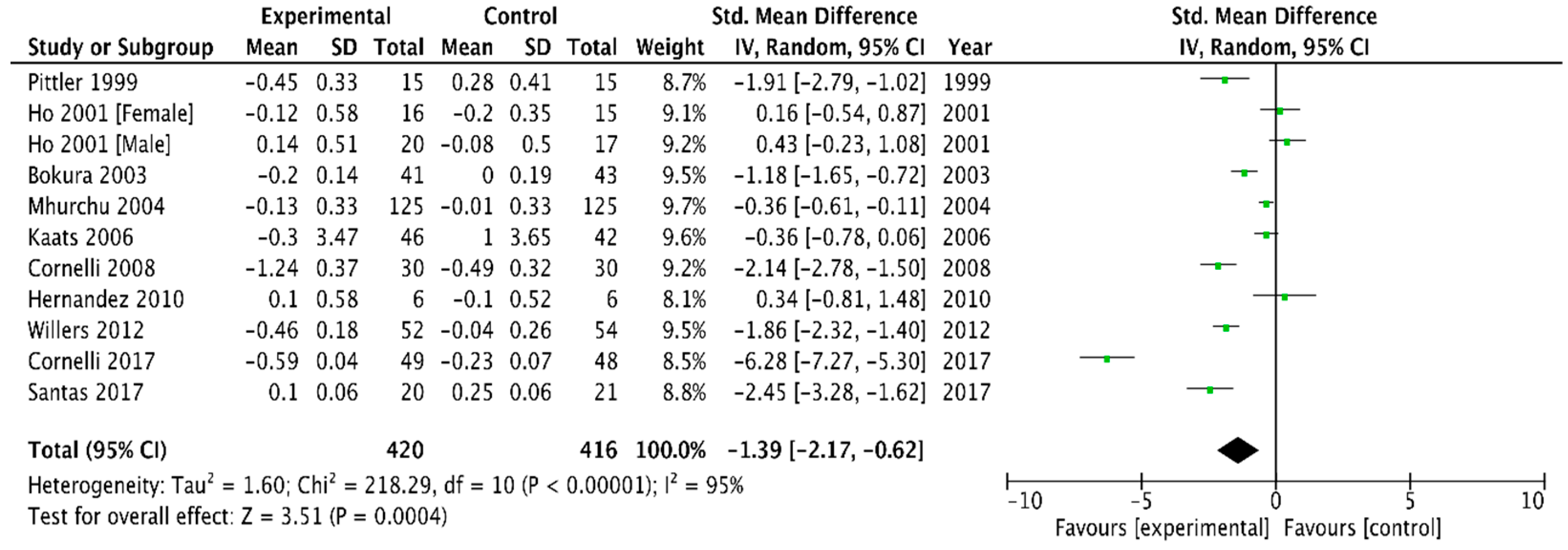
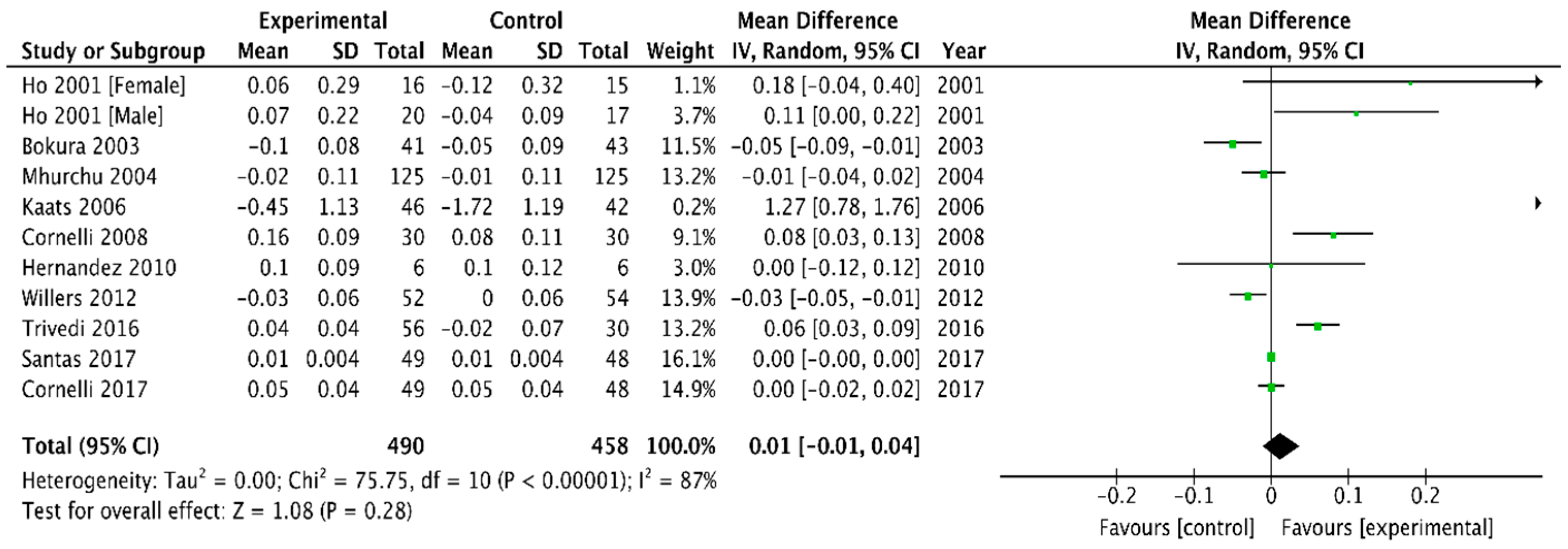
3.2.4. Plasma Cholesterol and Triglyceride Concentrations
Total Cholesterol
HDL Cholesterol
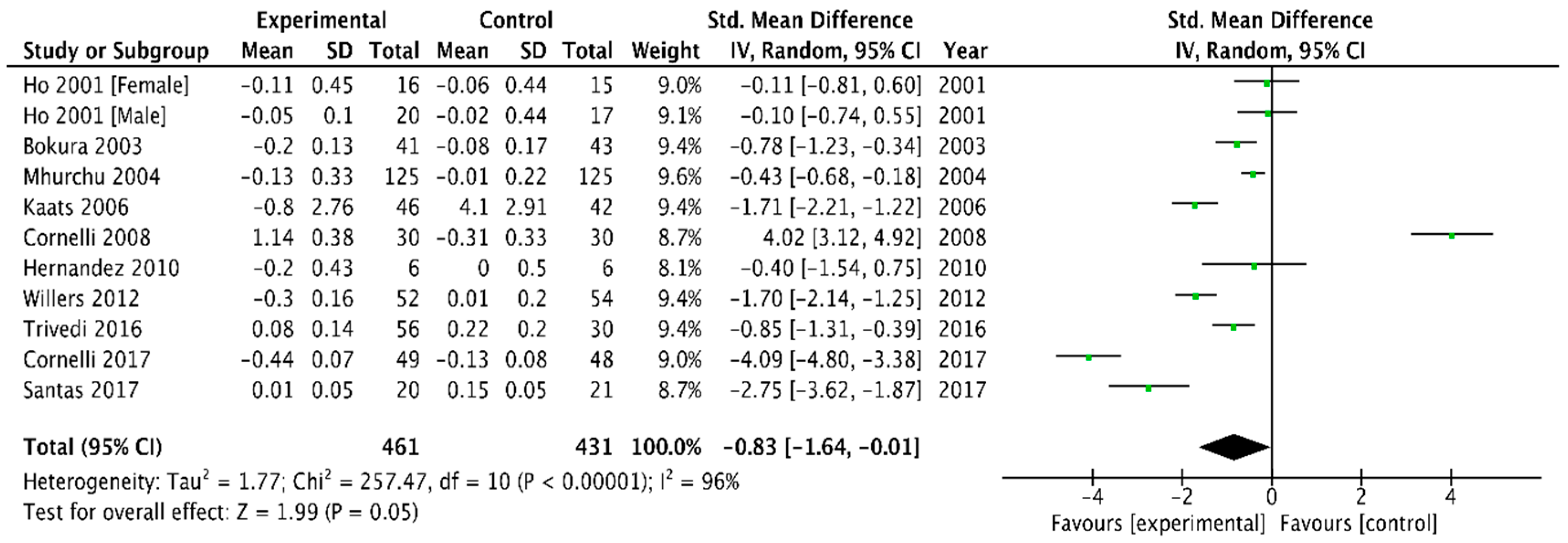
LDL Cholesterol
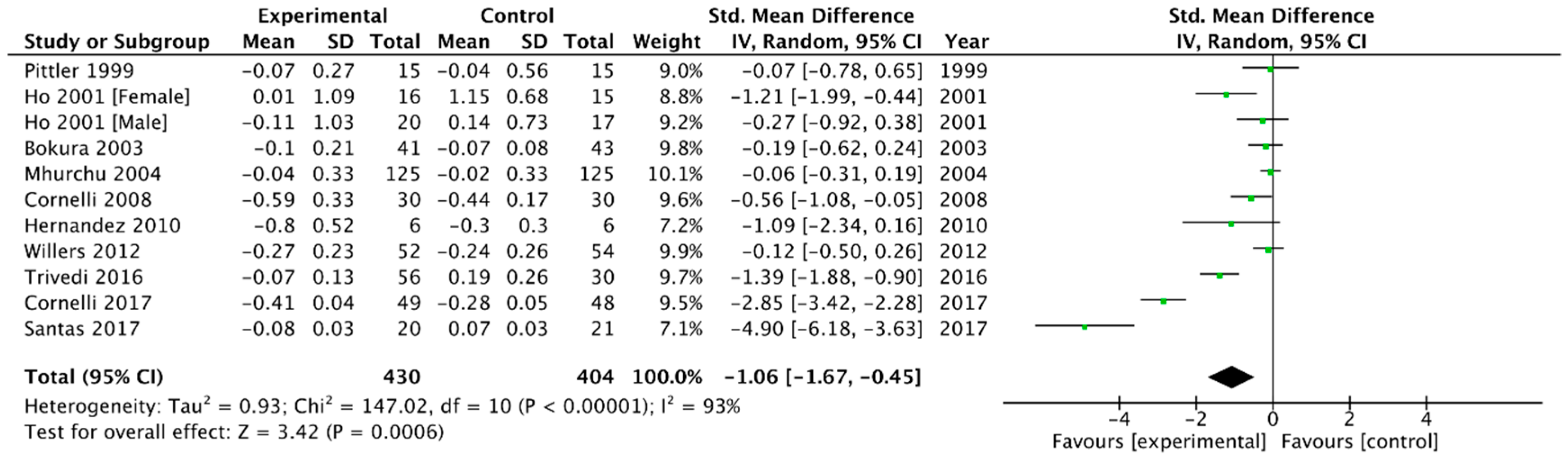
Triglycerides
4. Discussion
4.1. Comparison with Other Meta-Analysis
4.2. Strengths and Limitations of Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knight, J.A. Diseases and disorders associated with excess body weight. Ann. Clin. Lab. Sci. 2011, 41, 107–121. [Google Scholar] [PubMed]
- Praso, S.; Jusupovic, F.; Ramic, E.; Gledo, I.; Ferkovic, V.; Novakovic, B.; Hadzovic, E. Obesity as a risk factor for artherial hypertension. Mater. Socio-Med. 2012, 24, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Nessa, A.; Husain, M.F.; Wahed, F.; Khatun, N.; Yesmin, M.; Nasreen, S.; Tajkia, T. Effect of Obesity on Fasting Blood Sugar. Mymensingh Med. J. 2017, 26, 7–11. [Google Scholar] [PubMed]
- Khaodhiar, L.; Cummings, S.; Apovian, C.M. Treating diabetes and prediabetes by focusing on obesity management. Curr. Diabetes Rep. 2009, 9, 348–354. [Google Scholar] [CrossRef]
- Willers, J.; Plotz, S.C.; Hahn, A. The Combination of a High-protein Formula Diet and Polyglucosamine Decreases Body Weight and Parameters of Glucose and Lipid Metabolism in Overweight and Obese Men and Women. Eur. J. Food Res. Rev. 2012, 2, 29–45. [Google Scholar]
- Overweight and Obesity-BMI Statistics—Statistics Explained. 2014. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Overweight_and_obesity_-_BMI_statistics#Main_statistical_findings (accessed on 5 December 2018).
- Berghöfer, A.; Pischon, T.; Reinhold, T.; Apovian, C.M.; Sharma, A.M.; Wilich, S.N. Obesity prevalence from a European perspective: A systematic review. BMC Public Health 2008, 8, 200. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and managing the global epidemic: Report of a WHO consultation. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, D.B. Years of life lost due to obesity. JAMA 2003, 289, 187–193. [Google Scholar] [CrossRef]
- Picot, J.; Jones, J.; Colquitt, J.L.; Gospodarevskaya, E.; Loveman, E.; Baxter, L.; Clegg, A.J. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technol. Assess. 2009, 13, 1–190, 215–357. [Google Scholar] [CrossRef]
- Kleinert, S.; Horton, R. Rethinking and reframing obesity. Lancet 2015, 385, 2326–2328. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional and national prevalence of overweight and obesity in children and adults 1980–2013: A systematic analysis. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Brauer, P.; Connor Gorber, S.; Swan, E.; Singh, H.; Bell, N.; Shane, A.R.; Jaramillo, A.; Tonelli, M.; Canadian Task Force on Preventive Health Care. Recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. Can. Med. Assoc. J. 2015, 187, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chun, H.J. Endoscopic Treatment for Obesity: New Emerging Technology Trends. Gut Liver 2015, 9, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [PubMed]
- Sicinska, P.; Pytel, E.; Macczak, A.; Koter-Michalak, M. The use of various diet supplements in metabolic syndrome. Postepy Higieny i Medycyny Doswiadczalnej 2015, 69, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan—General Properties and Applications. Prog. Polym. Sci. 2007, 38, 603–632. [Google Scholar]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar]
- Park, J.K. Metabolic Pathway of Chitin and Its Oligosaccharides in Marine Bacterium Vibrios. In Chitin, Chitosan, Oligosaccharides and Their Derivatives; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 3–47. [Google Scholar]
- Gallaher, D.D.; Gallaher, C.M.; Mahrt, G.J.; Carr, T.P.; Hollingshead, C.H.; Hesselink, R., Jr.; Wise, J. A glucomannan and chitosan fiber supplement decreases plasma cholesterol and increases cholesterol excretion in overweight normocholesterolemic humans. J. Am. Coll. Nutr. 2002, 21, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Lutjohann, D.; Marinova, M.; Wolter, W.; Willinek, W.; Bitterlich, N.; Voenen, M.; Coch, C.; Stellaard, F. Influence of Chitosan Treatment on Surrogate Serum Markers of Cholesterol Metabolism in Obese Subjects. Nutrients 2018, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Abbot, N.C.; Harkness, E.F.; Ernst, E. Randomized, double-blind trial of chitosan for body weight reduction. Eur. J. Clin. Nutr. 1999, 53, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Lehtimaki, T.; Metso, S.; Ylitalo, R.; Rontu, R.; Nikkila, M.; Wuolijoki, E.; Ylitalo, P. Microcrystalline chitosan is ineffective to decrease plasma lipids in both apolipoprotein E epsilon 4 carriers and non-carriers: A long-term placebo-controlled trial in hypercholesterolaemic volunteers. Basic Clin. Pharmacol. Toxicol. 2005, 97, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Allaert, F.A. Effect of NaCl + Chitosan 3% vs. NaCl on high blood pressure parameters of healthy volunteers with prehypertension. Miner. Cardioangiol. 2017, 65, 563–576. [Google Scholar]
- Bokura, H.; Kobayashi, S. Chitosan decreases total cholesterol in women: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2003, 57, 721–725. [Google Scholar] [CrossRef] [PubMed]
- EFESA. Scientific Opinion on the substantiation of health claims related to chitosan and reduction in body weight (ID 679, 1499), maintenance of normal blood LDL-cholesterol concentrations (ID 4663), reduction of intestinal transit time (ID 4664) and reduction of inflammation (ID 1985) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2214. [Google Scholar] [CrossRef]
- Ylitalo, R.; Lehtinen, S.; Wuolijoki, E.; Ylitalo, P.; Lehtimaki, T. Cholesterol-lowering properties and safety of chitosan. Arzneimittelforschung 2002, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cornelli, U.; Belcaro, G.; Cesarone, M.R.; Coenlli, M. Use of polyglucosamine and physical activity to reduce body weight and dyslipidemia in moderately overweight subjects. Minerva Cardioangiol. 2008, 56, 71–78. [Google Scholar]
- Pokhis, K.; Bitterlich, N.; Coenelli, U.; Cassano, G. Efficacy of polyglucosamine for weight loss-confirmed in a randomized double-blind, placebo-controlled clinical investigation. BMC Obes. 2015, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, V.R.; Satia, M.C.; Deschamps, A.; Masquet, V.; Shah, R.B.; Zizuwadia, P.H.; Trivedi, J.V. Single-blind, placebo controlled randomised clinical study of chitosan for body weight reduction. Nutr. J. 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, S.O.; Gonzales-Ortiz, M.; Martinez-Abundis, E.; Robles-Cervantes, J.A. Chitosan improves insulin sensitivity as determined by the euglycemic-hyperinsulinemic clamp technique in obese subjects. Nutr. Res. 2010, 30, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Santas, J.; Lazaro, E.; Cune, J. Effect of a polysaccharide-rich hydrolysate from Saccharomyces cerevisiae (LipiGo(R)) in body weight loss: Randomised, double-blind, placebo-controlled clinical trial in overweight and obese adults. J. Sci. Food Agric. 2017, 97, 4250–4257. [Google Scholar] [CrossRef]
- Jull, A.B.; Nu Mhurchu, C.; Bennett, D.A.; Dunshea-Mooij, C.A.; Rodgers, A. Chitosan for overweight or obesity. Cochrane Database Syst. Rev. 2008, 3, CD003892. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.V. 9: Integrating Findings: The Meta-Analysis of Research. Rev. Res. Educ. 1977, 5, 351–379. [Google Scholar]
- Ni Mhurchu, C.; Poppitt, S.D.; McGill, A.T.; Leahy, F.E.; Bennett, D.A.; Lin, R.B.; Ormrod, D.; Ward, L.; Strik, C.; Rodgers, A. The effect of the dietary supplement, chitosan, on body weight: A randomised controlled trial in 250 overweight and obese adults. Int. J. Obes. 2004, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Schiller, R.N.; Barrager, E.; Schauss, A.G.; Nichols, E.J. A Randomized, Double-Blind, Placebo Controlled Study Examining the Effects of a Rapidly Soluble Chitosan Dietary Supplement on Weight Loss and Body Composition in Overweight and Mildly Obese Individuals. J. Am. Nutraceutical Assoc. 2001, 4, 42–49. [Google Scholar]
- Kaats, G.R.; Michalek, J.E.; Preuss, H.G. Evaluating efficacy of a chitosan product using a double-blinded, placebo-controlled protocol. J. Am. Coll. Nutr. 2006, 25, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Woodgate, D.E.; Conquer, J.A. Effects of a stimulant-free dietary supplement on body weight and fat loss in obese adults: A six-week exploratory study. Curr. Ther. Res. Clin. Exp. 2003, 64, 248–262. [Google Scholar] [CrossRef]
- Ho, S.C.; Tai, E.S.; Eng, P.H.; Tan, C.E.; Fok, A.C. In the absence of dietary surveillance, chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Singap. Med. J. 2001, 42, 6–10. [Google Scholar]
- Cornelli, U.; Belcaro, G.; Recchia, M.; D’Orazio, N. Long-Term Treatment of Overweight and Obesity with Polyglucosamine (PG L112): Randomized Study Compared with Placebo in Subjects after Caloric Restriction. Curr. Dev. Nutr. 2017, 1, e000919. [Google Scholar] [CrossRef]
- Chitosan Market Estimates and Trend Analysis by Application (Water Treatment, Pharmaceutical & Biomedical, Cosmetics, Food & Beverage), by Region (North America, Europe, Asia Pacific, RoW), by Country, And Segment Forecasts, 2018–2025. 2017. Available online: https://www.grandviewresearch.com/industry-analysis/global-chitosan-market (accessed on 12 May 2018).
- Colombo, P.; Sciutto, A.M. Nutritional aspects of chitosan employment in hypocaloric diet. Acta Toxicol. Ther. 1996, 17, 278–302. [Google Scholar]
- Giustina, A.; Ventura, P. Weight-reducing regimens in obese subjects: Effects of a new dietary fibre integrator. Acta Toxicol. Ther. 1995, 16, 199–214. [Google Scholar]
- Veneroni, G.; Veneroni, F.; Contos, S.; Tripodi, S.; De Bernardi, M.; Guarino, C.; Marletta, M. Effect of a new chitosan dietary integrator and hypocaloric diet on hyperlipidemia and overweight in obese patients. Acta Toxicol. Ther. 1996, 17, 53–70. [Google Scholar]
- Ernst, E.; Pittler, M.H. Chitosan as a treatment for body weight reduction: A meta-analysis. Perfusion 1998, 11, 461–465. [Google Scholar]
- Mhurchu, C.N.; Dunshea-Mooij, C.; Bennett, D.; Rodgers, A. Effect of chitosan on weight loss in overweight and obese individuals: A systematic review of randomized controlled trials. Obes. Rev. 2005, 6, 35–42. [Google Scholar] [CrossRef]
- Pyorala, K.; Pedersen, T.R.; Kjejshus, J.; Faergeman, O.; Olsson, A.G.; Thorgeirsson, G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997, 20, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Capes, S.E. Dysglycemia: A key cardiovascular risk factor. Semin. Vasc. Med. 2002, 2, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ausar, S.F.; Morcillo, M.; León, A.E.; Ribotta, P.D.; Masih, R.; Vilaro Mainero, M.; Amigone, J.L.; Rubin, G.; Lescano, C.; Castagna, L.F.; et al. Improvement of HDL- and LDL-cholesterol levels in diabetic subjects by feeding bread containing chitosan. J. Med. Food 2003, 6, 397–399. [Google Scholar] [CrossRef]
- Tai, T.S.; Sheu, W.H.; Lee, W.J.; Yao, H.T.; Chiang, M.T. Effect of chitosan on plasma lipoprotein concentrations in type 2 diabetic subjects with hypercholesterolemia. Diabetes Care 2000, 23, 1703–1704. [Google Scholar] [CrossRef]
- Guha, S.; Pal, S.K.; Chatterjee, N.; Sarkar, G.; Pal, S.; Guha, S.; Basu, A.K.; Banerjee, R. Effect of chitosan on lipid levels when administered concurrently with atorvastatin--a placebo-controlled study. J. Indian Med. Assoc. 2005, 103, 418, 420. [Google Scholar]
- Macchi, G. A new approach to the treatment of obesity: Chitosan’s effects on body weight reduction and plasma cholesterol’s levels [Un nuovo approccio al trattamento dell’obesita: Effetti del chitosano sulla riduzione del peso corporeo e sulla colesterolemia]. Acta Toxicol. Ther. 1996, 17, 301–302. [Google Scholar]
- Sciutto, A.M.; Colombo, P. Lipid-lowering effect of chitosan dietary integrator and hypocaloric diet in obese subjects [Effetto antilipemico dell’integratore dietetico Chitosano e della dieta ipocalorica in soggetti obesi]. Acta Toxicol. Ther. 1995, 16, 215–230. [Google Scholar]
| No.crt. | Reference (Author, Year) | No. of Participants | Study Design ** | Participants’ Age (Years) | Participants’ Gender | Participants’ Baseline BMI (kg/m2) | Chitosan Dosage Form | Chitosan Administration (g/Day) | Duration (Days) | Confounding Variables (Other Interventions or Active Ingredients) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pittler, 1999 | 30 | R, DB | 18–60 | M, F | treatment: 26.3 placebo: 26.9 | capsules | 1 | 28 | Participants were excluded if they were currently following a diet. |
| 2 | Schiller, 2001 | 59 | R, DB | 21–55 | F | treatment: 32.2 placebo: 31.8 | capsules | 3 | 56 | Subjects were instructed to continue their regular caloric intake. The chitosan capsules contained >90% chitosan and <10% succinic acid. |
| 3 | Ho 2001 (Male) | 37 | R, DB | treatment: 42.4 * placebo: 42.5 * | M | treatment: 25.7 placebo: 27.0 | capsules | 3.1 | 84 | No restrictions or monitoring of dietary habits. |
| Ho 2001 (Female) | 31 | R, DB | treatment: 42.8 * placebo: 44.3 * | F | treatment: 25.6 placebo: 24.6 | capsules | 3.1 | 84 | No restrictions or monitoring of dietary habits. | |
| 4 | Bokura 2003 | 84 | R, DB | 34–70 | F | treatment: 23.6 placebo: 22.3 | capsules | 1.2 | 56 | Subjects were instructed to continue their regular diet. |
| 5 | Woodgate, 2003 | 22 | R, DB | 20–50 | M, F | treatment: 36.8 placebo: 34.6 | capsules | no information available | 42 | Subjects were instructed to continue their regular diet and exercise patterns. The capsules contained additional active ingredients (glucomannan, chitosan, fenugreek, G sylvestre, and vitamin C) |
| 6 | Mhurchu, 2004 | 250 | R, DB | >18 | M, F | treatment: 34.8 placebo: 36 | capsules | 3 | 168 | All participants received standardized dietary and lifestyle advice for weight loss. |
| 7 | Kaats 2006 | 88 | R, DB | treatment: 43.9 * placebo: 48.7 * | M, F | not available not available | capsules | 3 | 60 | Subjects were asked to follow a behavior modification plan and their physical activity was monitored. The treatment group took the following substances in addition: Beta-glucan, sno white oat fiber, betaine hydrochloride and aloe saponins (1 mg of each). |
| 8 | Cornelli, 2008 | 56 | R, DB | 30–60 | M, F | treatment: 27.4 placebo: 27.4 | tablets | 2 | 122 | Intake of at least 1.5 L of water per day. Patients were asked to keep their habitual diet. In addition to chitosan, the capsules contained L-ascorbic acid (6%) and tartaric acid (3%). |
| 9 | Hernandez, 2010 | 12 | R, DB | 30–50 | M, F | treatment: 34.3 placebo: 32.7 | no information available | 2.25 | 91 | All patients received general recommendations about their medical nutritional therapy and were instructed to not modify their usual forms of exercise. |
| 10 | Willers, 2012 | 120 | R, DB | 30–60 | M, F | treatment: 31.7 placebo: 31.7 | tablets | 0.8 | 84 | One serving of protein-rich formula diet a day. |
| 11 | Pohkis 2015 | 87 | R, DB | 21–75 | M, F | treatment: 35 placebo: 35 | tablets | 3.4 | 175 | A daily calorie deficit (500 cal) and an increased daily physical activity (7 MET ***-h/ week). |
| 12 | Trivedi, 2016 | 96 | R, SB | 18–65 | M, F | treatment: 30.93 placebo: 30.91 | capsules | 2.5 | 90 | Subjects were advised to maintain their normal routine diet. |
| 13 | Cornelli, 2017 | 97 | R, DB | 25–65 | M, F | treatment: 33.9 placebo: 34.1 | tablets | 1.6 | 365 | 10% calorie restriction and an increase in physical activity (9 MET-h/wk). |
| 14 | Santas, 2017 | 41 | R, DB | 18–65 | M, F | treatment: 29.1 placebo: 29.2 | caps with solvable content | 0.343 | 84 | Diet was not controlled and participants were asked not to alter their dietary habits and physical activity. The administered caps contained a beta-glucan-chitin-chitosan fraction (BGCC) |
| Parameter | Jull [35] | Our Study |
|---|---|---|
| Weight | −1.71 (−2.09, −1.32) | −1.01 (−1.67, −0.34) |
| BMI | −0.35 (−0.55, −0.15) | −1.27 (−1.96, −0.57) |
| TC | −0.21 (−0.28, −0.13) | −1.39 (−2.17, −0.62) |
| HDL | 0.03 (0.01, 0.05) | 0.01 (−0.01, 0.04) |
| LDL | −0.16 (−0.23, −0.10) | −0.83 (−1.64, −0.01) |
| TG | −0.12 (−0.19, −0.06) | −1.06 (−1.67, −0.45) |
| SBP | −5.94 (−7.25, −4.63) | −2.68 (−4.19, −1.18) |
| DBP | −3.38 (−4.35, −2.42) | −2.14 (−4.14, −0.14) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraru, C.; Mincea, M.M.; Frandes, M.; Timar, B.; Ostafe, V. A Meta-Analysis on Randomised Controlled Clinical Trials Evaluating the Effect of the Dietary Supplement Chitosan on Weight Loss, Lipid Parameters and Blood Pressure. Medicina 2018, 54, 109. https://doi.org/10.3390/medicina54060109
Moraru C, Mincea MM, Frandes M, Timar B, Ostafe V. A Meta-Analysis on Randomised Controlled Clinical Trials Evaluating the Effect of the Dietary Supplement Chitosan on Weight Loss, Lipid Parameters and Blood Pressure. Medicina. 2018; 54(6):109. https://doi.org/10.3390/medicina54060109
Chicago/Turabian StyleMoraru, Cristina, Manuela Maria Mincea, Mirela Frandes, Bogdan Timar, and Vasile Ostafe. 2018. "A Meta-Analysis on Randomised Controlled Clinical Trials Evaluating the Effect of the Dietary Supplement Chitosan on Weight Loss, Lipid Parameters and Blood Pressure" Medicina 54, no. 6: 109. https://doi.org/10.3390/medicina54060109
APA StyleMoraru, C., Mincea, M. M., Frandes, M., Timar, B., & Ostafe, V. (2018). A Meta-Analysis on Randomised Controlled Clinical Trials Evaluating the Effect of the Dietary Supplement Chitosan on Weight Loss, Lipid Parameters and Blood Pressure. Medicina, 54(6), 109. https://doi.org/10.3390/medicina54060109






