Measurements of Inferior Vena Cava Diameter for Prediction of Hypotension and Bradycardia during Spinal Anesthesia in Spontaneously Breathing Patients during Elective Knee Joint Replacement Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lawicka, M.; Malek, A.; Antczak, D.; Wajlonis, A.; Owczuk, R. Non-invasive haemodynamic measurements with Nexfin predict the risk of hypotension following spinal anaesthesia. Anaesthesiol. Intensive Ther. 2015, 47, 403–408. [Google Scholar]
- Owczuk, R.; Wenski, W.; Polak-Krzeminska, A.; Twardowski, P.; Arszulowicz, R.; Dylczyk-Sommer, A.; Wujtewicz, M.A.; Sawicka, W.; Morzuch, E.; Smietanski, M.; et al. Ondansetron given intravenously attenuates arterial blood pressure drop due to spinal anesthesia: A double-blind, placebo-controlled study. Reg. Anesth. Pain Med. 2008, 33, 432–439. [Google Scholar]
- Carpenter, R.L.; Caplan, R.A.; Brown, D.L.; Stephenson, C.; Wu, R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology 1992, 76, 606–616. [Google Scholar] [CrossRef]
- Park, S.; Faiz, S.; Rahimzadeh, P.; Imani, F.; Bakhtiari, A.; Choi, B.; Bang, J.; Jung, K.; Lee, J.; Bae, H.; et al. Prediction of hypotension in spinal anesthesia. Korean J. Anesthesiol. 2013, 65, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Borodičienė, J.; Gudaitytė, J. Haemodynamic effects of central neural blocks. Acta Med. Litu. 2012, 19. [Google Scholar] [CrossRef]
- Šoštaric, S.; Oremuš, K. Sudden cardiorespiratory arrest following spinal anesthesia. Period. Biol. 2013, 115, 283–288. [Google Scholar]
- Kumari, A.; Gupta, R.; Bajwa, S.J.S.; Singh, A. Unanticipated cardiac arrest under spinal anesthesia: An unavoidable mystery with review of current literature. Anesth. Essays Res. 2014, 8, 19. [Google Scholar]
- Stienstra, R. Mechanisms behind and treatment of sudden, unexpected circulatory collapse during central neuraxis blockade. Acta Anaesthesiol. Scand. 2000, 44, 865–871. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Braslow, B.M.; Panebianco, N.L.; Kirkpatrick, J.N.; Gracias, V.H.; Hayden, G.E.; Dean, A.J. Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: Correlations with CVP. J. Am. Coll. Surg. 2009, 209, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Nagdev, A.D.; Merchant, R.C.; Tirado-Gonzalez, A.; Sisson, C.A.; Murphy, M.C. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann. Emerg. Med. 2010, 55, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Weekes, A.J.; Tassone, H.M.; Babcock, A.; Quirke, D.P.; Norton, H.J.; Jayarama, K.; Tayal, V.S. Comparison of serial qualitative and quantitative assessments of caval index and left ventricular systolic function during early fluid resuscitation of hypotensive emergency department patients. Acad. Emerg. Med. 2011, 18, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.M.; Blair, J.E.; Goonewardena, S.; Ronan, A.; Shah, D.; Vasaiwala, S.; Kirkpatrick, J.N.; Spencer, K.T. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J. Am. Soc. Echocardiogr. 2007, 20, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Bobbia, X.; Toumi, M.; Louart, G.; Molinari, N.; Ragonnet, B.; Quintard, H.; Leone, M.; Zoric, L.; Lefrant, J.Y. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit. Care 2012, 16, 5–7. [Google Scholar] [CrossRef] [PubMed]
- De Valk, S.; Olgers, T.J.; Holman, M.; Ismael, F.; Ligtenberg, J.J.M.; ter Maaten, J.C. The caval index: An adequate non-invasive ultrasound parameter to predict fluid responsiveness in the emergency department? BMC Anesthesiol. 2014, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Airapetian, N.; Maizel, J.; Alyamani, O.; Mahjoub, Y.; Lorne, E.; Levrard, M.; Ammenouche, N.; Seydi, A.; Tinturier, F.; Lobjoie, E.; et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit. Care 2015, 19, 400. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.M.; Ronan, A.; Goonewardena, S.; Blair, J.E.; Hammes, M.; Shah, D.; Vasaiwala, S.; Kirkpatrick, J.N.; Spencer, K.T. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin. J. Am. Soc. Nephrol. Am. Soc. Nephrol. 2006, 1, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Veering, B. Physiological Aspects of Central Blockade; Euroanaesthesia Austria: Vienna, Austria, 2005; pp. 23–27. [Google Scholar]
- Huang, S.J.; McLean, A.S. Appreciating the strengths and weaknesses of transthoracic echocardiography in hemodynamic assessments. Cardiol. Res. Pract. 2012, 2012, 894308. [Google Scholar] [CrossRef] [PubMed]
- Cowie, B. Three years’ experience of focused cardiovascular ultrasound in the peri-operative period. Anaesthesia 2011, 66, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Canty, D.; Royse, C.; Kilpatrick, D.; Bowman, L.; Royse, A. The impact of focused transthoracic echocardiography in the pre-operative clinic. Anaesthesia 2012, 67, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Lynes, H.; Griffiths, R. Focused transthoracic echocardiography in hip fracture surgery. Anaesthesia 2013, 68, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Steinberg, D.; Thom, C.; Shirazi, M.; Papanagnou, D.; Ku, B.S.; Fields, J.M. Ultrasound measurement of inferior vena cava collapse predicts propofol-induced hypotension. Am. J. Emerg. Med. 2016, 34, 6125–6128. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Kelly, N.; Papadimos, T.J.; Bahner, D.P.; Stawicki, S.P. Correlations between Venous Collapsibility and Common Hemodynamic and Ventilatory Parameters: A Multi-Variable Assessment. OPUS 12 Sci. 2007, 63, 495–500. [Google Scholar]
- Zhang, Z.; Xu, X.; Ye, S.; Xu, L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: Systematic review and meta-analysis. Ultrasound Med. Biol. 2014, 40, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Corl, K.; Napoli, A.M.; Gardiner, F. Bedside sonographic measurement of the inferior vena cava caval index is a poor predictor of fluid responsiveness in emergency department patients. Emerg. Med. Australas. 2012, 24, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Laborda, A.; Sierre, S.; Malvè, M.; De Blas, I.; Ioakeim, I.; Kuo, W.T.; De Gregorio, M.A. Influence of breathing movements and Valsalva maneuver on vena caval dynamics. World J. Radiol. 2014, 6, 1033. [Google Scholar] [CrossRef] [PubMed]
- Kimura, B.J.; Dalugdugan, R.; Gilcrease, G.W.; Phan, J.N.; Showalter, B.K.; Wolfson, T. The effect of breathing manner on inferior vena caval diameter. Eur. Heart J. Cardiovasc. Imaging 2011, 12, 220–223. [Google Scholar] [CrossRef] [PubMed]
| Total | Hypotensive | Nonhypotensive | p Value * | |
|---|---|---|---|---|
| Sex, n (%) Male Female | 14 (23.3) 46 (76.7) | 4 (28.6) 11 (23.9) | 11 (71.4) 35 (76.1) | 0.487 |
| Age, mean (SD), years | 69.35 (9.14) | 66.13 (7.3) | 70.4 (9.5) | 0.16 |
| ASA status, n (%) I II | 8 (100) 52 (100) | 2 (25) 13 (25) | 6 (75) 39 (75) | 0.65 |
| Body mass index, mean (SD), kg/m2 | 30.97 (4.77) | 33 (4.1) | 30 (4.8) | 0.706 |
| Body surface area, mean (SD), m2 | 2.043 (0.182) | 2.1 (0.159) | 2.01 (0.183) | 0.98 |
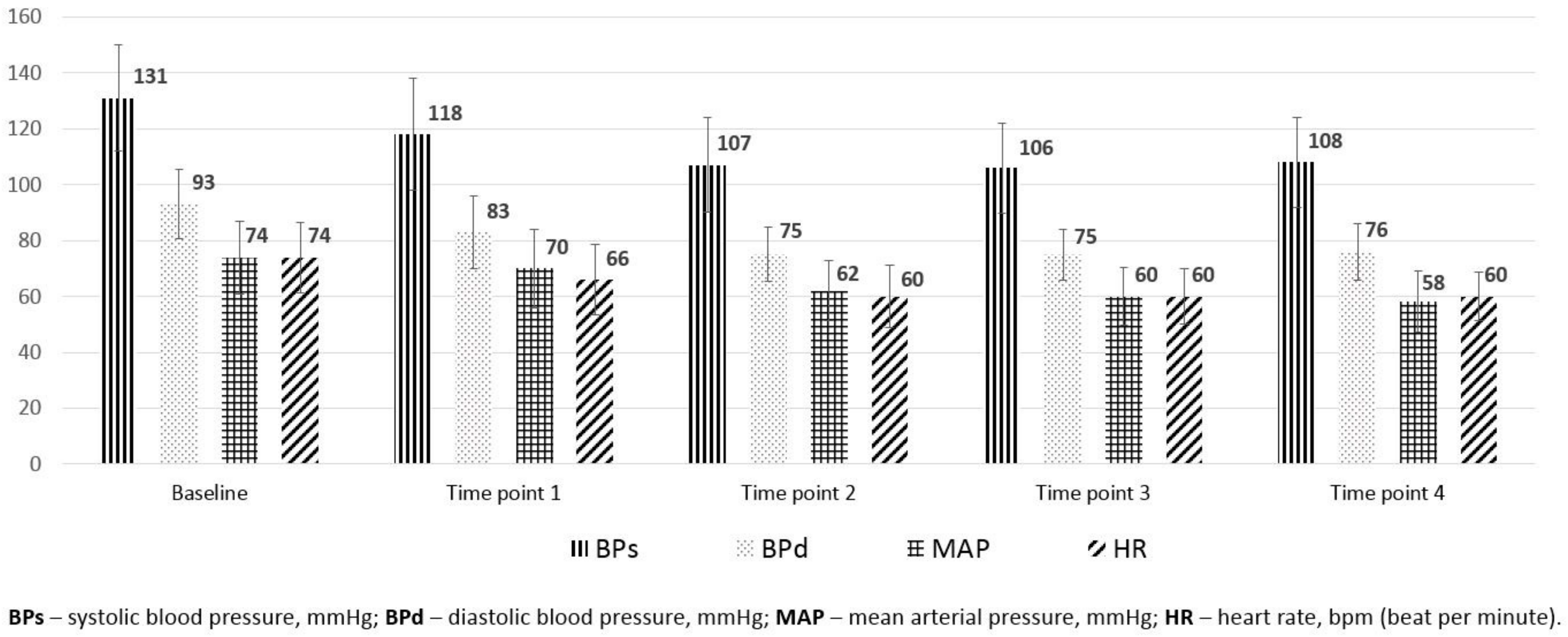
| MAP before spinal anesthesia (SA), mean (SD), mmHg | 93 (12.9) | 101 (13.15) | 90 (11.72) | 0.34 |
| HR before SA, mean (SD), bpm | 73 (12.5) | 72 (11.4) | 74 (12.9) | 0.54 |
| Breathing rate, mean (SD), b/min | 14 (2) | 13 (1.6) | 14 (2.5) | 0.25 |
| IVCex baseline, mean (SD), mm | 13.9 (5.5) | 13.7 (3.9) | 13.9 (5.9) | 0.911 |
| IVCin baseline, mean (SD), mm | 8.9 (4.3) | 8.5 (3.3) | 9 (4.6) | 0.676 |
| IVC-CI baseline, mean (SD), % | 35.6 (18.7) | 38.4 (18.5) | 34.7 (18.8) | 0.521 |
| Total | Bradycardic | Nonbradycardic | pValue † | |
| Sex, n (%) Male Female | 14 (23.3) 46 (76.7) | 4 (28.6) 7 (15.2) | 10 (71.4) 39 (84.8) | 0.258 |
| Age, mean (SD), years | 69.35 (9.14) | 70.09 (7.4) | 69.18 (9.5) | 0.769 |
| ASA status, n (%) I II | 8 (100) 52 (100) | 0 (0) 11 (21.2) | 8 (100) 41 (78.8) | 0.061 |
| Body mass index, mean (SD), kg/m2 | 30.97 (4.77) | 33 (4.9) | 30 (4.65) | 0.715 |
| Body surface area, mean (SD), m2 | 2.043 (0.182) | 2.14 (0.171) | 2.02 (0.178) | 0.727 |
| MAP before SA, mean (SD), mmHg | 93 (12.9) | 94.39 (11.8) | 92.6 (13.3) | 0.697 |
| HR before SA, mean (SD), bpm | 73 (12.5) | 67 (9.1) | 76 (11.3) | 0.508 |
| Breathing rate, mean (SD), b/min | 14 (2) | 13 (1.6) | 14 (2.5) | 0.25 |
| IVCex baseline, mean (SD), mm | 13.9 (5.5) | 13 (5.8) | 13.9 (5.4) | 0.894 |
| IVCin baseline, mean (SD), mm | 8.9 (4.3) | 7.6 (3.7) | 9.2 (4.4) | 0.266 |
| IVC-CI baseline, mean (SD), % | 35.6 (18.7) | 43.7 (18.4) | 33.8 (18.5) | 0.15 |
| Parameters | Baseline | Time Point 1 | Time Point 2 | Time Point 3 | Time Point 4 | p Value |
|---|---|---|---|---|---|---|
| IVCex, median (range), mm | 12.5 (5.67–32.2) | 13 (6.1–33.1) | 13 (6.88–24.8) | 13.7 (5.23–22.9) | 12.8 (5.23–23.4) | >0.05 |
| IVCin, median (range), mm | 8.2 (2.29–22.9) | 9.2 (3.03–24.8) | 8.7 (2.7–17.4) | 8.3 (3.1–19.8) | 8.3 (2.5–15.7) | >0.05 |
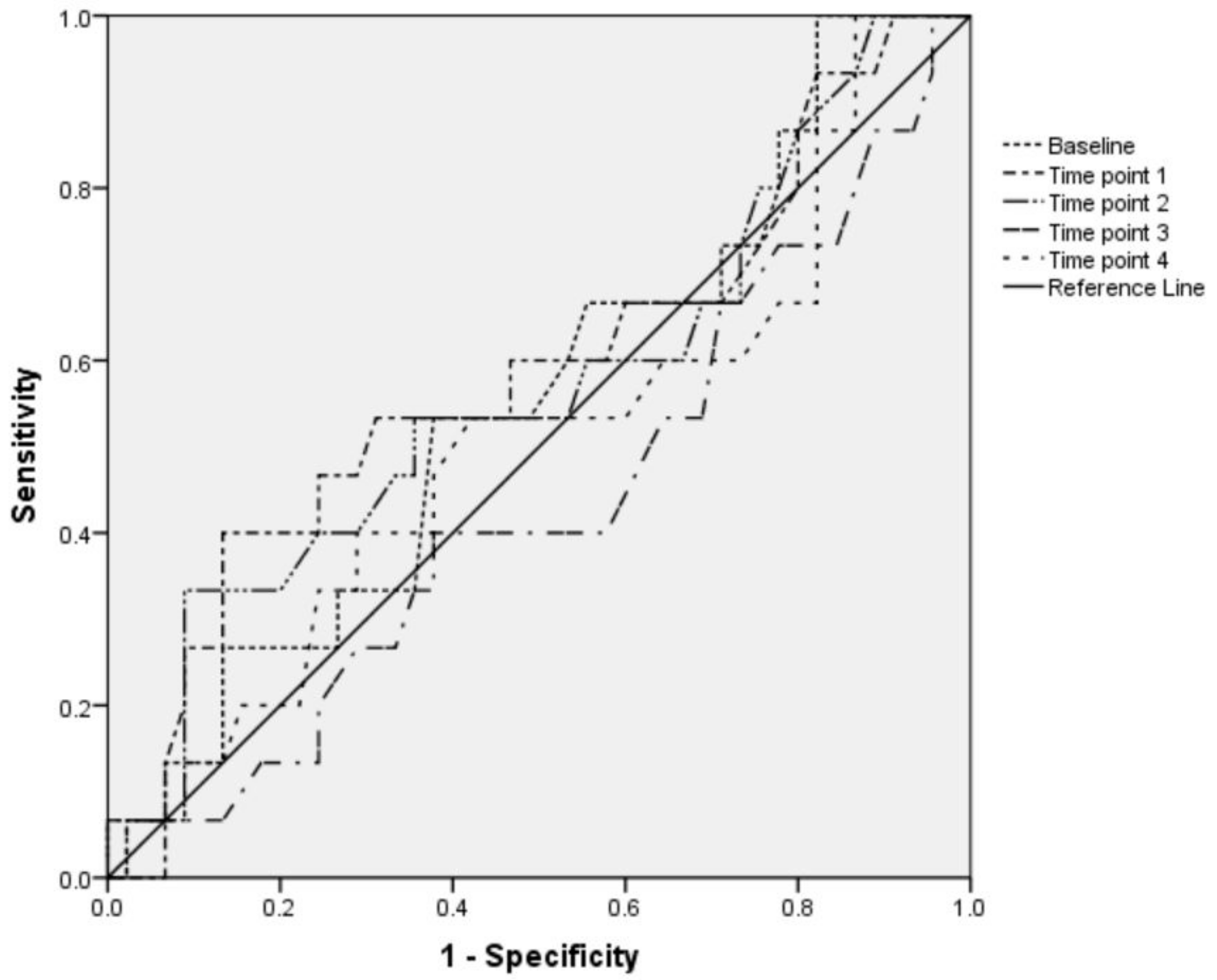
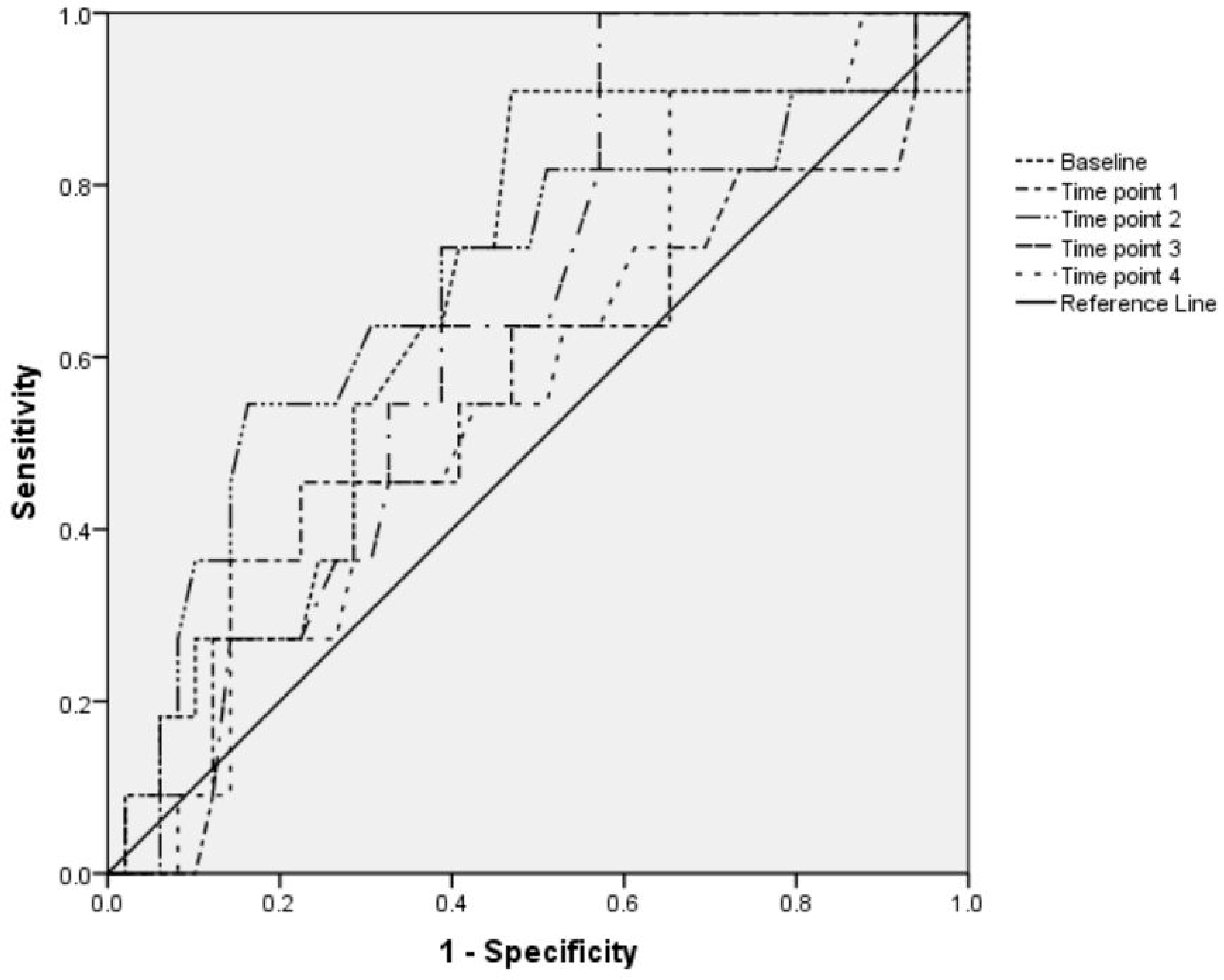
| Time Point | IVC-CI, % | |||||
|---|---|---|---|---|---|---|
| Hypotensive Group | Nonhypotensive Group | p Value * | Bradycardic Group | Nonbradycardic Group | p Value * | |
| Baseline | 38.4 (18.5) | 34.8 (18.9) | 0.984 | 43.7 (18.5) | 33.8 (18.5) | 0.55 |
| Time point 1 | 35.6 (19.1) | 30.22 (17.7) | 0.34 | 35.2 (19) | 30.8 (17.9) | 0.629 |
| Time point 2 | 37.9 (21.2) | 31.16 (16.2) | 0.104 | 41.7 (18.5) | 30.8 (17) | 0.578 |
| Time point 3 | 29.4 (18.4) | 32.13 (16.7) | 0.794 | 38.2 (13.7) | 29.9 (17.5) | 0.25 |
| Time point 4 | 32.6 (17.2) | 30.93 (17.4) | 0.878 | 34 (17.9) | 30.8 (14.5) | 0.632 |
| p value † | >0.05 | >0.05 | >0.05 | >0.05 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mačiulienė, A.; Gelmanas, A.; Jaremko, I.; Tamošiūnas, R.; Smailys, A.; Macas, A. Measurements of Inferior Vena Cava Diameter for Prediction of Hypotension and Bradycardia during Spinal Anesthesia in Spontaneously Breathing Patients during Elective Knee Joint Replacement Surgery. Medicina 2018, 54, 49. https://doi.org/10.3390/medicina54030049
Mačiulienė A, Gelmanas A, Jaremko I, Tamošiūnas R, Smailys A, Macas A. Measurements of Inferior Vena Cava Diameter for Prediction of Hypotension and Bradycardia during Spinal Anesthesia in Spontaneously Breathing Patients during Elective Knee Joint Replacement Surgery. Medicina. 2018; 54(3):49. https://doi.org/10.3390/medicina54030049
Chicago/Turabian StyleMačiulienė, Asta, Arūnas Gelmanas, Inna Jaremko, Ramūnas Tamošiūnas, Alfredas Smailys, and Andrius Macas. 2018. "Measurements of Inferior Vena Cava Diameter for Prediction of Hypotension and Bradycardia during Spinal Anesthesia in Spontaneously Breathing Patients during Elective Knee Joint Replacement Surgery" Medicina 54, no. 3: 49. https://doi.org/10.3390/medicina54030049
APA StyleMačiulienė, A., Gelmanas, A., Jaremko, I., Tamošiūnas, R., Smailys, A., & Macas, A. (2018). Measurements of Inferior Vena Cava Diameter for Prediction of Hypotension and Bradycardia during Spinal Anesthesia in Spontaneously Breathing Patients during Elective Knee Joint Replacement Surgery. Medicina, 54(3), 49. https://doi.org/10.3390/medicina54030049






