Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in hemodialysis patients
Abstract
1. Introduction
2. Materials and methods
3. Results
4. Discussion
5. Conclusions
Conflict of interest
Authors’ contributions
Acknowledgments
R E F E R E N C E S
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guidelines for anemia in chronic kidney disease. Kidney Int Suppl 2012, 2, 279–335. [Google Scholar]
- Palmer, SC; Navaneethan, SD; Craig, JC; Johnson, DW; Tonelli, M; Garg, AX; et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 2010, 153(July (1)), 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, IA; Macdougall, IC; Richards, S; Jones, V; Marcelli, D; Froissart, M; et al. Factors precipitating erythropoiesis-stimulating agent responsiveness in a European haemodialysis cohort: case-crossover study. Pharmacoepidemiol Drug Saf 2015, 24(April (4)), 414–26. [Google Scholar] [CrossRef] [PubMed]
- Canavesi, E; Alfieri, C; Pelusi, S; Valenti, L. Hepcidin and HFE protein: iron metabolism as a target for the anemia of chronic kidney disease. World J Nephrol 2012, 1(December (6)), 166–76. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L; Messa, P; Pelusi, S; Campostrini, N; Girelli, D. Hepcidin levels in chronic hemodialysis patients: a critical evaluation. Clin Chem Lab Med 2014, 52(May (5)), 613–9. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood 2011, 117(April (17)), 4425–33. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, LT. Iron deficiency syndromes and iron-restricted erythropoiesis (CME). Transfusion 2012, 52(July (7)), 1584–92. [Google Scholar] [CrossRef] [PubMed]
- Kato, A; Tsuji, T; Luo, J; Sakao, Y; Yasuda, H; Hishida, A. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol 2008, 28(1), 115–21. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, T; Shimonaka, Y; Kida, A; Furuta, M; Nanami, M; Otaki, Y; et al. Determinants of hepcidin in patients on maintenance hemodialysis: role of inlammation. Am J Nephrol 2010, 31(6), 534–40. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G; Theurl, I; Eder, S; Koppelstaetter, C; Kurz, K; Sonnweber, T; et al. Serum hepcidin concentration in chronic haemodialysis patients: associations and effects of dialysis, iron and erythropoietin therapy. Eur J Clin Invest 2009, 39(October (10)), 883–90. [Google Scholar] [CrossRef] [PubMed]
- van der Weerd, NC; Grooteman, MP; Bots, ML; van den Dorpel, MA; den Hoedt, CH; Mazairac, AH; et al. Hepcidin-25 in chronic hemodialysis patients is related to residual kidney function and not to treatment with erythropoiesis stimulating agents. PLoS ONE 2012, 7(7), e39783. [Google Scholar] [CrossRef] [PubMed]
- Ashby, DR; Gale, DP; Busbridge, M; Murphy, KG; Duncan, ND; Cairns, TD; et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int 2009, 75(May (9)), 976–81. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T; Hasuike, Y; Otaki, Y; Kida, A; Nonoguchi, H; Kuragano, T. Hepcidin: another culprit for complications in patients with chronic kidney disease? Nephrol Dial Transplant 2011, 26(October (10)), 3092–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van der Weerd, NC; Grooteman, MP; Nube, MJ; ter Wee, PM; Swinkels, DW; Gaillard, CA. Hepcidin in chronic kidney disease: not an anaemia management tool, but promising as a cardiovascular biomarker. Neth J Med 2015, 73(March (3)), 108–18. [Google Scholar] [PubMed]
- van der Weerd, NC; Grooteman, MP; Bots, ML; van den Dorpel, MA; den Hoedt, CH; Mazairac, AH; et al. Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol Dial Transplant 2013, 28(December (12)), 3062–71. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S; Mathew, A; Vaziri, ND. Iron toxicity: relevance for dialysis patients. Nephrol Dial Transplant 2014, 29(February (2)), 255–9. [Google Scholar] [CrossRef] [PubMed]
- Zumbrennen-Bullough, K; Babitt, JL. The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients. Nephrol Dial Transplant 2014, 29(February (2)), 263–73. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomez, JM; Portoles, JM; Aljama, P. Factors that condition the response to erythropoietin in patients on hemodialysis and their relation to mortality. Kidney Int Suppl 2008, (December (111)), S75–81. [Google Scholar] [CrossRef] [PubMed]
- Vega, A; Ruiz, C; Abad, S; Quiroga, B; Velazquez, K; Yuste, C; et al. Body composition affects the response to erythropoiesis-stimulating agents in patients with chronic kidney disease in dialysis. Ren Fail 2014, 36(August (7)), 1073–7. [Google Scholar] [CrossRef] [PubMed]
- Ziginskiene, E; Kuzminskis, V; Petruliene, K; Vaiciuniene, R; Stankuviene, A; Bumblyte, IA. Renal anemia control in Lithuania: influence of local conditions and local guidelines. Sci World J 2013, 2013(December), 260915. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M; Ashby, D. Hepcidin – a well-known iron biomarker with prognostic implications in chronic kidney disease. Nephrol Dial Transplant 2013, 28(December (12)), 2936–9. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, BD; Critchlow, CW; Weir, MR; Stewart, R; Krishnan, M; Hakim, RH. Impact of elevated C-reactive protein levels on erythropoiesis-stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transplant 2009, 24(March (3)), 919–25. [Google Scholar] [CrossRef] [PubMed]
- Rattanasompattikul, M; Molnar, MZ; Zaritsky, JJ; Hatamizadeh, P; Jing, J; Norris, KC; et al. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant 2013, 28(July (7)), 1936–45. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L; Girelli, D; Valenti, GF; Castagna, A; Como, G; Campostrini, N; et al. HFE mutations modulate the effect of iron on serum hepcidin-25 in chronic hemodialysis patients. Clin J Am Soc Nephrol 2009, 4(August (8)), 1331–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sany, D; Elsawy, AE; Elshahawy, Y. Hepcidin and regulation of iron homeostasis in maintenance hemodialysis patients. Saudi J Kidney Dis Transpl 2014, 25(September (5)), 967–73. [Google Scholar] [CrossRef] [PubMed]
- Coyne, DW. Hepcidin: clinical utility as a diagnostic tool and therapeutic target. Kidney Int 2011, 80(August (3)), 240–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swinkels, DW; Wetzels, JF. Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease? Nephrol Dial Transplant 2008, 23(August (8)), 2450–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, E; Swinkels, DW; Laarakkers, CM; Rocha-Pereira, P; Rocha, S; Reis, F; et al. Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in haemodialysis patients. Acta Haematol 2009, 122(4), 226–9. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G; Griuncelli, M; Loridon, C; Couprie, R; Benmaadi, A; Bounhiol, C; et al. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: a MRI study. Am J Med 2012, 125(October (10)), 991–999.e1. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A; Tetta, C; Merello, JI; Palomares, I; Perez-Garcia, R; Maduell, F; et al. Cumulative iron dose and resistance to erythropoietin. J Nephrol 2015, 28(October (5)), 603–13. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, N; Girelli, D; Campostrini, N; Bedogna, V; Pietro Solero, G; Castagna, A; et al. Hepcidin is not useful as a biomarker for iron needs in haemodialysis patients on maintenance erythropoiesis-stimulating agents. Nephrol Dial Transplant 2010, 25(December (12)), 3996–4002. [Google Scholar] [CrossRef] [PubMed]
- do Sameiro-Faria, M; Ribeiro, S; Rocha-Pereira, P; Fernandes, J; Reis, F; Bronze-da-Rocha, E; et al. Body mass index and resistance to recombinant human erythropoietin therapy in maintenance hemodialysis patients. Ren Fail 2013, 35(10), 1392–8. [Google Scholar] [CrossRef] [PubMed]
- Neven, E; De Schutter, TM; Behets, GJ; Gupta, A; D’Haese, PC. Iron and vascular calcification. Is there a link? Nephrol Dial Transplant 2011, 26(April (4)), 1137–45. [Google Scholar] [CrossRef] [PubMed]
- Pelusi, S; Girelli, D; Rametta, R; Campostrini, N; Alfieri, C; Traglia, M; et al. The A736V TMPRSS6 polymorphism influences hepcidin and iron metabolism in chronic hemodialysis patients: TMPRSS6 and hepcidin in hemodialysis. BMC Nephrol 2013, 14(February), 48. [Google Scholar] [CrossRef] [PubMed]
- Rubab, Z; Amin, H; Abbas, K; Hussain, S; Ullah, MI; Mohsin, S. Serum hepcidin levels in patients with end-stage renal disease on hemodialysis. Saudi J Kidney Dis Transpl 2015, 26(January (1)), 19–25. [Google Scholar] [PubMed]
- Kali, A; Yayar, O; Erdogan, B; Eser, B; Buyukbakkal, M; Ercan, Z; et al. Is hepcidin-25 a predictor of atherosclerosis in hemodialysis patients? Hemodial Int 2016, 20(April (2)), 191–7. [Google Scholar] [CrossRef] [PubMed]
- Kotanko, P; Thijssen, S; Levin, NW. Association between erythropoietin responsiveness and body composition in dialysis patients. Blood Purif 2008, 26(1), 82–9. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M; Perazella, MA; Kasapoglu, B; Koroglu, M; Covic, A. Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif 2010, 29(1), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Movilli, E; Cancarini, GC; Zani, R; Camerini, C; Sandrini, M; Maiorca, R. Adequacy of dialysis reduces the doses of recombinant erythropoietin independently from the use of biocompatible membranes in haemodialysis patients. Nephrol Dial Transplant 2001, 16(January (1)), 111–4. [Google Scholar] [CrossRef]
- Movilli, E; Cancarini, GC; Vizzardi, V; Camerini, C; Brunori, G; Cassamali, S; et al. Epoetin requirement does not depend on dialysis dose when Kt/N > 1.33 in patients on regular dialysis treatment with cellulosic membranes and adequate iron stores. J Nephrol 2003, 16(July–August (4)), 546–51. [Google Scholar] [PubMed]
- Green, D; Kalra, PR; Kalra, PA. Echocardiographic abnormalities in dialysis patients with normal ejection fraction. Nephrol Dial Transplant 2012, 27(December (12)), 4256–9. [Google Scholar] [CrossRef] [PubMed]
- Kainz, A; Mayer, B; Kramar, R; Oberbauer, R. Association of ESA hypo-responsiveness and haemoglobin variability with mortality in haemodialysis patients. Nephrol Dial Transplant 2010, 25(November (11)), 3701–6. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V; Rosati, A; Bigazzi, R; Paoletti, S; Mantuano, E; Beati, S; et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant 2011, 26(August (8)), 2641–8. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M; Komatsu, M; Kawaguchi, H; Tsuchiya, K; Nitta, K. Erythropoietin resistance index and the all-cause mortality of chronic hemodialysis patients. Blood Purif 2014, 37(2), 106–12. [Google Scholar] [CrossRef] [PubMed]
- Vaiciuniene, R. Evaluation of predisposing factors for hospitalization in hemodialysis patients. Doctorial dissertation: biomedical sciences, medicine, Lithuanian University of Health Sciences, 2010. [Google Scholar]
- Saeed, O; Otsuka, F; Polavarapu, R; Karmali, V; Weiss, D; Davis, T; et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol 2012, 32(February (2)), 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, T; Itoh, K; Shimonaka, Y; Kida, A; Furuta, M; Kitamura, R; et al. Hepcidin as well as TNF-alpha are significant predictors of arterial stiffness in patients on maintenance hemodialysis. Nephrol Dial Transplant 2011, 26(August (8)), 2663–7. [Google Scholar] [CrossRef] [PubMed]
- Gaweda, AE; Ginzburg, YZ; Chait, Y; Germain, MJ; Aronoff, GR; Rachmilewitz, E. Iron dosing in kidney disease: inconsistency of evidence and clinical practice. Nephrol Dial Transplant 2015, 30(February (2)), 187–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, CC; Vaja, V; Babitt, JL; Lin, HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol 2012, 87(April (4)), 392–400. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E. Anti-hepcidin therapy for iron-restricted anemias. Blood 2013, 122(October (17)), 2929–31. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M; Del Vecchio, L; Sirolli, V; Locatelli, F. New treatment approaches for the anemia of CKD. Am J Kidney Dis 2016, 67(January (1)), 133–42. [Google Scholar] [CrossRef] [PubMed]

| Ferritin (µg/L or ng/mL) | IV iron therapy |
|---|---|
| <100 | 100–500 mg per day, not exceed 1000 mg. |
| Repeat ferritin test one week after the last dose of IV iron, if it is still less 100 mg/L, repeat the same treatment course | |
| 100–300 | 100 mg of iron per week. Measure ferritin concentration every 3 months |
| >300–500 | 100 mg of iron every second week. Measure ferritin concentration every 3 months |
| >500 | Suspend IV iron therapy. Measure ferritin concentration after 1–3 months |
| Characteristic | Total population (n = 173) | Nonresponders, ERI > 15 (n = 20) | Responders, ERI ≤ 15 (n = 153) | P |
|---|---|---|---|---|
| Demographic and dialysis-related data | ||||
| Age, years | 63.19 ± 15.60 | 62.19 ± 15.73 | 63.5 ± 15.59 | 0.082 |
| Males, % | 51.2 | 43.2 | 53.7 | 0.475 |
| Time on dialysis, min per week | 682.2 ± 79.66 | 667.50 ± 118.44 | 685.63 ± 67.92 | 0.668 |
| UF, kg | 2.17 ± 0.84 | 2.04 ± 0.73 | 2.21 ± 0.87 | 0.404 |
| Kt/V (single pool) | 1.42 ± 0.18 | 1.35 ± 0.17 | 1.43 ± 0.17 | 0.039 |
| CVC versus fistula, % | 14.3 | 25 | 11.8 | 0.095 |
| Diabetic patients, % | 22.2 | 13 | 24.3 | 0.242 |
| Malignancy, % | 9.5 | 26.1 | 5.8 | 0.003 |
| Treatment characteristics | ||||
| ESA dose, IU/week | 6210.59 ± 5276.89 | 14,973.25 ± 4902.54 | 4168.80 ± 2588.68 | <0.001 |
| ESA dose, IU/kg/week | 88.65 ± 77.31 | 216.75 ± 72.89 | 58.81 ± 37.74 | <0.001 |
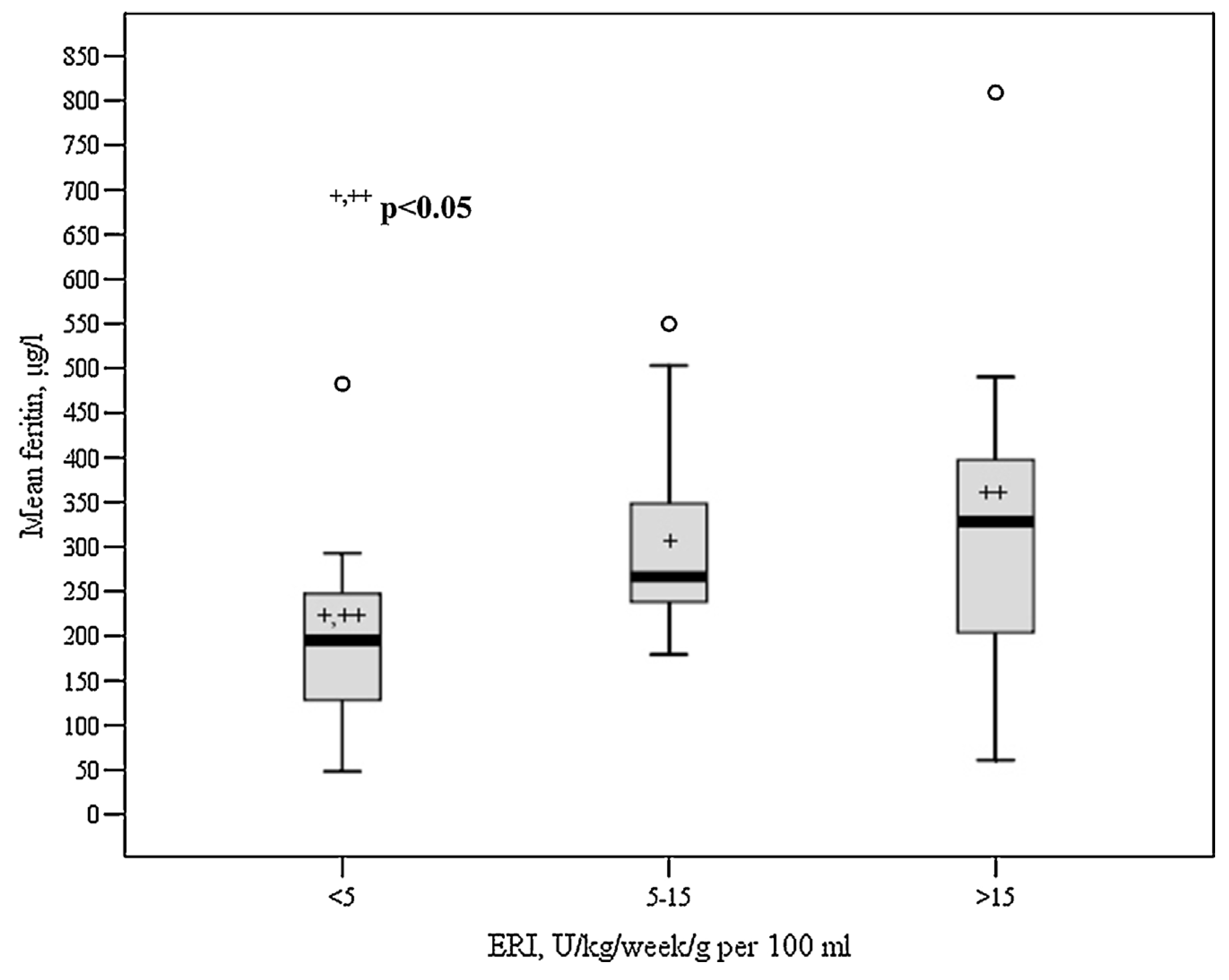
| ERI, IU/kg/week per 100 mL | 12.29 ± 10.9 | 23.9 ± 8.9 | 5.4 ± 3.6 | 0.001 |
| Cardiovascular parameters | ||||
| Systolic blood pressure, mmHg | 140.1 ± 17.06 | 141.10 ± 11.09 | 139.13 ± 17.65 | 0.617 |
| Diastolic blood pressure, mmHg | 76.34 ± 11.02 | 77.25 ± 10.18 | 75.05 ± 11.17 | 0.399 |
| LVMM, g | 260.36 ± 92.19 | 239.40 ± 50.37 | 264.47 ± 98.19 | 0.461 |
| LVMI, g/m2 | 143.28 ± 51.57 | 141.51 ± 29.36 | 143.62 ± 55.11 | 0.912 |
| LVEF, % | 49.60 ± 9.16 | 51.79 ± 9.58 | 49.16 ± 9.06 | 0.194 |
| No. of antihypertensive medications | 2.09 ± 1.71 | 2.13 ± 1.56 | 2.08 ± 1.75 | 0.901 |
| Prescription of RAS inhibitors, % | 48.2 | 53.8 | 47.1 | 0.657 |
| Hematological data | ||||
| Erythrocytes, ×1012/L | 3.31 ± 0.32 | 3.0 ± 0.38 | 3.37 ± 0.28 | <0.001 |
| Hemoglobin, g/L | 98.8 ± 9.8 | 91.1 ± 9.8 | 103.9 ± 5.5 | <0.001 |
| Hematocrit, % | 30.32 ± 3.23 | 28.24 ± 2.72 | 32.01 ± 2.92 | <0.001 |
| MCV, fL | 94.86 ± 5.71 | 95.6 ± 5.9 | 94.34 ± 5.6 | 0.479 |
| MCH, pg | 30.87 ± 2.2 | 31.11 ± 2.27 | 30.70 ± 2.21 | 0.556 |
| Platelets, ×109/L | 223.66 ± 94.06 | 235.43 ± 81.82 | 220.95 ± 96.83 | 0.23 |
| Iron metabolism | ||||
| Cumulative iron dose, mg per year | 2668.04 ± 1300.69 | 3560.00 ± 1576.98 | 2504.88 ± 1183.62 | 0.003 |
| Ferritin, µg/L | 283.1 ± 139.8 | 318.87 ± 168.8 | 260.16 ± 114.98 | 0.206 |
| Hepcidin-25, ng/mL | 135.79 ± 115.52 | 158.51 ± 162.57 | 120.65 ± 67.28 | 0.33 |
| Inflammatory and nutritional status | ||||
| Body mass index, kg/m2 | 26.53 ± 6.37 | 24.83 ± 4.89 | 26.93 ± 6.62 | 0.067 |
| Albumin, g/L | 36.39 ± 4.13 | 33.91 ± 4.12 | 37.98 ± 3.33 | 0.001 |
| Protein, g/L | 66.10 ± 4.25 | 64.64 ± 5.19 | 66.44 ± 3.97 | 0.13 |
| CRP, mg/L | 18.17 ± 21.67 | 27.16 ± 29.24 | 12.39 ± 12.50 | 0.048 |
| Cholesterol, mmol/L | 4.89 ± 1.12 | 4.78 ± 1.17 | 4.95 ± 1.10 | 0.638 |
| Calcium-phosphorus metabolism | ||||
| Phosphorus, mmol/L | 1.78 ± 0.38 | 1.82 ± 0.41 | 1.76 ± 0.38 | 0.64 |
| Calcium, mmol/L | 2.26 ± 0.17 | 2.29 ± 0.13 | 2.22 ± 0.22 | 0.27 |
| PTH, pmol/L | 29.22 ± 25.67 | 25.44 ± 22.93 | 31.65 ± 27.41 | 0.412 |
| Hepatic enzymes | ||||
| AST, U/L | 20.15 ± 10.38 | 20.31 ± 11.96 | 20.12 ± 10.06 | 0.982 |
| ALT, U/L | 19.89 ± 12.40 | 19.51 ± 12.16 | 19.99 ± 12.51 | 0.77 |
| Alkaline phosphatase, U/L | 97.08 ± 55.85 | 93.32 ± 43.99 | 97.94 ± 58.39 | 0.647 |
| Hospitalization | ||||
| Hospitalization rate per year | 1.53 ± 1.5 | 2.35 ± 1.8 | 1.04 ± 1.04 | 0.011 |
| Mean length of one hospitalization | 16.22 ± 19.90 | 25.12 ± 21.26 | 10.82 ± 17.25 | 0.012 |
| Values are mean ± SD, unless otherwise stated. | ||||
| UF, ultrafiltration; CVC, central venous catheter; LVMM, left ventricular myocardium mass; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; RAS, rennin angiotensin aldosterone system; CRP, C-reactive protein; PTH, parathyroid hormone; AST, asparatate aminotransferase; ALT, alanine aminotransferase. | ||||
| Parameter | r | P | Unstandardized B coefficient (95% CI) |
|---|---|---|---|
| Demographic and dialysis-related data | |||
| Age, years | −0.061 | 0.495 | |
| Time on dialysis, min per week | −0.067 | 0.451 | |
| Kt/V (single pool) | −0.048 | 0.598 | |
| UF, kg | −0.102 | 0.284 | |
| Hematological data | |||
| Erythrocytes, ×1012/L | −0.444 | <0.001 | −12.84 (−16.98;−8.701) |
| Hematocrit, % | −0.537 | <0.001 | −1.29 (−1.704;−0.876) |
| Platelets, ×109/L | −0.031 | 0.732 | |
| Iron metabolism | |||
| MCV, fl | 0.028 | 0.779 | |
| MCH, pg | −0.102 | 0.303 | |
| Ferritin, µg/L | 0.057 | 0.524 | |
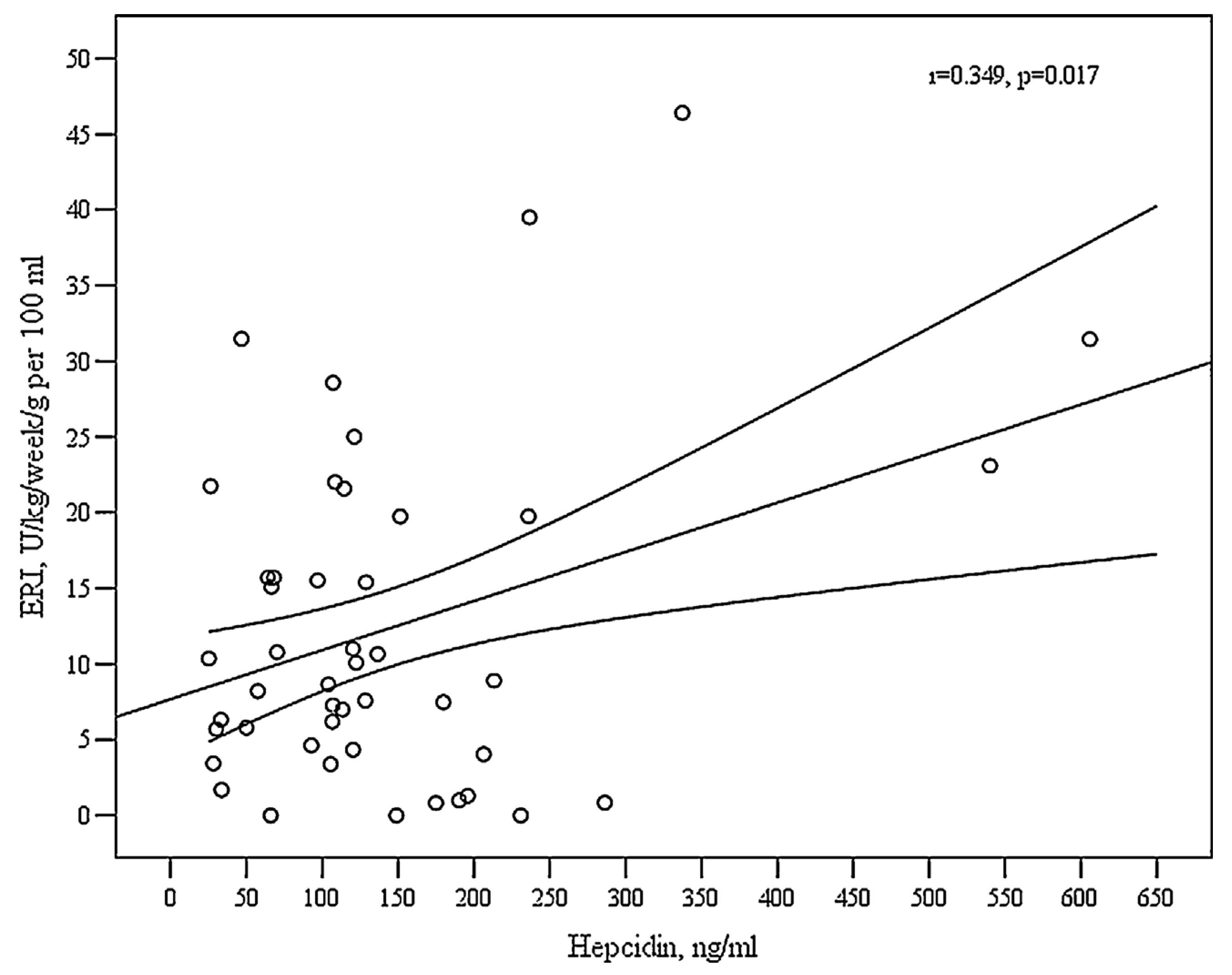
| Hepcidin, ng/mL | 0.349 | 0.017 | 0.032 (0.006;0.059) |
| Cumulative iron dose, mg per year | 0.1 | 0.442 | |
| Inflammatory and nutritional status | |||
| Body mass index, kg/m2 | −0.344 | <0.001 | −0.368 (−0.598;−0.137) |
| CRP, mg/L | 0.217 | 0.015 | 0.148 (0.083;0.213) |
| Protein, g/L | −0.09 | 0.32 | −0.640 (−0.994;−0.286) |
| Albumin, g/L | −0.181 | 0.044 | |
| Cholesterol, mmol/L | −0.04 | 0.661 | |
| Calcium-phosphorus metabolism | |||
| Calcium, mmol/L | −0.026 | 0.776 | |
| Phosphorus, mmol/L | 0.144 | 0.109 | |
| PTH, pmol/L | −0.077 | 0.398 | |
| Hepatic enzymes | |||
| AST, U/L | −0.067 | 0.461 | |
| ALT, U/L | −0.06 | 0.508 | |
| Alkaline phosphatase, U/L | −0.057 | 0.42 | |
| Hospitalization | |||
| Hospitalization rate per year | 0.459 | <0.001 | 3.725 (2.73;4.74) |
| Mean length of one hospitalization | 0.406 | <0.001 | 0.25 (0.173;0.327) |
| UF, ultrafiltration; CRP, C-reactive protein; PTH, parathyroid hormone; AST, aspartate aminotransferase; ALT, alanine aminotransferase. | |||
| Independent variables | Unstandardized B coefficient (95% CI) | Standardized B coefficient | P |
|---|---|---|---|
| CRP | 0.078 (0.021;0.134) | 0.198 | 0.007 |
| Albumin | −0.436 (−0.73;−0.142) | −0.209 | 0.004 |
| Body mass index | −0.374 (−0.553;−0.195) | −0.28 | <0.001 |
| Hospitalization rate per year | 3.017 (2.077;3.957) | 0.446 | <0.001 |
| Constant | 30.959 (18.099;43.818) | <0.001 | |
| r2 = 0.46. | |||
| Variable | r | P | Unstandardized B coefficient (95% CI) |
|---|---|---|---|
| Hematological data | |||
| Hemoglobin | −0.4 | 0.013 | −3.404 (−5.554;−1.255) |
| Hematocrit | −0.382 | 0.008 | −11.976 (−18.945;−5.008) |
| Erythrocytes | −0.3 | 0.041 | −78.895 (−136.14;−21.647) |
| Platelets | 0.015 | 0.921 | |
| MCV | 0.036 | 0.816 | |
| MCH | 0.084 | 0.587 | |
| EAS therapy | |||
| ESA dose, IU/week | 0.4 | 0.008 | 0.006 (0.002;0.011) |
| ERI, IU/kg/week per 100 mL | 0.349 | 0.017 | 3.756 (0.691;6.821) |
| Iron metabolism | |||
| Ferritin | 0.5 | <0.001 | 0.334 (0.212;0.457) |
| TSAT | −0.43 | 0.025 | −4.642 (−9.101;−0.183) |
| Cumulative iron dose, mg per month | −0.096 | 0.485 | |
| Inflammatory and nutritional status | |||
| Body mass index, kg/m2 | 0.11 | 0.25 | |
| Albumin | −0.4 | 0.013 | −8.34 (−14.846;−1.833) |
| Protein | 0.097 | 0.51 | |
| Cholesterol | −0.11 | 0.607 | |
| CRP | 0.213 | 0.15 | |
| Calcium-phosphorus metabolism | |||
| Calcium | −0.179 | 0.223 | |
| Calcium corrected | 0.147 | 0.138 | |
| Phosphorus | −0.138 | 0.351 | |
| CaxP | −0.24 | 0.1 | |
| PTH | 0.113 | 0.444 | |
| Hepatic enzymes | |||
| AST | 0.118 | 0.261 | |
| ALT | 0.14 | 0.18 | |
| Alkaline phosphatase | −0.034 | 0.745 | |
| ESAs, erythropoiesis-stimulating agents; ERI, ESA resistance index; TSAT, transferrin saturation; CRP, C-reactive protein; PTH, parathyroid hormone; AST, aspartate aminotransferase; ALT, alanine aminotransferase. | |||
| Independent variables | Unstandardized B coefficient (95% CI) | Standardized B coefficient | P |
|---|---|---|---|
| ERI, IU/kg/week per 100 mL | 4.869 (1.844;7.895) | 0.439 | 0.002 |
| Ferritin | 0.242 (0.086;0.399) | 0.422 | 0.003 |
| r2 = 0.64. | |||
© 2017 The Lithuanian University of Health Sciences. Production and hosting by Elsevier Sp. z o.o. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Share and Cite
Petrulienė, K.; Žiginskienė, E.; Kuzminskis, V.; Nedzelskienė, I.; Bumblytė, I.A. Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Medicina 2017, 53, 90-100. https://doi.org/10.1016/j.medici.2017.03.001
Petrulienė K, Žiginskienė E, Kuzminskis V, Nedzelskienė I, Bumblytė IA. Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Medicina. 2017; 53(2):90-100. https://doi.org/10.1016/j.medici.2017.03.001
Chicago/Turabian StylePetrulienė, Kristina, Edita Žiginskienė, Vytautas Kuzminskis, Irena Nedzelskienė, and Inga Arūnė Bumblytė. 2017. "Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in hemodialysis patients" Medicina 53, no. 2: 90-100. https://doi.org/10.1016/j.medici.2017.03.001
APA StylePetrulienė, K., Žiginskienė, E., Kuzminskis, V., Nedzelskienė, I., & Bumblytė, I. A. (2017). Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Medicina, 53(2), 90-100. https://doi.org/10.1016/j.medici.2017.03.001




