Rapid Screening and Identification of BSA Bound Ligands from Radix astragali Using BSA Immobilized Magnetic Nanoparticles Coupled with HPLC-MS
Abstract
:1. Introduction
2. Results and Discussion
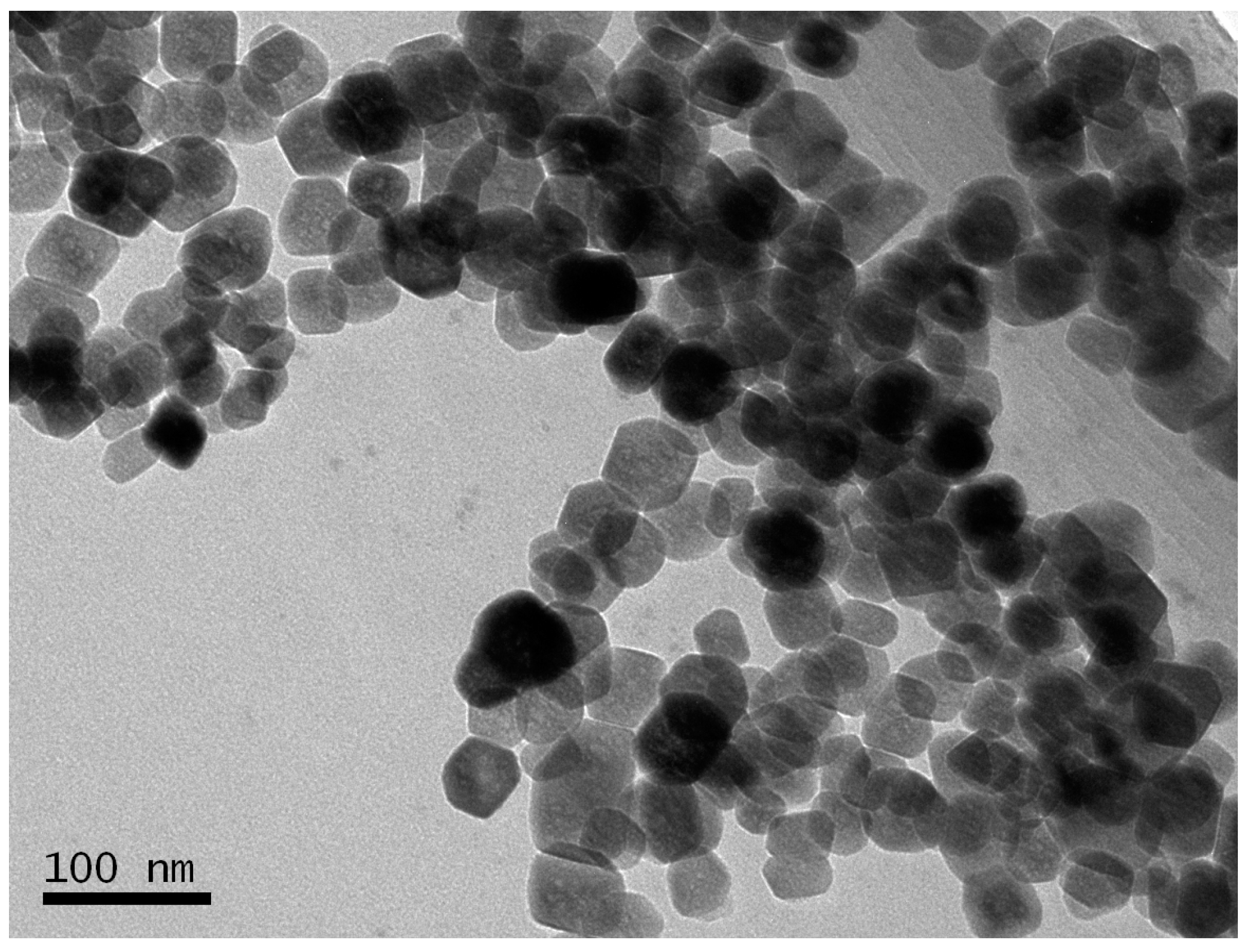
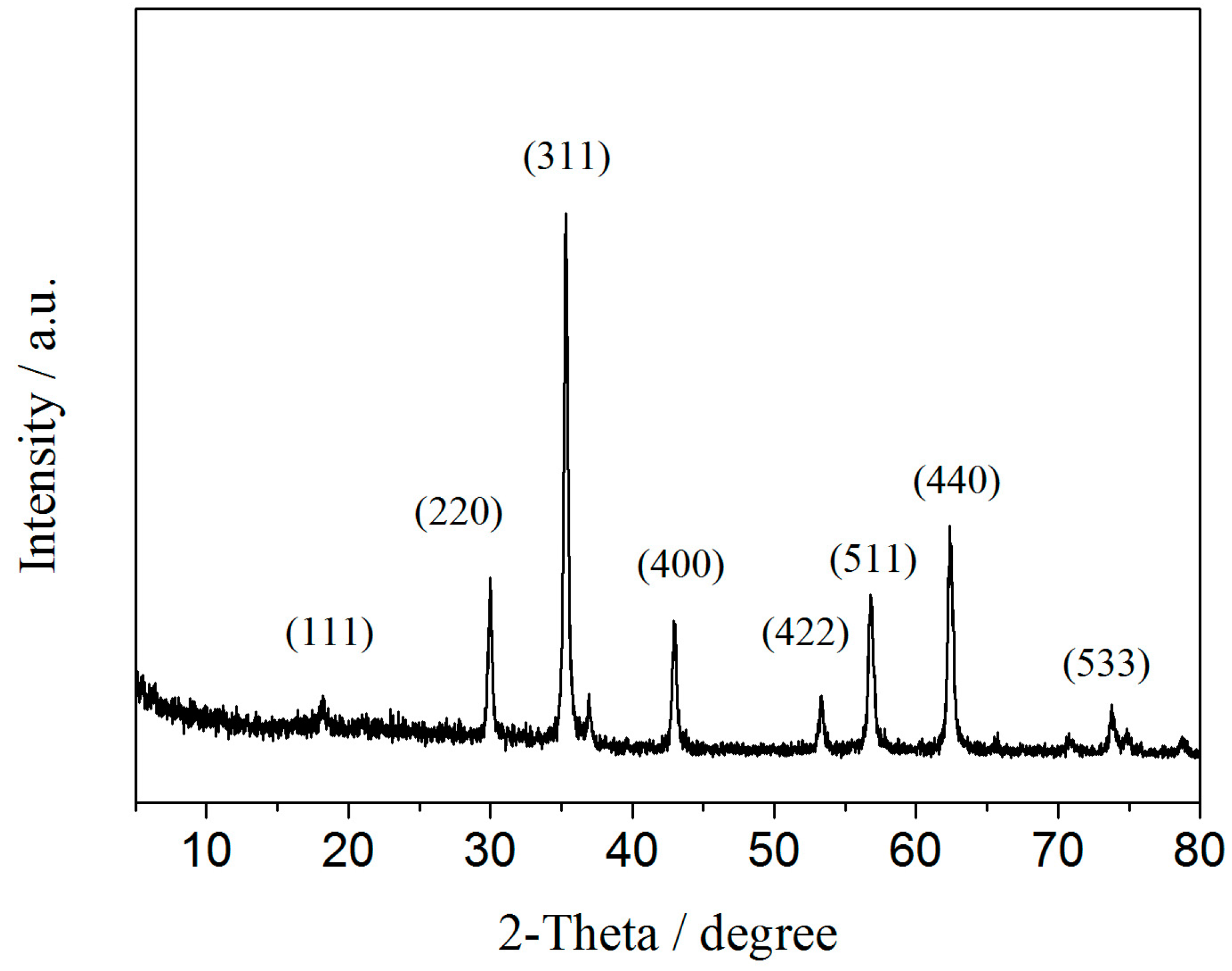
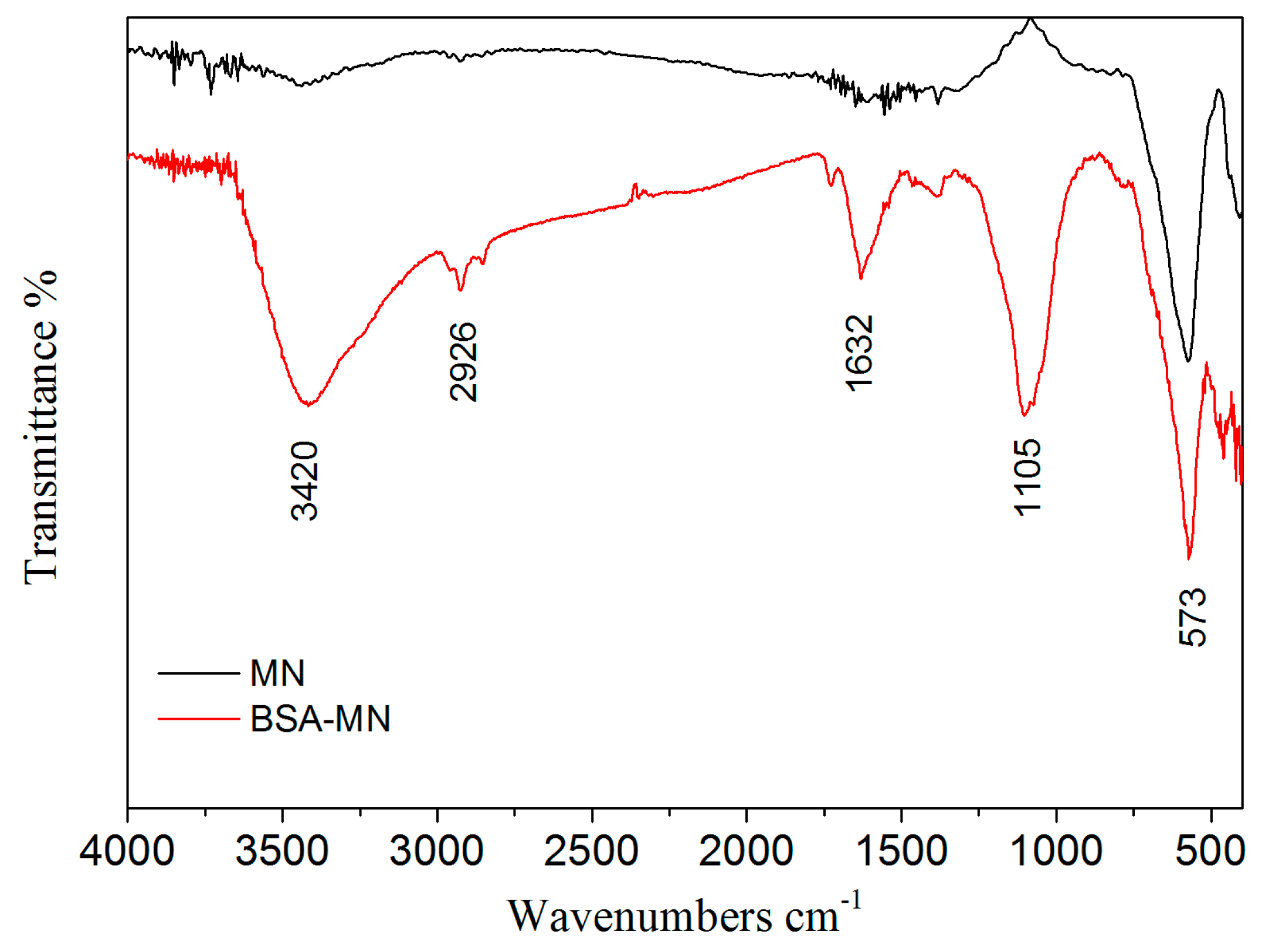
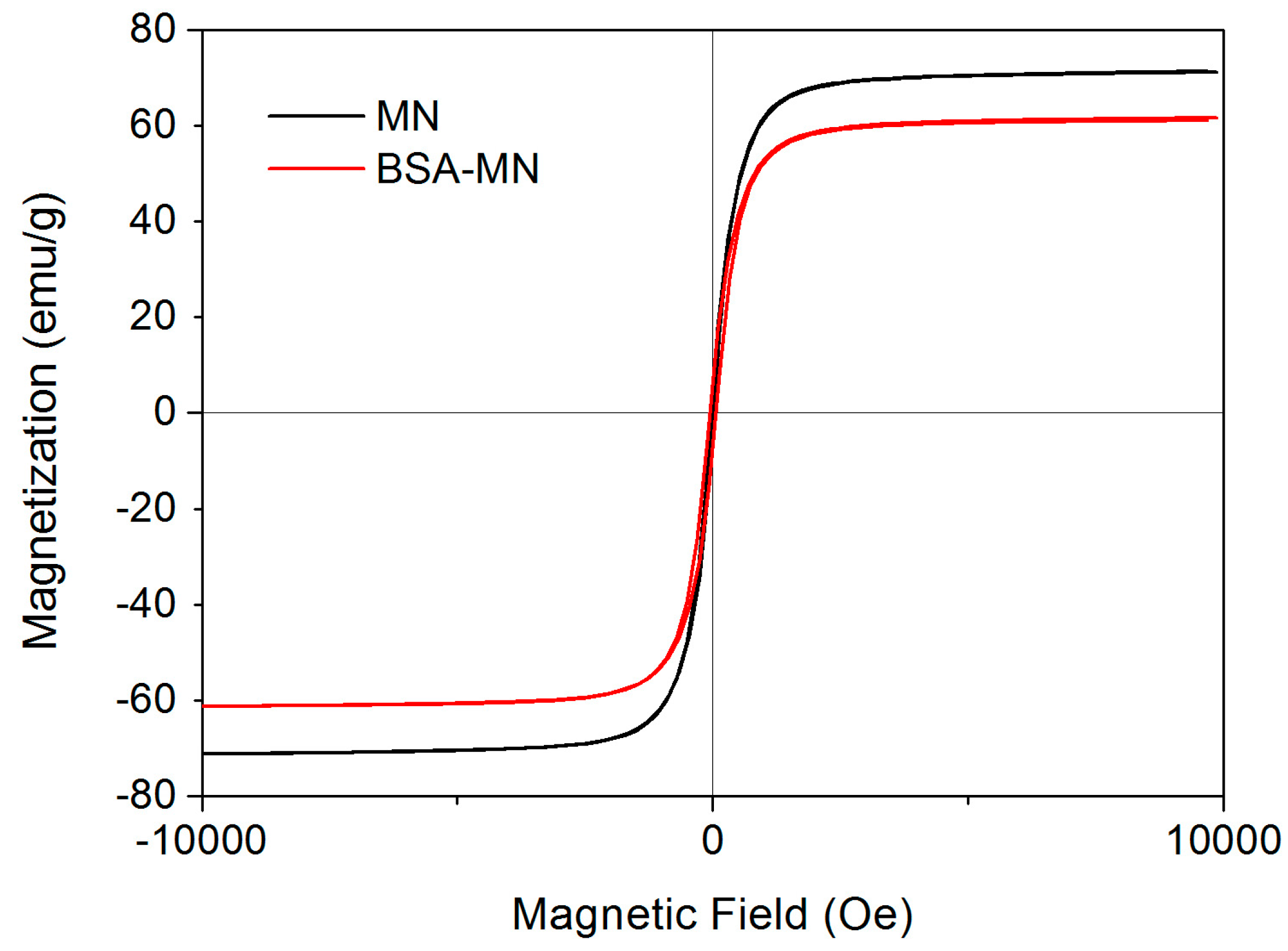
2.1. Characterizations of BSA-MN
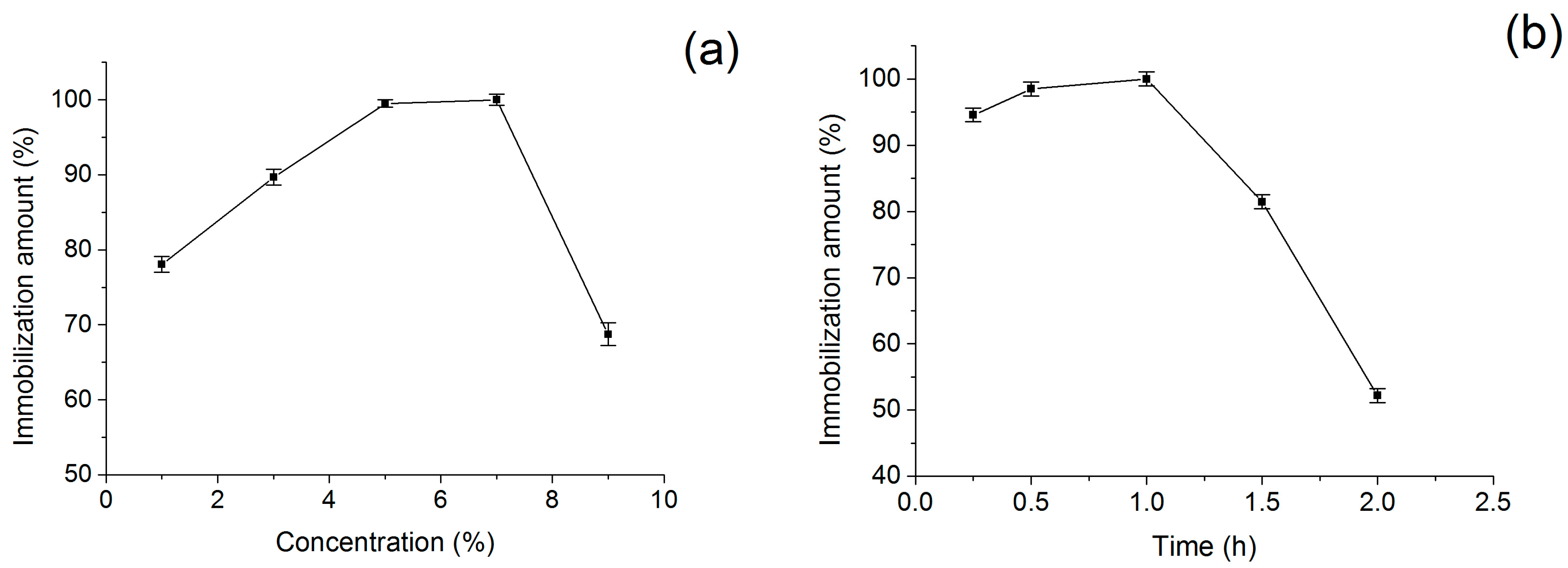
2.2. Optimization of Immobilization Conditions
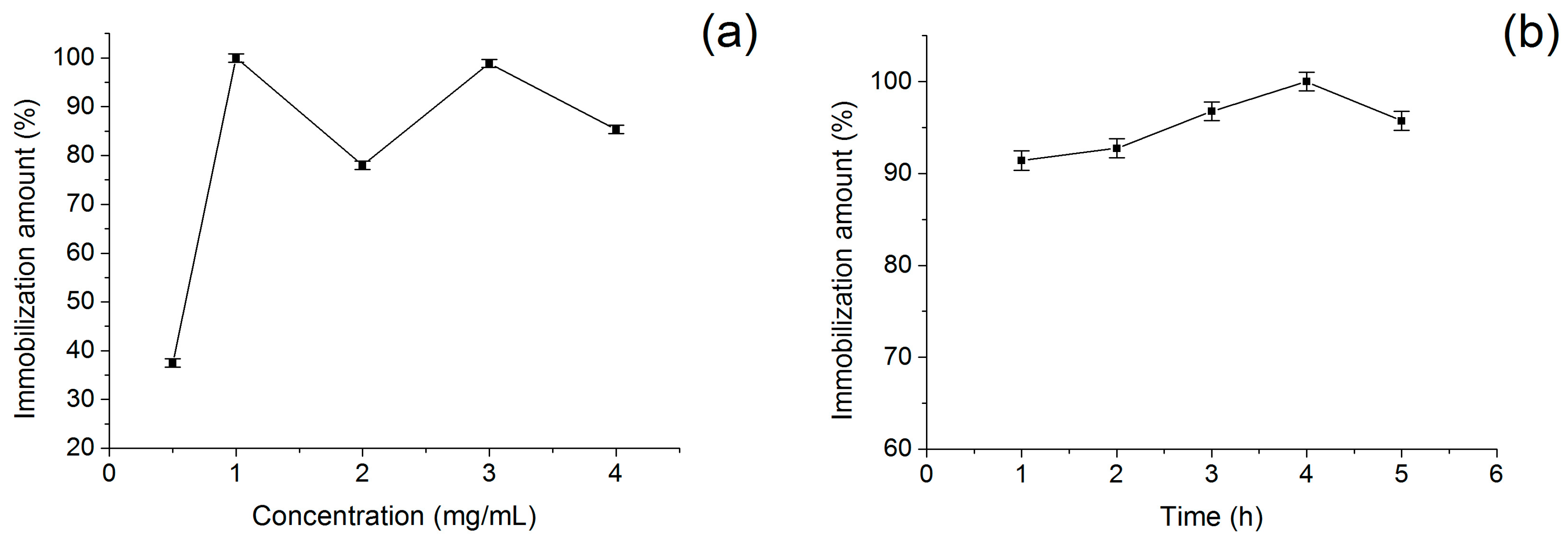
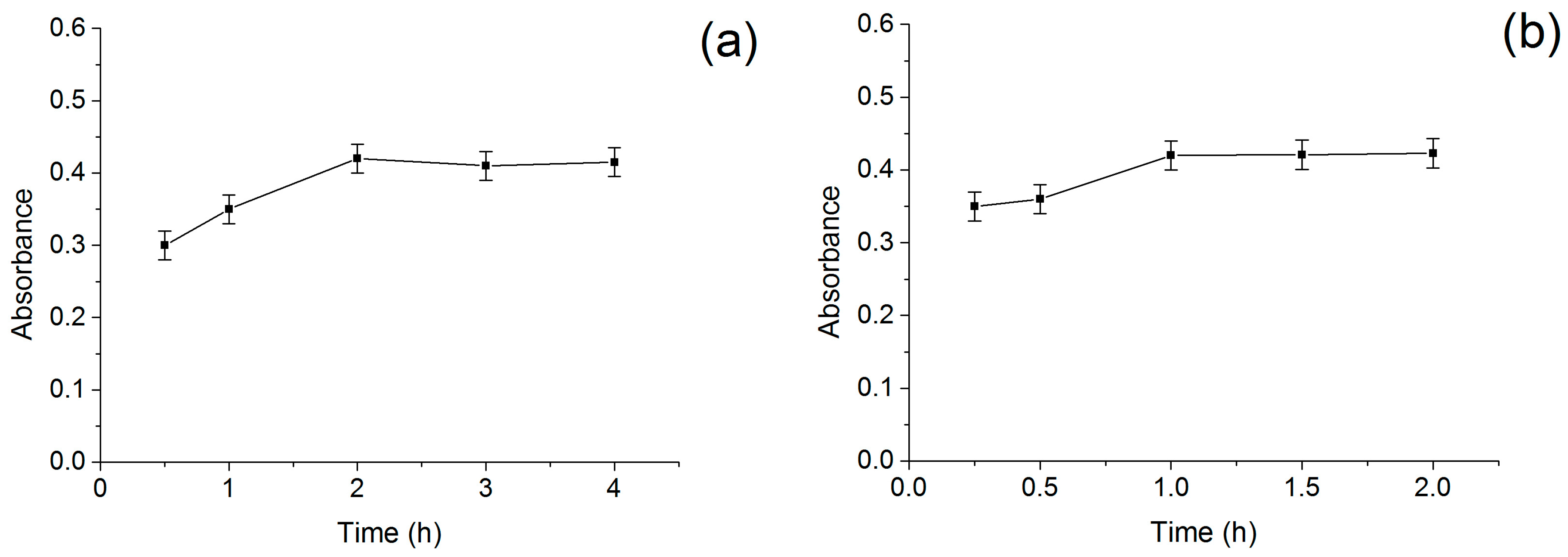
2.3. Optimization of Screening Conditions
2.4. Screening and Identification of Bound Ligands from Radix astragali Extract
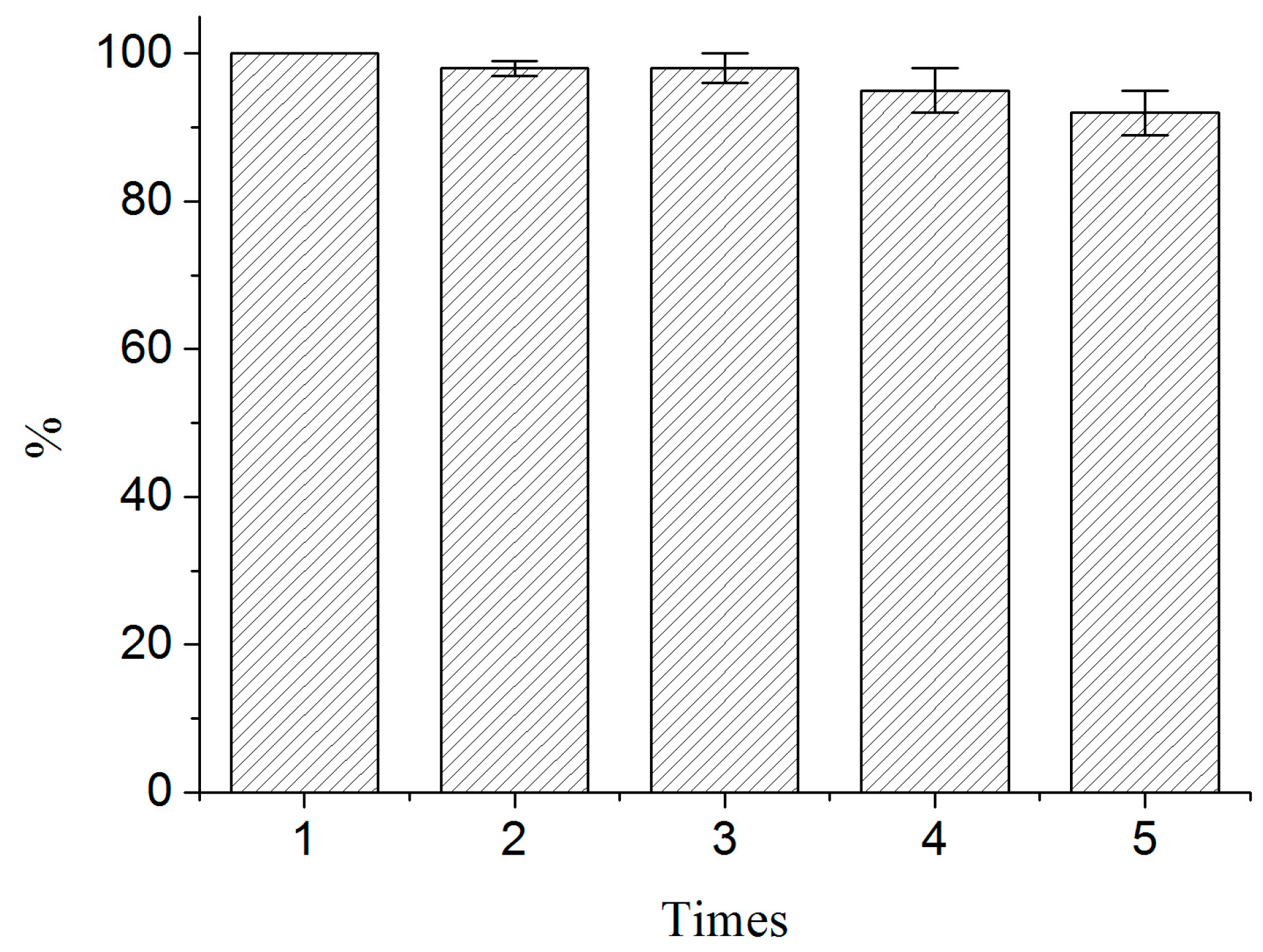
2.5. Reusability of BSA-MN
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of BSA-MN
3.3. Preparation of Radix astragali Extract
3.4. BSA Bound Ligands Screening
3.5. HPLC-MS Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Lu, F.; Luo, G.; Qiao, L.; Jiang, L.; Li, G.; Zhang, Y. Virtual Screening for Potential Allosteric Inhibitors of Cyclin-Dependent Kinase 2 from Traditional Chinese Medicine. Molecules 2016, 21, 1259. [Google Scholar] [CrossRef]
- Xie, Y.; Song, X.; Sun, X.; Huang, J.; Zhong, M.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016, 473, 775–780. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Wu, S. A combination strategy for extraction and isolation of multi-component natural products by systematic two-phase solvent extraction-13C nuclear magnetic resonance pattern recognition and following conical counter-current chromatography separation: Podophyllotoxins and flavonoids from Dysosma versipellis (Hance) as examples. J. Chromatogr. A 2016, 1431, 184–196. [Google Scholar]
- Li, S.P.; Zhao, J.; Yang, B. Strategies for quality control of Chinese medicines. J. Pharm. Biomed. 2011, 55, 802–809. [Google Scholar] [CrossRef]
- Wang, B.C.; Deng, J.; Gao, Y.M.; Zhu, L.C.; He, R.; Xu, Y.Q. The screening toolbox of bioactive substances from natural products: A review. Fitoterapia 2011, 82, 1141–1151. [Google Scholar] [CrossRef]
- Yang, X.X.; Wang, Y.W.; Zhang, X.X.; Chang, R.M.; Li, X.N. Screening vasoconstriction inhibitors from traditional Chinese medicines using a vascular smooth muscle/cell membrane chromatography-offline-liquid chromatography-mass spectrometry. J. Sep. Sci. 2011, 34, 2586–2593. [Google Scholar] [CrossRef]
- Zhao, A.; Li, L.; Li, B.; Zheng, M.; Tsao, R. Ultrafiltration LC-ESI-MSn screening of 5-lipoxygenase inhibitors from selected Chinese medicinal herbs Saposhnikovia divaricata, Smilax glabra, Pueraria lobata and Carthamus tinctorius. J. Funct. Foods 2016, 24, 244–253. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, X.; Jiang, F.; Guo, Z.; Xie, J.; Fu, L. Application of the ultrafiltration-based LC-MS approach for screening PTP1B inhibitors from Chinese red yeast rice. Anal. Methods 2016, 8, 353–361. [Google Scholar] [CrossRef]
- Triñanes, S.; Casais, M.C.; Mejuto, M.C.; Cela, R. Matrix solid-phase dispersion followed by liquid chromatography tandem mass spectrometry for the determination of selective ciclooxygenase-2 inhibitors in sewage sludge samples. J. Chromatogr. A 2016, 1462, 35–43. [Google Scholar] [CrossRef]
- Li, D.Q.; Zhao, J.; Li, S.P. High-performance liquid chromatography coupled with post-column dual-bioactivity assay for simultaneous screening of xanthine oxidase inhibitors and free radical scavengers from complex mixture. J. Chromatogr. A 2014, 1345, 50–56. [Google Scholar] [CrossRef]
- Rea, V.; Falck, D.; Kool, J.; de Kanter, F.J.J.; Commandeur, J.N.M.; Vermeulen, N.P.E.; Niessen, W.M.A.; Honing, M. Combination of biotransformation by P450 BM3 mutants with on-line post-column bioaffinity and mass spectrometric profiling as a novel strategy to diversify and characterize p38α kinase inhibitors. MedChemComm 2013, 4, 371–377. [Google Scholar] [CrossRef]
- Lu, S.; Luo, Q.; Li, X.; Wu, J.; Liu, J.; Xiong, S.; Feng, Y.Q.; Wang, F. Inhibitor screening of protein kinases using MALDI-TOF MS combined with separation and enrichment of phosphopeptides by TiO2 nanoparticle deposited capillary column. Analyst 2010, 135, 2858–2863. [Google Scholar] [CrossRef]
- Wang, E.K.; Guo, S.J.; Li, D.; Zhang, L.M.; Li, J. Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery. Biomaterials 2009, 30, 1881–1889. [Google Scholar]
- Jiang, J.S.; Gan, Z.F.; Yang, Y.; Du, B.; Qian, M.; Zhang, P. Immobilization of homing peptide on magnetite nanoparticles and its specificity in vitro. J. Biomed. Mater. Res. A 2008, 84, 10–18. [Google Scholar]
- Ichikawa, S.; Kuroiwa, T.; Noguchi, Y.; Nakajima, M.; Sato, S.; Mukataka, S. Production of chitosan oligosaccharides using chitosanase immobilized on amylose-coated magnetic nanoparticles. Process Biochem. 2008, 43, 62–69. [Google Scholar]
- Pati, S.S.; Singh, L.H.; Guimarães, E.M.; Mantilla, J.; Coaquira, J.A.H.; Oliveira, A.C.; Sharma, V.K.; Garg, V.K. Magnetic chitosan-functionalized Fe3O4@Au nanoparticles: Synthesis and characterization. J. Alloys Compd. 2016, 684, 68–74. [Google Scholar] [CrossRef]
- Atacan, K.; Çakıroğlu, B.; Özacar, M. Improvement of the stability and activity of immobilized trypsin on modified Fe3O4 magnetic nanoparticles for hydrolysis of bovine serum albumin and its application in the bovine milk. Food Chem. 2016, 212, 460–468. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Jang, S.; Lee, H.; Sung, D.; Chang, J.H. High efficient chromogenic catalysis of tetramethylbenzidine with horseradish peroxidase immobilized magnetic nanoparticles. Biochem. Eng. J. 2016, 105, 406–411. [Google Scholar] [CrossRef]
- Casado-Carmona, F.A.; del Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Magnetic nanoparticles coated with ionic liquid for the extraction of endocrine disrupting compounds from waters. Microchem. J. 2016, 128, 347–353. [Google Scholar] [CrossRef]
- Cao, Y.; Wen, L.; Svec, F.; Tan, T.; Lv, Y. Magnetic AuNP@Fe3O4 nanoparticles as reusable carriers for reversible enzyme immobilization. Chem. Eng. J. 2016, 286, 272–281. [Google Scholar] [CrossRef]
- Siurdyban, E.; Brotin, T.; Talaga, D.; Heuzé, K.; Vellutini, L.; Buffeteau, T. Immobilization of Cryptophane Derivatives onto γ-Fe2O3 Core–Shell Magnetic Nanoparticles. J. Phys. Chem. C 2016, 120, 6583–6590. [Google Scholar] [CrossRef]
- Larsen, G.K.; Farr, W.; Hunyadi Murph, S.E. Multifunctional Fe2O3–Au Nanoparticles with Different Shapes: Enhanced Catalysis, Photothermal Effects, and Magnetic Recyclability. J. Phys. Chem. C 2016, 120, 15162–15172. [Google Scholar] [CrossRef]
- Nazari Serenjeh, F.; Hashemi, P.; Naeimi, H.; Zakerzadeh, E.; Ghiasvand, A.R. Spherical agarose-coated magnetic nanoparticles functionalized with a new salen for magnetic solid-phase extraction of uranyl ion. Microchim. Acta 2016, 183, 2449–2455. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, Z.; Zhang, Y.; Wang, Y.; Cheng, Y. Immobilized magnetic beads based multi-target affinity selection coupled with high performance liquid chromatography–mass spectrometry for screening anti-diabetic compounds from a Chinese medicine “Tang-Zhi-Qing”. J. Pharm. Biomed. 2013, 78–79, 190–201. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 2013, 27, 148–155. [Google Scholar] [CrossRef]
- Chu, C.; Qi, L.W.; Liu, E.H.; Li, B.; Gao, W.; Li, P. Radix astragali (Astragalus): Latest Advancements and Trends in Chemistry, Analysis, Pharmacology and Pharmacokinetics. Curr. Org. Chem. 2010, 14, 1792–1807. [Google Scholar] [CrossRef]
- Luo, Y.; Qin, Z.; Hong, Z.; Zhang, X.; Ding, D.; Fu, J.H.; Zhang, W.D.; Chen, J. Astragaloside IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neurosci. Lett. 2004, 363, 218–223. [Google Scholar] [CrossRef]
- Gui, S.Y.; Wei, W.; Wang, H.; Wu, L.; Sun, W.Y.; Chen, W.B.; Wu, C.Y. Effects and mechanisms of crude astragalosides fraction on liver fibrosis in rats. J. Ethnopharmacol. 2006, 103, 154–159. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Yu, L.; Zhao, Y.; Ding, W. Antifibrotic effect of the Chinese herbs, Astragalus mongholicus and Angelica sinensis, in a rat model of chronic puromycin aminonucleoside nephrosis. Life Sci. 2004, 74, 1645–1658. [Google Scholar] [CrossRef]
- Qi, L.W.; Cao, J.; Li, P.; Yu, Q.T.; Wen, X.D.; Wang, Y.X.; Li, C.Y.; Bao, K.D.; Ge, X.X.; Cheng, X.L. Qualitative and quantitative analysis of Radix astragali products by fast high-performance liquid chromatography-diode array detection coupled with time-of-flight mass spectrometry through dynamic adjustment of fragmentor voltage. J. Chromatogr. A 2008, 1203, 27–35. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; de Freitas, V. Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary α-Amylase (HSA) by Fluorescence Quenching. J. Agric. Food Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; Campos-Terán, J.; Hernández-Arana, A.; McClements, D.J. Characterization of flavonoid-protein interactions using fluorescence spectroscopy: Binding of pelargonidin to dairy proteins. Food Chem. 2016, 213, 431–439. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Song, L.N.; Zhao, Y.; Zang, F.L.; Zhao, Z.H.; Chen, N.H.; Xu, X.J.; Wang, C.J. Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA). Molecules 2015, 20, 16491–16523. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Deng, C.; Yang, P.; Zhang, X. Immobilization of Trypsin on Superparamagnetic Nanoparticles for Rapid and Effective Proteolysis. J. Proteome Res. 2007, 6, 3849–3855. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Chen, X.; Xiong, X.; Shi, S. Screening and identification of BSA bound ligands from Puerariae lobata flower by BSA functionalized Fe3O4 magnetic nanoparticles coupled with HPLC-MS/MS. J. Chromatogr. B 2012, 887–888, 55–60. [Google Scholar] [CrossRef]
- Mahesh, M.; Arivizhivendhan, K.V.; Maharaja, P.; Boopathy, R.; Hamsavathani, V.; Sekaran, G. Production, purification and immobilization of pectinase from Aspergillus ibericus onto functionalized nanoporous activated carbon (FNAC) and its application on treatment of pectin containing wastewater. J. Mol. Catal. B Enzym. 2016, 133, 43–54. [Google Scholar] [CrossRef]
- Ozseker, E.E.; Akkaya, A. Development of a new antibacterial biomaterial by tetracycline immobilization on calcium-alginate beads. Carbohydr. Polym. 2016, 151, 441–451. [Google Scholar] [CrossRef]
- Waifalkar, P.P.; Parit, S.B.; Chougale, A.D.; Sahoo, S.C.; Patil, P.S.; Patil, P.B. Immobilization of invertase on chitosan coated γ-Fe2O3 magnetic nanoparticles to facilitate magnetic separation. J. Colloid Interface Sci. 2016, 482, 159–164. [Google Scholar] [CrossRef]
- Golfam, G.; Karen, C.W. Capillary Electrophoretic Peptide Mapping to Probe the Immobilization/Digestion Conditions of Glutaraldehyde-crosslinked Chymotrypsin. Curr. Anal. Chem. 2016, 12, 65–73. [Google Scholar]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef]
- Aybaster, O.; Sahin, S.; Isk, E.; Demir, C. Determination of total phenolic content in Prunella L. by horseradish peroxidase immobilized onto chitosan beads. Anal. Methods 2011, 3, 2289–2297. [Google Scholar] [CrossRef]
- Kauldhar, B.S.; Dhau, J.S.; Sooch, B.S. Covalent linkage of alkalothermophilic catalase onto functionalized cellulose. RSC Adv. 2016, 6, 39364–39375. [Google Scholar] [CrossRef]
- Jian, H.; Wang, Y.; Bai, Y.; Li, R.; Gao, R. Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Flow Reactors. Molecules 2016, 21, 895. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, J.; Wang, Y.; Li, Z.; Li, X.; Sun, C.; Zheng, L. Immobilization of Cyclooxygenase-2 on Silica Gel Microspheres: Optimization and Characterization. Molecules 2015, 20, 19971–19983. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Li, S.; Yang, X.; Qin, Y.; Zhang, Y.; Liu, C. Screening and isolation of potential lactate dehydrogenase inhibitors from five Chinese medicinal herbs: Soybean, Radix pueraria, Flos pueraria, Rhizoma belamcandae, and Radix astragali. J. Sep. Sci. 2016, 39, 2043–2049. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, L.; Lin, Z.; Hou, C.; Liu, W.; Yan, T.; Zhu, L.; Wang, Y.; Lu, L.; Liu, Z. Study of pharmacokinetic profiles and characteristics of active components and their metabolites in rat plasma following oral administration of the water extract of Astragali radix using UPLC-MS/MS. J. Ethnopharmacol. 2015, 169, 183–194. [Google Scholar] [CrossRef]
- Pan, H.; Li, X.; Cheng, X.; Wang, X.; Fang, C.; Zhou, T.; Chen, J. Evidence of calycosin-7-O-β-d-glucoside’ role as a major antioxidant molecule of Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao plants under freezing stress. Environ. Exp. Bot. 2015, 109, 1–11. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhao, J.B.; Guo, L.; Yang, Y.L.; Hu, F.; Zhu, R.J.; Feng, S.L. Simultaneous Determination of Calycosin-7-O-β-d-Glucoside, Ononin, Calycosin, Formononetin, Astragaloside IV, and Astragaloside II in Rat Plasma After Oral Administration of Radix astragali Extraction for Their Pharmacokinetic Studies by Ultra-Pressure Liquid Chromatography with Tandem Mass Spectrometry. Cell Biochem. Biophys. 2014, 70, 677–686. [Google Scholar]
- Fu, S.; Gu, Y.; Jiang, J.Q.; Chen, X.; Xu, M.; Chen, X.; Shen, J. Calycosin-7-O-β-d-glucoside regulates nitric oxide /caveolin-1/matrix metalloproteinases pathway and protects blood–brain barrier integrity in experimental cerebral ischemia–reperfusion injury. J. Ethnopharmacol. 2014, 155, 692–701. [Google Scholar] [CrossRef]
- Xiao, J.B.; Wu, M.; Kai, G.; Wang, F.; Cao, H.; Yu, X. ZnO-ZnS QDs interfacial heterostructure for drug and food delivery application: enhancement of the binding affinities of flavonoid aglycones to bovine serum albumin. Nanomed. Nanotechnol. 2011, 7, 850–858. [Google Scholar] [CrossRef]
- Liu, E.H.; Qi, L.W.; Li, P. Structural Relationship and Binding Mechanisms of Five Flavonoids with Bovine Serum Albumin. Molecules 2010, 15, 9092–9103. [Google Scholar] [CrossRef]
- Wen, X.D.; Qi, L.W.; Chen, J.; Song, Y.; Yi, L.; Yang, X.W.; Li, P. Analysis of interaction property of bioactive components in Danggui Buxue Decoction with protein by microdialysis coupled with HPLC–DAD–MS. J. Chromatogr. B 2007, 852, 598–604. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
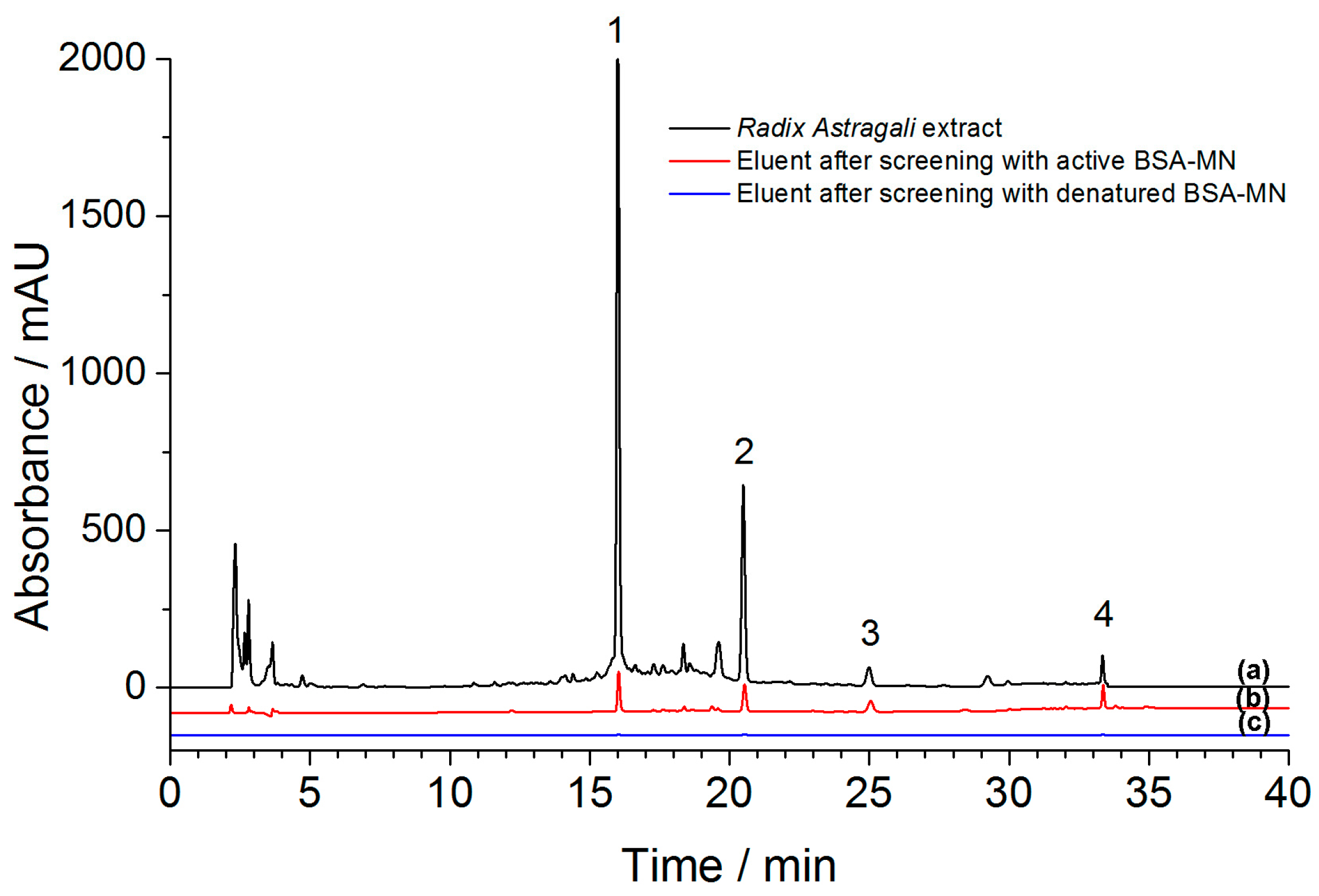
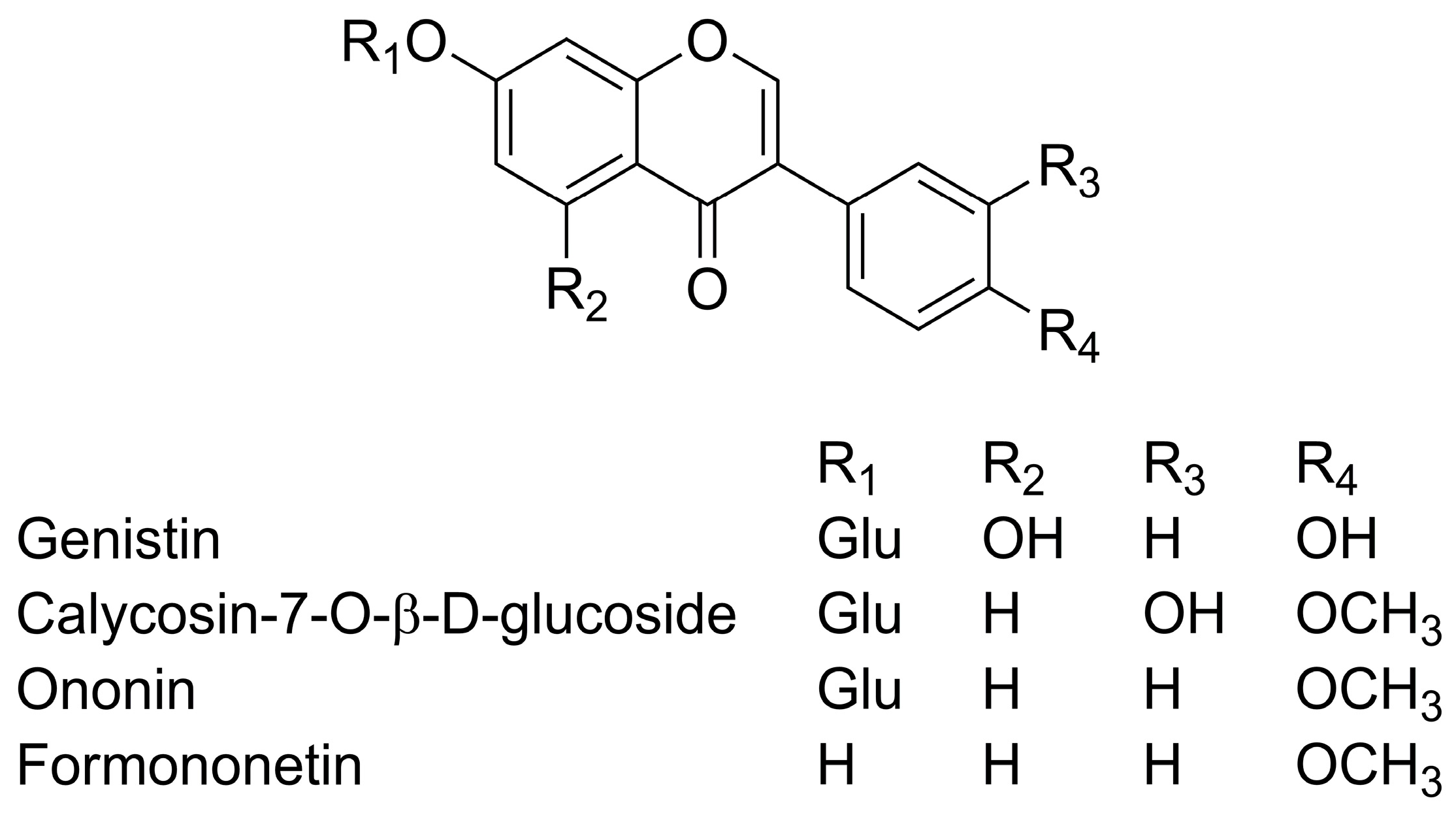
| No. | Identification | Rt (min) | Proposed Ions (m/z) | λmax (nm) | |
|---|---|---|---|---|---|
| 1 | Genistin | 16.02 | [M + H]− | 433 | 260 |
| [M − glc + H]− | 271 | ||||
| 2 | Calycosin-7-O-β-d-glucoside | 20.50 | [M + H]− | 447 | 260, 290 |
| [M − glc + H]− | 285 | ||||
| 3 | Ononin | 25.01 | [M + H]− | 431 | 260, 310 |
| [M − glc + H]− | 269 | ||||
| 4 | Formononetin | 33.36 | [M + H]− | 269 | 260, 315 |
| [M − CH3 + H]− | 254 | ||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Leng, J.; Yang, X.; Liao, L.; Cen, Y.; Xiao, A.; Ma, L. Rapid Screening and Identification of BSA Bound Ligands from Radix astragali Using BSA Immobilized Magnetic Nanoparticles Coupled with HPLC-MS. Molecules 2016, 21, 1471. https://doi.org/10.3390/molecules21111471
Liu L, Leng J, Yang X, Liao L, Cen Y, Xiao A, Ma L. Rapid Screening and Identification of BSA Bound Ligands from Radix astragali Using BSA Immobilized Magnetic Nanoparticles Coupled with HPLC-MS. Molecules. 2016; 21(11):1471. https://doi.org/10.3390/molecules21111471
Chicago/Turabian StyleLiu, Liangliang, Juan Leng, Xiai Yang, Liping Liao, Yin Cen, Aiping Xiao, and Lei Ma. 2016. "Rapid Screening and Identification of BSA Bound Ligands from Radix astragali Using BSA Immobilized Magnetic Nanoparticles Coupled with HPLC-MS" Molecules 21, no. 11: 1471. https://doi.org/10.3390/molecules21111471
APA StyleLiu, L., Leng, J., Yang, X., Liao, L., Cen, Y., Xiao, A., & Ma, L. (2016). Rapid Screening and Identification of BSA Bound Ligands from Radix astragali Using BSA Immobilized Magnetic Nanoparticles Coupled with HPLC-MS. Molecules, 21(11), 1471. https://doi.org/10.3390/molecules21111471






