Tryptophan Levels as a Marker of Auxins and Nitric Oxide Signaling
Abstract
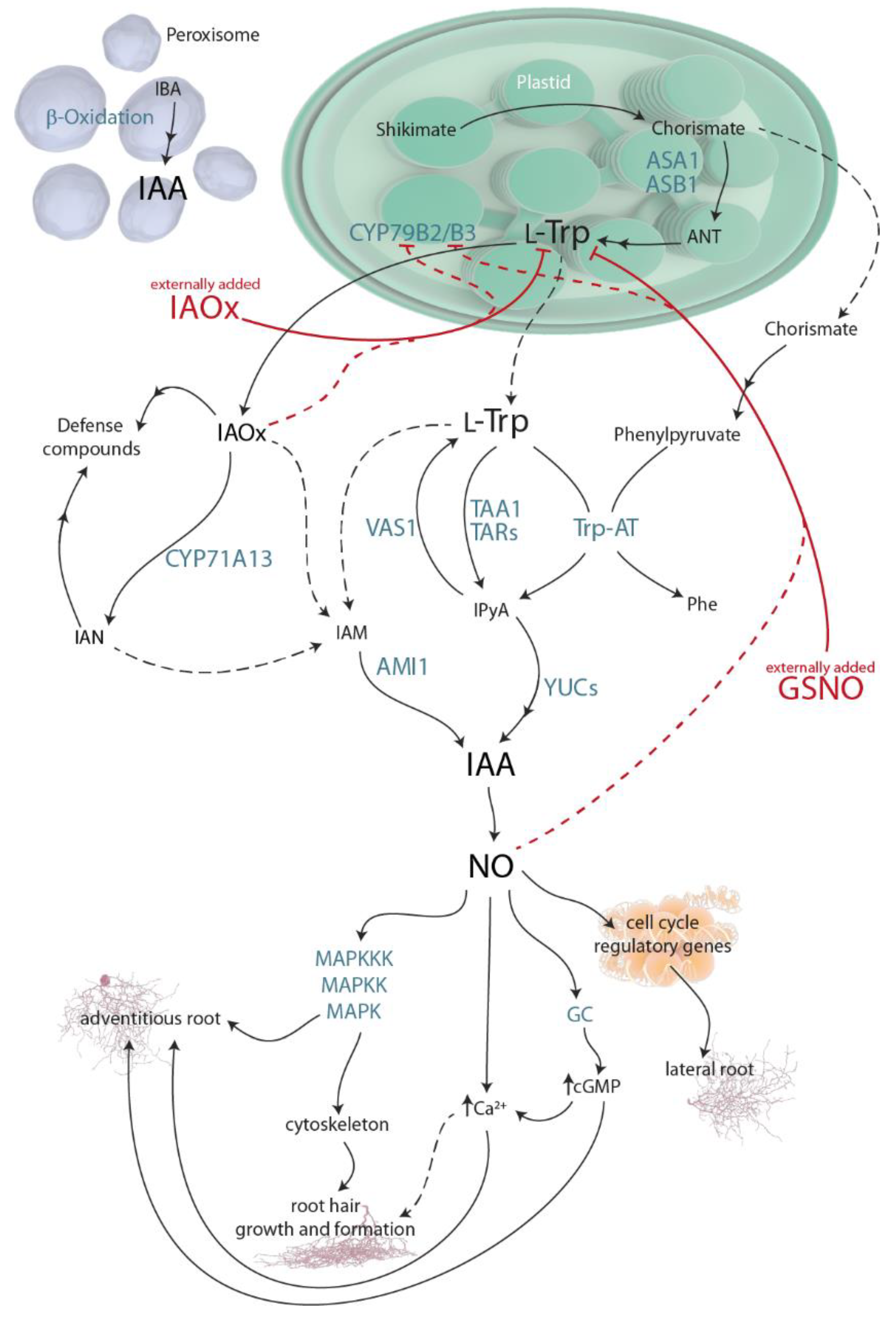
:1. Introduction
2. Results
2.1. Ionic Phases Separation of Plant Cell Contents
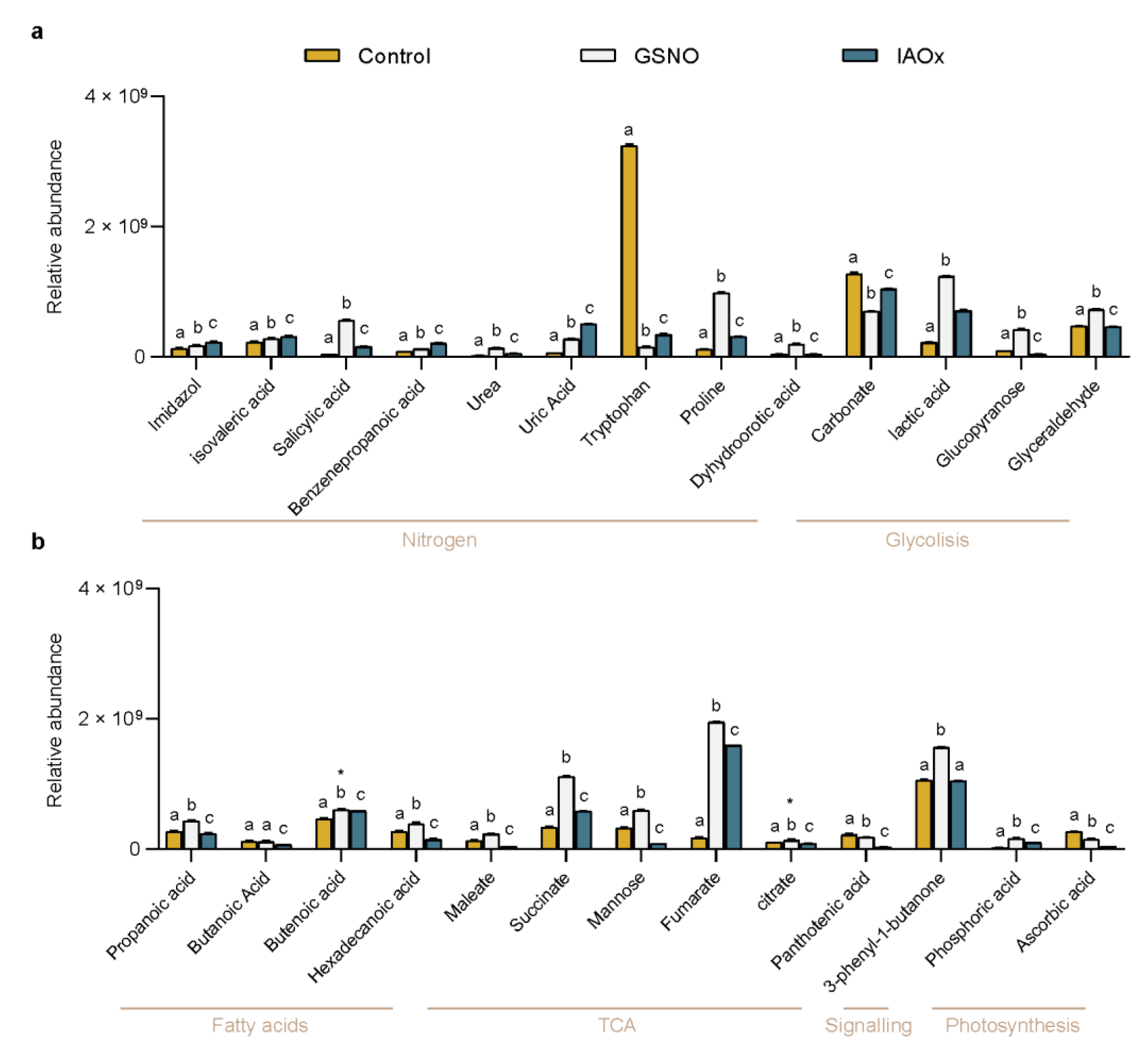
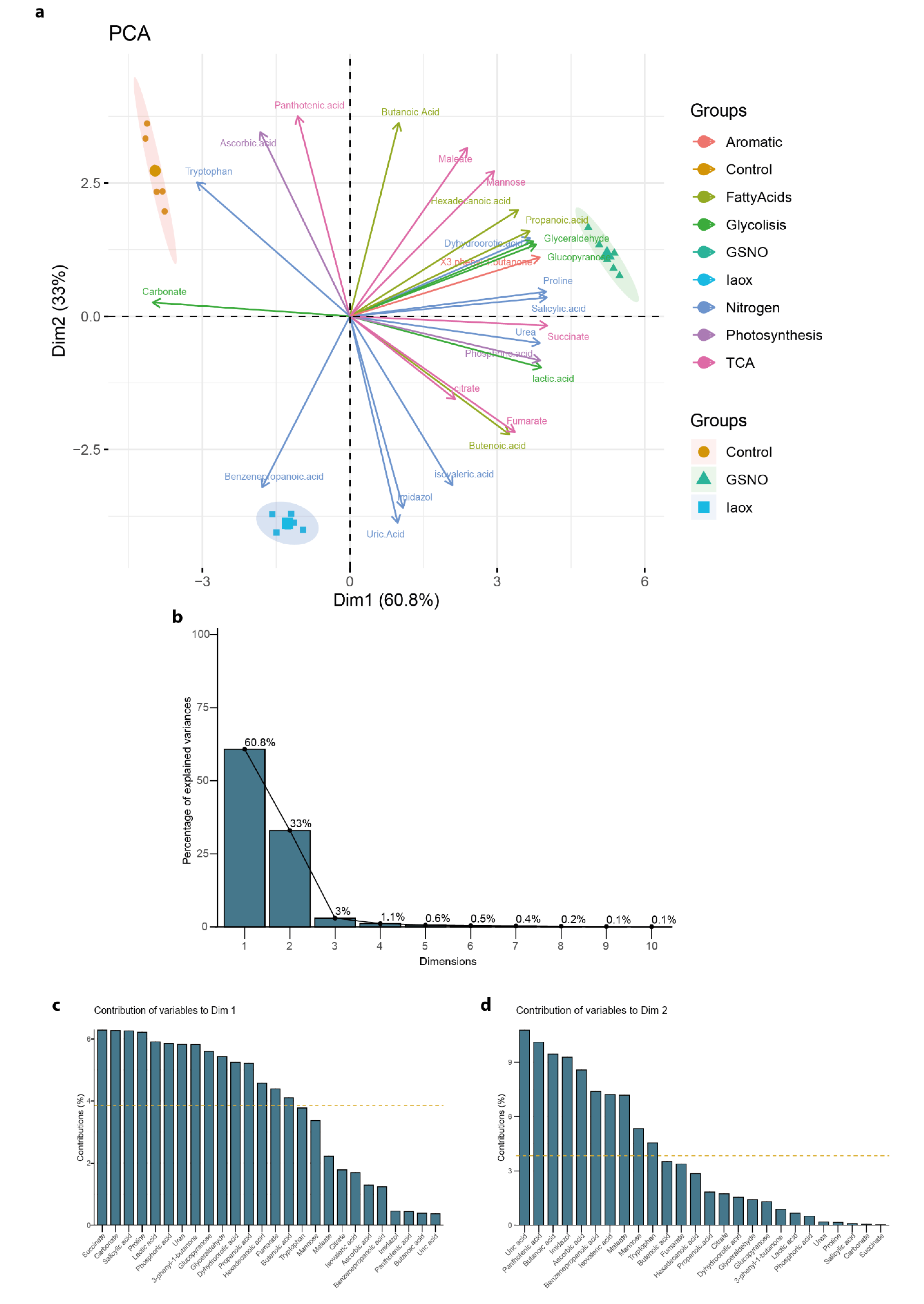
2.2. Modification of Several Molecular Contents by IAOx and GSNO Exposure
3. Discussion
3.1. Ionic Phases Separation as an Accuracy Booster for GC-MS of Plant Material
3.2. Trp Mainly Defines the Observed Differences in the Molecular Contents Induced by NO
4. Materials and Methods
4.1. Plant Cell Culturing
4.2. IAOx and GSNO Cell Exposition
4.3. Separation of Sample Phases by Ion Exchange Columns
4.4. Molecules Preparation for GC-MS and Derivatization
4.5. GC-MS Parameters
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Hull, A.K.; Vij, R.; Celenza, J.L. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2379–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, M.D.; Hansen, C.H.; Wittstock, U.; Halkier, B.A. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J. Biol. Chem. 2000, 275, 33712–33717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hull, A.K.; Gupta, N.R.; Goss, K.A.; Alonso, J.; Ecker, J.R.; Normanly, J.; Chory, J.; Celenza, J.L. Trp-dependent auxin biosynthesis in Arabidopsis: Involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002, 16, 3100–3112. [Google Scholar] [CrossRef] [Green Version]
- Casanova-Sáez, R.; Mateo-Bonmatí, E.; Ljung, K. Auxin metabolism in plants. Cold Spring Harb. Perspect. Biol. 2021, 11, a039867. [Google Scholar] [CrossRef]
- Sugawara, S.; Hishiyama, S.; Jikumaru, Y.; Hanada, A.; Nishimura, T.; Koshiba, T.; Zhao, Y.; Kamiya, Y.; Kasahara, H. Biochemical analyses of indole-3-acetaldoximedependent auxin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 5430–5435. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, M.; Neilson, E.H.J.; Møller, B.L. Oximes: Unrecognized Chameleons in General and Specialized Plant Metabolism. Mol. Plant 2018, 11, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Buezo, J.; Esteban, R.; Cornejo, A.; López-Gómez, P.; Marino, D.; Chamizo-Ampudia, A.; Gil, M.J.; Martínez-Merino, V.; Moran, J.F. IAOx induces the SUR phenotype and differential signalling from IAA under different types of nitrogen nutrition in Medicago truncatula roots. Plant Sci. 2019, 287, 110176. [Google Scholar] [CrossRef]
- Nafisi, M.; Goregaoker, S.; Botanga, C.J.; Glawischnig, E.; Olsen, C.E.; Halkier, B.A.; Glazebrook, J. Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis. Plant Cell 2007, 19, 2039–2052. [Google Scholar] [CrossRef] [Green Version]
- Glawischnig, E.; Hansen, B.G.; Olsen, C.E.; Halkier, B.A. Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 8245–8250. [Google Scholar] [CrossRef] [Green Version]
- Gaupels, F.; Kuruthukulangarakoola, G.T.; Durner, J. Upstream and downstream signals of nitric oxide in pathogen defence. Curr. Opin. Plant Biol. 2011, 14, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, O.; Bertoldo, J.B.; Besson-Bard, A.; Rosnoblet, C.; Aimé, S.; Hichami, S.; Terenzi, H.; Wendehenne, D. Protein S-nitrosylation: Specificity and identification strategies in plants. Front. Chem. 2015, 2, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.J.; Igamberdiev, A.U. Compartmentalization of Reactive Oxygen Species and Nitric Oxide Production in Plant Cells: An Overview. In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants. Signaling and Communication in Plants; Gupta, K., Igamberdiev, A., Eds.; Springer: Cham, Switzerland, 2015; Volume 23. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Astier, J.; Loake, G.; Velikova, V.; Gaupels, F. Editorial: Interplay between NO signaling, ROS, and the antioxidant system in plants. Front. Plant Sci. 2016, 7, 1731. [Google Scholar] [CrossRef] [Green Version]
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997–1019. [Google Scholar] [CrossRef] [Green Version]
- Demecsová, L.; Tamás, L. Reactive oxygen species, auxin and nitric oxide in metal-stressed roots: Toxicity or defence. BioMetals 2019, 32, 717–744. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef]
- Del Castello, F.; Nejamkin, A.; Cassia, R.; Correa-Aragunde, N.; Fernández, B.; Foresi, N.; Lombardo, C.; Ramirez, L.; Lamattina, L. The era of nitric oxide in plant biology: Twenty years tying up loose ends. Nitric Oxide Biol. Chem. 2019, 85, 17–27. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Auxin and Nitric Oxide: A Counterbalanced Partnership Ensures the Redox Cue Control Required for Determining Root Growth Pattern. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2016; Volume 77, pp. 41–54. [Google Scholar]
- Pagnussat, G.C.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002, 129, 954–956. [Google Scholar] [CrossRef] [Green Version]
- Pagnussat, G.C.; Lanteri, M.L.; Lamattina, L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003, 132, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Pagnussat, G.C.; Lanteri, M.L.; Lombardo, M.C.; Lamattina, L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 2004, 135, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Lamattina, L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friebe, A.; Koesling, D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ. Res. 2003, 93, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mulaudzi, T.; Ludidi, N.; Ruzvidzo, O.; Morse, M.; Hendricks, N.; Iwuoha, E.; Gehring, C. Identification of a novel Arabidopsis thaliana nitric oxide-binding molecule with guanylate cyclase activity in vitro. FEBS Lett. 2011, 585, 2693–2697. [Google Scholar] [CrossRef] [Green Version]
- Gross, I.; Durner, J. In search of enzymes with a role in 3′, 5′-Cyclic guanosine monophosphate metabolism in plants. Front. Plant Sci. 2016, 7, 576. [Google Scholar] [CrossRef] [Green Version]
- Astier, J.; Mounier, A.; Santolini, J.; Jeandroz, S.; Wendehenne, D. The evolution of nitric oxide signalling diverges between animal and green lineages. J. Exp. Bot. 2019, 70, 4355–4364. [Google Scholar] [CrossRef]
- Astier, J.; Lindermayr, C. Nitric oxide-dependent posttranslational modification in plants: An update. Int. J. Mol. Sci. 2012, 13, 15193–15208. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, D.J.; MdIsa, N.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; Marín-delaRosa, N.; VicenteConde, J.; SousaCorreia, C.; Pearce, S.P.; et al. Nitric Oxide Sensing in Plants Is Mediated by Proteolytic Control of Group VII ERF Transcription Factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Cárdenas, M.L.; Ryan, C.A. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 2002, 130, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Planchet, E.; Gupta, K.J.; Sonoda, M.; Kaiser, W.M. Nitric oxide emission from tobacco leaves and cell suspensions: Rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 2005, 41, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Amoroso, G.; Köhle, H.; Sültemeyer, D.F. Non-invasive online detection of nitric oxide from plants and some other organisms by mass spectrometry. Plant J. 2004, 38, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.J.; Santosa, I.E.; Laarhoven, L.J.J.; Holton, N.J.; Harren, F.J.M.; Smith, A.R. Laser photoacoustic detection allows in planta detection of nitric oxide in tobacco following challenge with avirulent and virulent Pseudomonas syringae pathovars. Plant Physiol. 2005, 138, 1247–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, H.; Sakihama, Y.; Takahashi, S. An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci. 1999, 4, 128–129. [Google Scholar] [CrossRef]
- Guo, F.Q.; Okamoto, M.; Crawford, N.M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 2003, 302, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G.; Miranda, K.M.; Thomas, D.D.; Wink, D.A. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radic. Biol. Med. 2002, 33, 827–834. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Soszynski, M.; Bartosz, G. On the specificity of 4-amino-5-methylamino-2′,7′-difluorofluorescein as a probe for nitric oxide. Free Radic. Biol. Med. 2005, 39, 327–335. [Google Scholar] [CrossRef]
- Arita, N.O.; Cohen, M.F.; Tokuda, G.; Yamasaki, H. Fluorometric Detection of Nitric Oxide with Diaminofluoresceins (DAFs): Applications and Limitations for Plant NO Research. In Nitric Oxide in Plant Growth, Development and Stress Physiology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 269–280. [Google Scholar]
- Jain, P.; David, A.; Bhatla, S.C. A novel protocol for detection of nitric oxide in plants. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1424, pp. 69–79. [Google Scholar]
- Ivanov, V.M. The 125th anniversary of the Griess reagent. J. Anal. Chem. 2004, 59, 1002–1005. [Google Scholar] [CrossRef]
- Pinder, A.G.; Rogers, S.C.; Khalatbari, A.; Ingram, T.E.; James, P.E. The measurement of nitric oxide and its metabolites in biological samples by ozone-based chemiluminescence. Methods Mol. Biol. 2008, 476, 11–28. [Google Scholar] [CrossRef]
- Birkemeyer, C.; Kolasa, A.; Kopka, J. Comprehensive chemical derivatization for gas chromatography-mass spectrometry-based multi-targeted profiling of the major phytohormones. J. Chromatogr. A 2003, 993, 89–102. [Google Scholar] [CrossRef]
- Schummer, C.; Delhomme, O.; Appenzeller, B.M.R.; Wennig, R.; Millet, M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 2009, 77, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Tachikawa, M.; Kanamori-Kataoka, M.; Sasamoto, K.; Ochiai, N. Target analysis of tert-butyldimethylsilyl derivatives of nerve agent hydrolysis products by selectable one-dimensional or two-dimensional gas chromatography–mass spectrometry. J. Chromatogr. A 2017, 1501, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Katona, Z.F.; Sass, P.; Molnár-Perl, I. Simultaneous determination of sugars, sugar alcohols, acids and amino acids in apricots by gas chromatography-mass spectrometry. J. Chromatogr. A 1999, 847, 91–102. [Google Scholar] [CrossRef]
- Jones, J.K.N.; Wall, R.A. The separation of sugars on ion-exchange resins: Part II. separation of monosaccharides. Can. J. Chem. 1960, 38, 2290–2294. [Google Scholar] [CrossRef]
- Acikara, O.B. Ion-Exchange Chromatography and Its Applications. Column Chromatogr. 2013, 10, 55744. [Google Scholar]
- Lee, K.; Choi, G.-H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Takemura, S.; Imaoka, S.; Funae, Y.; Tanimoto, Y.; Inoue, M. Irreversible inhibition of cytochrome P450 by nitric oxide. J. Pharmacol. Exp. Ther. 1997, 283, 1479–1485. [Google Scholar] [CrossRef]
- Koikov, L.N.; Alexeeva, N.V.; Lisitza, E.A.; Krichevsky, E.S.; Grigoryev, N.B.; Danilov, A.V.; Severina, I.S.; Pyatakova, N.V.; Granik, V.G. Oximes, amidoximes and hydroxamic acids as nitric oxide donors. Mendeleev Commun. 1998, 8, 165–168. [Google Scholar] [CrossRef]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and purine biosynthesis and degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef]
- Hou, A.X.; Tsuruta, H. Nitrous oxide and nitric oxide fluxes from an upland field in Japan: Effect of urea type, placement, and crop residues. Nutr. Cycl. Agroecosyst. 2003, 65, 191–200. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Wang, K.; Zhao, Y.; Zhai, Y.; Gao, M.; An, Z.; Liu, J.; Hu, J. Foliar urea application affects nitric oxide burst and glycinebetaine metabolism in two maize cultivars under drought. Pakistan J. Bot. 2012, 44, 81–86. [Google Scholar]
- Gaihre, Y.K.; Singh, U.; Islam, S.M.M.; Huda, A.; Islam, M.R.; Satter, M.A.; Sanabria, J.; Islam, M.R.; Shah, A.L. Impacts of urea deep placement on nitrous oxide and nitric oxide emissions from rice fields in Bangladesh. Geoderma 2015, 259–260, 370–379. [Google Scholar] [CrossRef]
- Witte, C.P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.M.; Gaihre, Y.K.; Biswas, J.C.; Singh, U.; Ahmed, M.N.; Sanabria, J.; Saleque, M.A. Nitrous oxide and nitric oxide emissions from lowland rice cultivation with urea deep placement and alternate wetting and drying irrigation. Sci. Rep. 2018, 8, 17623. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2016, 2, 16050. [Google Scholar] [CrossRef]
- Rohdich, F.; Wiese, A.; Feicht, R.; Simon, H.; Bacher, A. Enoate Reductases of Clostridia: Cloning, sequencing, and expression. J. Biol. Chem. 2001, 276, 5779–5787. [Google Scholar] [CrossRef] [Green Version]
- La Camera, S.; Gouzerh, G.; Dhondt, S.; Hoffmann, L.; Fritig, B.; Legrand, M.; Heitz, T. Metabolic reprogramming in plant innate immunity: The contributions of phenylpropanoid and oxylipin pathways. Immunol. Rev. 2004, 198, 267–284. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.M.A.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum esculentum Moench) Sprouts. Molecules 2017, 22, 374. [Google Scholar] [CrossRef] [Green Version]
- Poór, P. Effects of salicylic acid on the metabolism of mitochondrial reactive oxygen species in plants. Biomolecules 2020, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Silva, H.; Klessig, D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, N.J.; Masakapalli, S.K.; Ratcliffe, R.G. Assessing metabolic flux in plants with radiorespirometry. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1670, pp. 1–16. ISBN 978-1-4939-7291-3. [Google Scholar]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]

| Kegg Entry | Name | Structure | Formula | Related Pathways | Pathways KEGG Maps | Tag |
|---|---|---|---|---|---|---|
| C01589 | Imidazole |  | C3H4N2 | Histidine and purine synthesis | map00340; map00230 | Nitrogen |
| C08262 | Isovaleric acid | 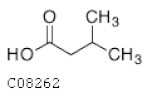 | C5H10O2 | Biosynthesis of alkaloids derived from histidine and purine | map01065; map01110; map04974 | Nitrogen |
| C00805 | Salicylic acid |  | C7H6O3 | Phenylalanine metabolism | map00360; map00621; map00624; map00626; map01053; map01061; map01070; map01100; map01110; map01120; map01220; map04075; map04976 | Nitrogen |
| C05629 | Benzenepropanoic acid | 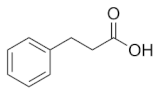 | C9H10O2 | Phenylalanine metabolism | map00360; map01100; map01120; map01220 | Nitrogen |
| C00086 | Urea | 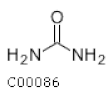 | CH4N2O | Arginine and proline biosynthesis Purine metabolism Pyrimidine metabolism | map00220; map00230; map00240; map00330; map00780; map00791; map01100; map01120; map02010; map05120 | Nitrogen |
| C00366 | Uric Acid | 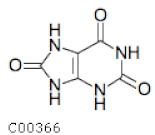 | C5H4N4O3 | Purine metabolism | map00230; map01100; map01120; map04976 | Nitrogen |
| C00078 | Tryptophan |  | C11H12N2O2 | Glycine, serine, and threonine metabolism Tryptophan metabolism | map00260; map00380; map00400; map00404; map00901; map00966; map00970; map00998; map01060; map01061; map01063; map01070; map01100; map01110; map01210; map01230; map01240; map04361; map04726; map04974; map04978; map05143; map05230 | Nitrogen |
| C00148 | Proline | 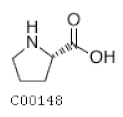 | C5H9NO2 | Arginine and proline metabolism | map00330; map00332; map00333; map00401; map00404; map00970; map01100; map01110; map01230; map02010; map04974; map04978 | Nitrogen |
| C00337 | Dyhydroorotic acid | 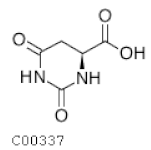 | C5H6N2O4 | Pyrimidine metabolism | map00240; map01100; map01240 | Nitrogen |
| - | Carbonate | 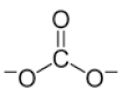 | - | - | Glycolisis | |
| C00186 | Lactic acid | 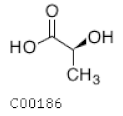 | C3H6O3 | Glycolisis and gluconeoge-nesis Fructose and mannose metabolism | map00010; map00051; map00620; map00640; map00643; map01100; map01110; map01120; map04024; map04066; map04922; map05230 | Glycolisis |
| C00031 | Glucopyranose | 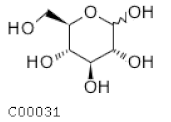 | C6H12O6 | Glycolisis and gluconeoge-nesis Pentose phosphate pathway | map00010; map00030; map00052; map00500; map00520; map00521; map00524; map00901; map01100; map01110; map02010; map02020 | Glycolisis |
| C00577 | Glyceraldehyde |  | C3H6O3 | Pentose phosphate pathway Fructose and mannose metabolism | map00030; map00051; map00052; map00561; map01100; map01120; map01200 | Glycolisis |
| C00163 | Propanoic acid | 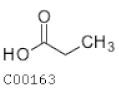 | C3H6O2 | Propanoate metabolism Ethylbenzene degradation | map00640; map00642; map00760; map01100; map01120; map01220; map04973; map04974 | Fatty Acids |
| C00246 | Butanoic Acid | 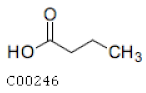 | C4H8O2 | Butanoate metabolism Carbohydrate digestion and absorption | map00650; map01100; map04973; map04974 | Fatty Acids |
| C01771 | Butenoic acid | 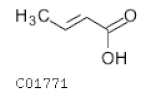 | C4H6O2 | Carbohydrate digestion and absorption | - | Fatty Acids |
| C00249 | Hexadecanoic acid | 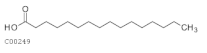 | C16H32O2 | Fatty acid biosynthesis, elongation, and degradation | map00061; map00062; map00071; map00073; map01040; map01060; map01100; map01212 | Fatty Acids |
| C01384 | Maleate |  | C4H4O4 | Citrate cycle | map00350; map00650; map00760; map01100 | TCA |
| C00042 | Succinate | 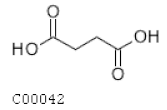 | C4H6O4 | Citrate cycle | map00020; map00190; map00250; map00310; map00350; map00360; map00361; map00620; map00630; map00640; map00650; map00720; map00760; map00920; map01060; map01061; map01062; map01063; map01064; map01065; map01066; map01070; map01100; map01110 | TCA |
| C00159 | Mannose | 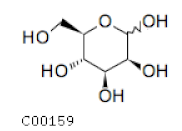 | C6H12O6 | Fructose and mannose metabolism Galactose metabolism | map00051; map00052; map00520; map01100; map02010 | TCA |
| C00122 | Fumarate | 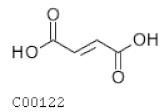 | C4H4O4 | Citrate cycle | map00020; map00190; map00220; map00250; map00350; map00360; map00620; map00643; map00650; map00760; map01060; map01061; map01062; map01063; map01064; map01065; map01066; map01070; map01100 | TCA |
| C00158 | Citrate | 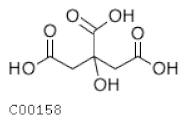 | C6H8O7 | Citrate cycle | map00020; map00250; map00630; map00997; map01060; map01061; map01062; map01063; map01064; map01065; map01066; map01070; map01100; map01110; map01120; map01200; map01210; map01230; map01240 | TCA |
| C00864 | Panthotenic acid | 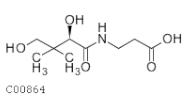 | C9H17NO5 | β-Alanine metabolism Pantothenate and CoA biosynthesis | map00410; map00770; map01100; map01110; map01240; map04977 | TCA |
| - | 3-phenyl-1-butanone | 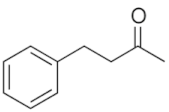 | C10H12O | Attractant compound in flowers | - | Signaling |
| C00009 | Phosphoric acid | 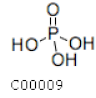 | H3PO4 | Photosynthesis | map00190; map00195; map02010; map04928 | Photosynthesis |
| C00072 | Ascorbic acid | 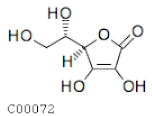 | C6H8O6 | Ascorbate and aldarate metabolism | map00053; map00480; map01100; map01110; map01240 | Photosynthesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, P.; Smith, E.N.; Bota, P.; Cornejo, A.; Urra, M.; Buezo, J.; Moran, J.F. Tryptophan Levels as a Marker of Auxins and Nitric Oxide Signaling. Plants 2022, 11, 1304. https://doi.org/10.3390/plants11101304
López-Gómez P, Smith EN, Bota P, Cornejo A, Urra M, Buezo J, Moran JF. Tryptophan Levels as a Marker of Auxins and Nitric Oxide Signaling. Plants. 2022; 11(10):1304. https://doi.org/10.3390/plants11101304
Chicago/Turabian StyleLópez-Gómez, Pedro, Edward N. Smith, Pedro Bota, Alfonso Cornejo, Marina Urra, Javier Buezo, and Jose F. Moran. 2022. "Tryptophan Levels as a Marker of Auxins and Nitric Oxide Signaling" Plants 11, no. 10: 1304. https://doi.org/10.3390/plants11101304









