MoWhi2 Mediates Mitophagy to Regulate Conidiation and Pathogenesis in Magnaporthe oryzae
Abstract
:1. Introduction
2. Results
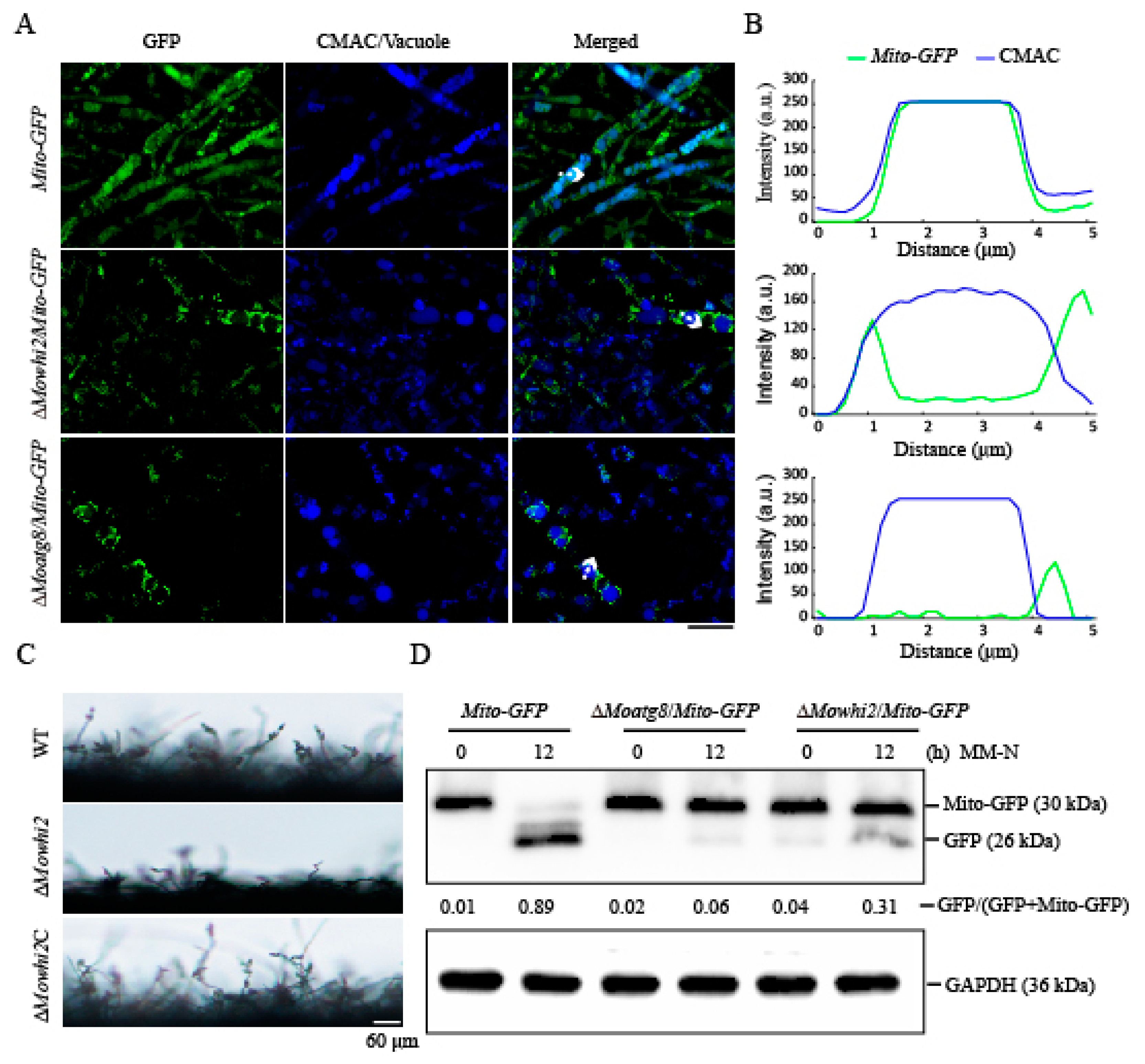
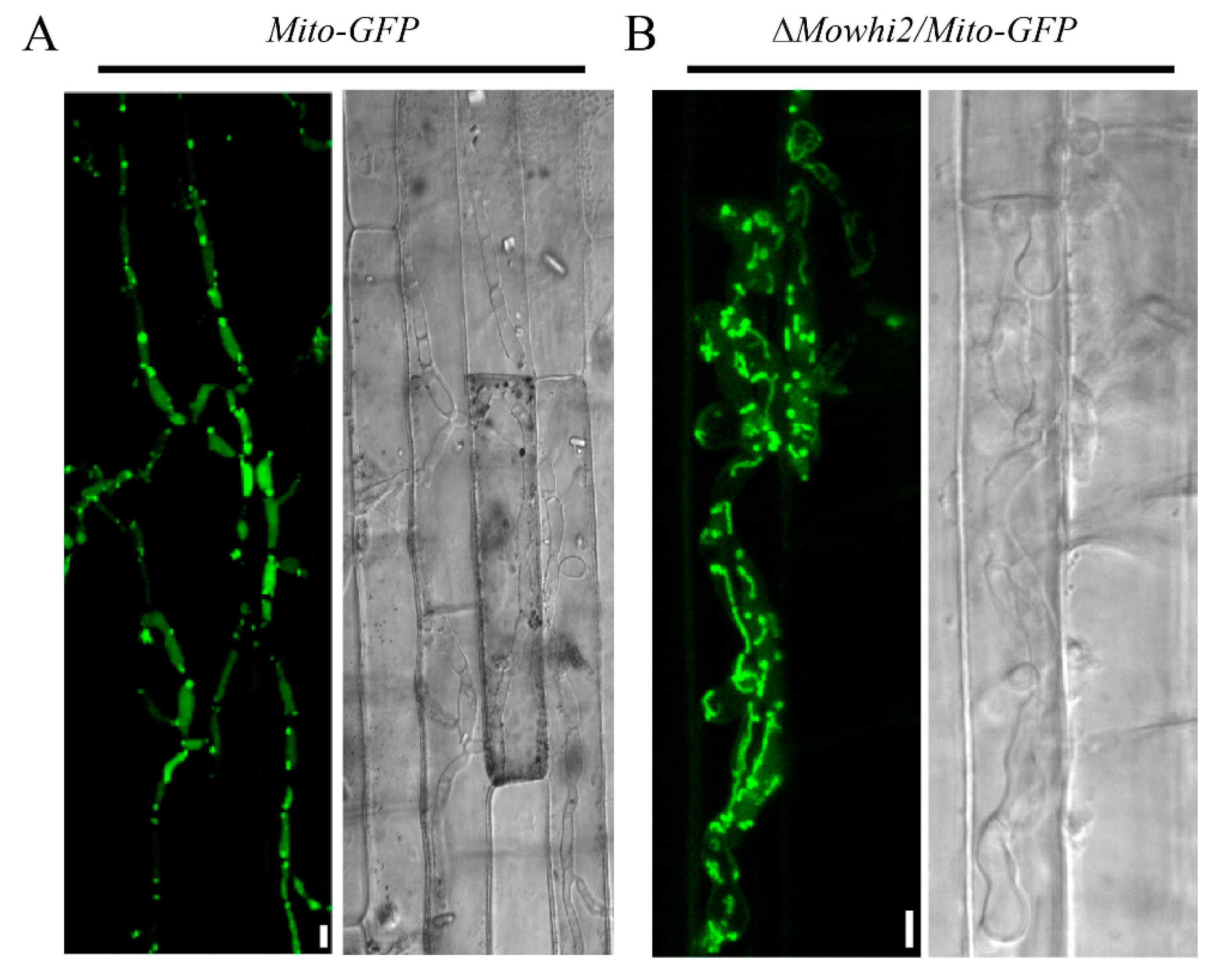
2.1. MoWHI2 Is Required for Mitophagy in M. oryzae
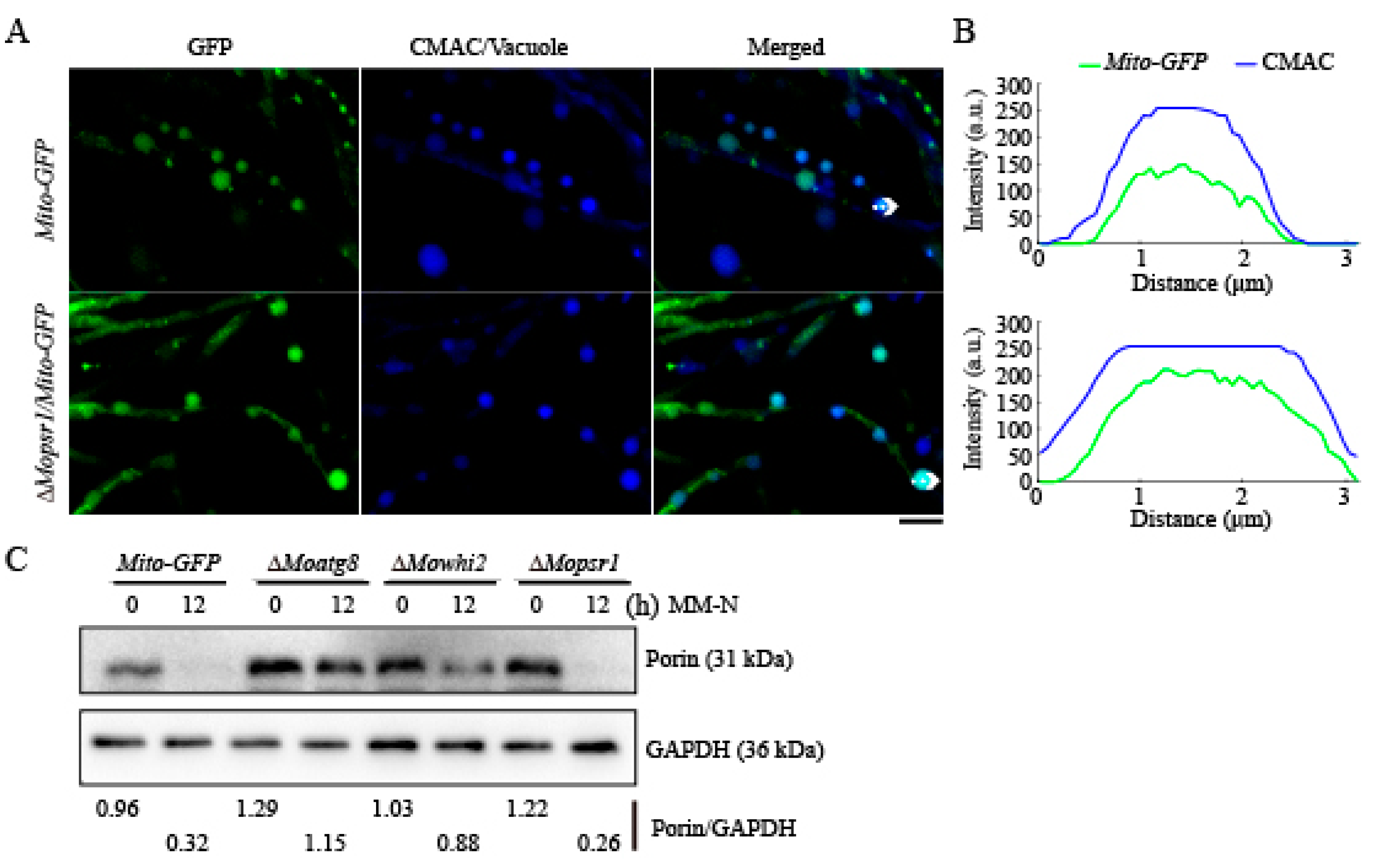
2.2. MoPsr1 Is Not Necessary for Mitophagy in M. oryzae
2.3. Deletion of MoWHI2 Did Not Affect Autophagy (Aacroautophagy) upon Nitrogen Starvation
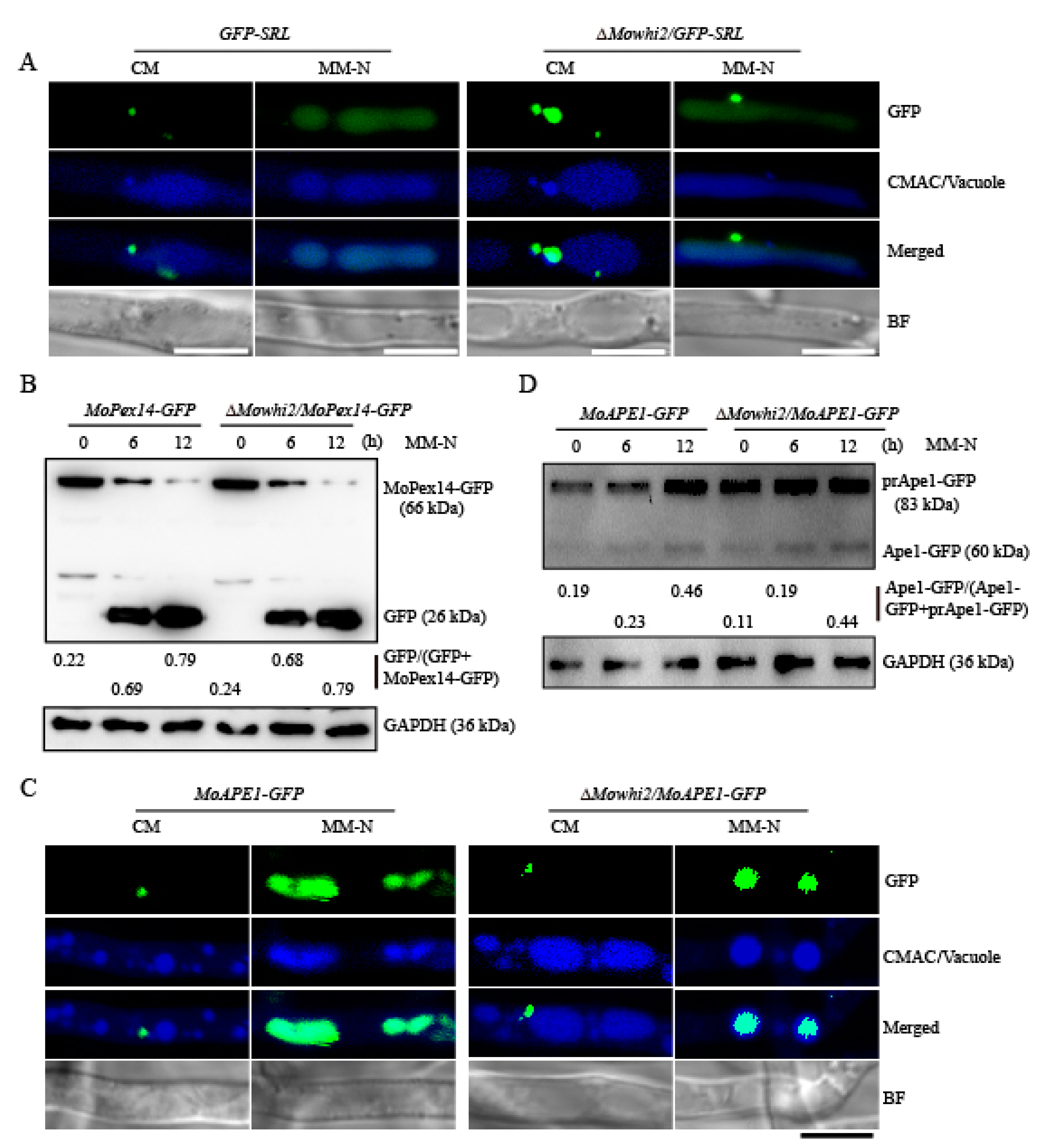
2.4. MoWHI2 Is Not Necessary for Pexophagy and the Cytoplasm-to-Vacuole Targeting (CVT) Pathway under Nitrogen Starvation
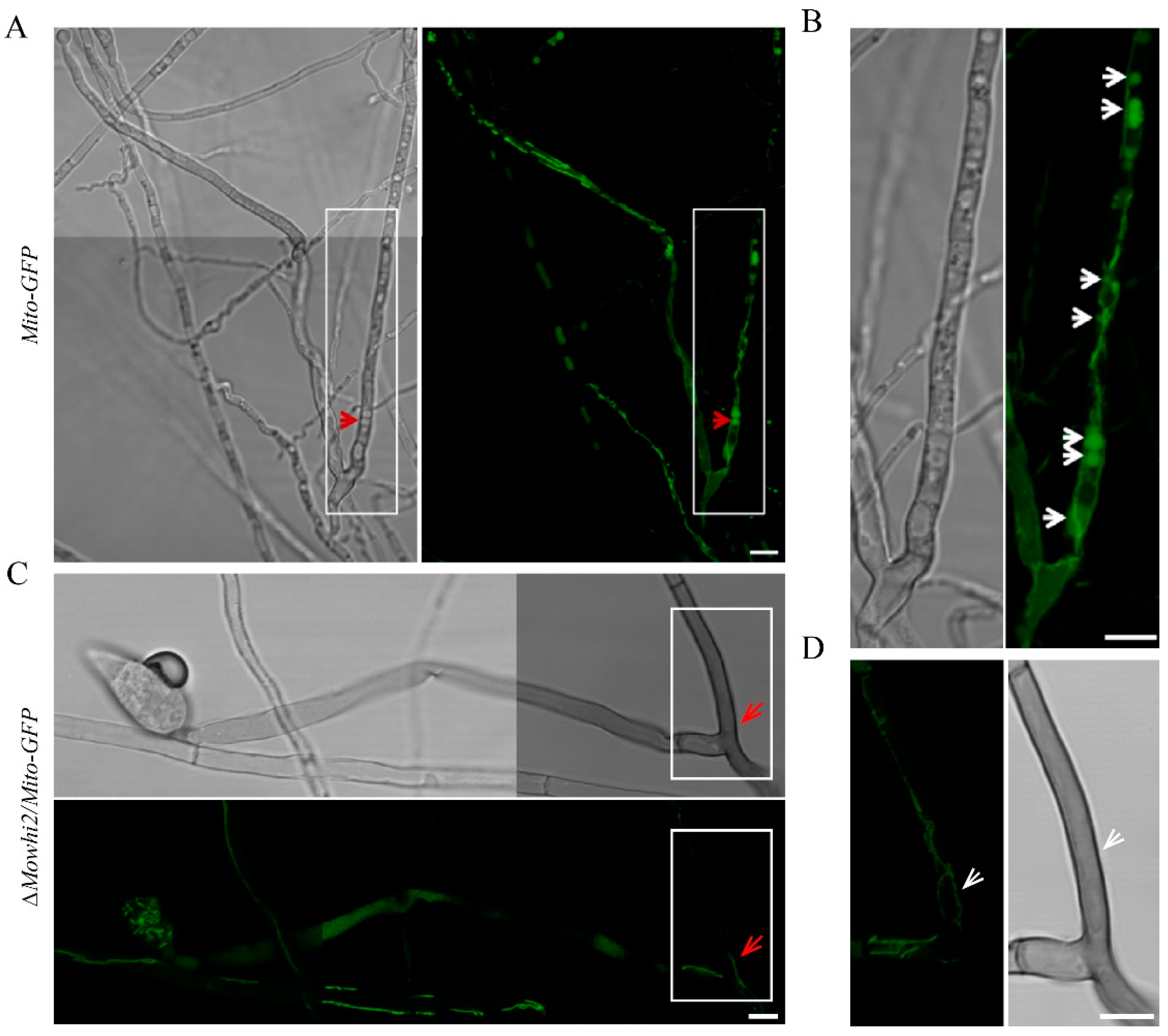
2.5. MoWhi2 Is Necessary for Mitophagy in Foot Cells during Conidiation
2.6. MoWhi2 Is Required for Mitophagy during Invasive Growth in M. oryzae
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Media
4.2. Plasmid Constructions
4.3. Mitophagy, Autophagy, Pexophagy and CVT Pathway Analyses
4.4. Fluorescence Microscopy
4.5. Western Blot Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanki, T.; Wang, K.; Baba, M.; Bartholomew, C.R.; Lynch-Day, M.A.; Du, Z.; Geng, J.; Mao, K.; Yang, Z.; Yen, W.L.; et al. A genomic screen for yeast mutants defective in selective mitochondria autophagy. Mol. Biol. Cell 2009, 20, 4730–4738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zutphen, T.; Veenhuis, M.; van der Klei, I.J. Pex14 is the sole component of the peroxisomal translocon that is required for pexophagy. Autophagy 2008, 4, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagernath, J.S.; Meng, S.; Qiu, J.; Shi, H.; Kou, Y. Selective degradation of mitochondria by mitophagy in pathogenic fungi. Am. J. Mol. Biol. 2021, 11, 15–27. [Google Scholar] [CrossRef]
- Kanki, T.; Wang, K.; Cao, Y.; Baba, M.; Klionsky, D.J. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell 2009, 17, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, M.; Lorenzi, I.; Dudek, J. The degradation pathway of the mitophagy receptor Atg32 is re-routed by a posttranslational modification. PLoS ONE 2016, 11, e0168518. [Google Scholar] [CrossRef]
- Liu, Y.; Okamoto, K. Regulatory mechanisms of mitophagy in yeast. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129858. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Kumar, R.; Reichert, A.S. Common principles and specific mechanisms of mitophagy from yeast to humans. Int. J. Mol. Sci. 2021, 22, 4363. [Google Scholar] [CrossRef] [PubMed]
- Leadsham, J.E.; Miller, K.; Ayscough, K.R.; Colombo, S.; Martegani, E.; Sudbery, P.; Gourlay, C.W. Whi2p links nutritional sensing to actin-dependent Ras-cAMP-PKA regulation and apoptosis in yeast. J. Cell Sci. 2009, 122, 706–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendl, N.; Occhipinti, A.; Muller, M.; Wild, P.; Dikic, I.; Reichert, A.S. Mitophagy in yeast is independent of mitochondrial fission and requires the stress response gene WHI2. J. Cell Sci. 2011, 124, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saul, D.J.; Sudbery, P.E. Molecular cloning of WHI2, a gene involved in the regulation of cell proliferation in Saccharomyces cerevisiae. J. Gen. Microbiol. 1985, 131, 1797–1806. [Google Scholar] [CrossRef] [Green Version]
- Kaida, D.; Yashirodaa, H.; Toh-e, A.; Kikuchi, Y. Yeast Whi2 and Psr1-phosphatase form a complex and regulate STRE-mediated gene expression. Genes Cells 2002, 7, 543–552. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Zhang, Y.; Dayhoff-Brannigan, M.; Diny, N.L.; Zhao, M.; He, G.; Sing, C.N.; Metz, K.A.; Stolp, Z.D.; et al. Whi2 is a conserved negative regulator of TORC1 in response to low amino acids. PLoS Genet. 2018, 14, e1007592. [Google Scholar] [CrossRef]
- Muller, M.; Reichert, A.S. Mitophagy, mitochondrial dynamics and the general stress response in yeast. Biochem. Soc. Trans. 2011, 39, 1514–1519. [Google Scholar] [CrossRef] [Green Version]
- Harata, K.; Nishiuchi, T.; Kubo, Y. Colletotrichum orbiculare WHI2, a yeast stress-response regulator homolog, controls the biotrophic stage of hemibiotrophic infection through TOR signaling. Mol. Plant Microbe Interact. 2016, 29, 468–483. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Meng, S.; Qiu, J.; Wang, C.; Shu, Y.; Luo, C.; Kou, Y. MoWhi2 regulates appressorium formation and pathogenicity via the MoTor signalling pathway in Magnaporthe oryzae. Mol. Plant Pathol. 2021, 22, 969–983. [Google Scholar] [CrossRef]
- He, Y.; Deng, Y.Z.; Naqvi, N.I. Atg24-assisted mitophagy in the foot cells is necessary for proper asexual differentiation in Magnaporthe oryzae. Autophagy 2013, 9, 1818–1827. [Google Scholar] [CrossRef] [Green Version]
- Kou, Y.; He, Y.; Qiu, J.; Shu, Y.; Yang, F.; Deng, Y.; Naqvi, N.I. Mitochondrial dynamics and mitophagy are necessary for proper invasive growth in rice blast. Mol. Plant Pathol. 2019, 20, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, K.; Li, X.; Le, X.; Kong, X.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoDnm1 dynamin mediating peroxisomal and mitochondrial fission in complex with MoFis1 and MoMdv1 is important for development of functional appressorium in Magnaporthe oryzae. PLoS Pathog. 2016, 12, e1005823. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, L.; Zhang, T.; Zhou, R.; Ren, Y.; Li, X.; Shu, H.; Ye, W.; Zheng, X.; Zhang, Z.; et al. Transcription factor MoMsn2 targets the putative 3-methylglutaconyl-CoA hydratase-encoding gene MoAUH1 to govern infectious growth via mitochondrial fusion/fission balance in Magnaporthe oryzae. Environ. Microbiol. 2021, 23, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Patkar, R.N.; Ramos-Pamplona, M.; Gupta, A.P.; Fan, Y.; Naqvi, N.I. Mitochondrial beta-oxidation regulates organellar integrity and is necessary for conidial germination and invasive growth in Magnaporthe oryzae. Mol. Microbiol. 2012, 86, 1345–1363. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Dunn, W.A., Jr.; Cregg, J.M.; Kiel, J.A.; van der Klei, I.J.; Oku, M.; Sakai, Y.; Sibirny, A.A.; Stasyk, O.V.; Veenhuis, M. Pexophagy: The selective autophagy of peroxisomes. Autophagy 2005, 1, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, J.; Chen, H.; Chai, R.; Zhang, Z.; Mao, X.; Qiu, H.; Jiang, H.; Wang, Y.; Sun, G. Pex14/17, a filamentous fungus-specific peroxin, is required for the import of peroxisomal matrix proteins and full virulence of Magnaporthe oryzae. Mol. Plant. Pathol. 2017, 18, 1238–1252. [Google Scholar] [CrossRef] [Green Version]
- Lv, W.; Xu, Z.; Talbot, N.J.; Wang, Z. The sorting nexin FgAtg20 is involved in the Cvt pathway, non-selective macroautophagy, pexophagy and pathogenesis in Fusarium graminearum. Cell Microbiol. 2020, 22, e13208. [Google Scholar] [CrossRef]
- Deng, Y.; Qu, Z.; Naqvi, N.I. The role of snx41-based pexophagy in magnaporthe development. PLoS ONE 2013, 8, e79128. [Google Scholar] [CrossRef]
- Nair, U.; Cao, Y.; Xie, Z.; Klionsky, D.J. Roles of the lipid-binding motifs of Atg18 and Atg21 in the cytoplasm to vacuole targeting pathway and autophagy. J. Biol. Chem. 2010, 285, 11476–11488. [Google Scholar] [CrossRef] [Green Version]
- Mao, K.; Liu, X.; Feng, Y.; Klionsky, D.J. The progression of peroxisomal degradation through autophagy requires peroxisomal division. Autophagy 2014, 10, 652–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Sakakibara, K.; Chen, Q.; Okamoto, K. Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res. 2014, 24, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Chiang, W.C.; Sumpter, R., Jr.; Mishra, P.; Levine, B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 2017, 168, 224–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timpano, H.; Chan Ho Tong, L.; Gautier, V.; Lalucque, H.; Silar, P. The PaPsr1 and PaWhi2 genes are members of the regulatory network that connect stationary phase to mycelium differentiation and reproduction in Podospora anserina. Fungal Genet. Biol. 2016, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Naqvi, N.I. Sulfonylurea resistance reconstitution as a novel strategy for ILV2-specific integration in Magnaporthe oryzae. Fungal Genet. Biol. 2014, 68, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Veneault-Fourrey, C.; Barooah, M.; Egan, M.; Wakley, G.; Talbot, N.J. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 2006, 312, 580–583. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, S.; Jagernath, J.S.; Luo, C.; Shi, H.; Kou, Y. MoWhi2 Mediates Mitophagy to Regulate Conidiation and Pathogenesis in Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 5311. https://doi.org/10.3390/ijms23105311
Meng S, Jagernath JS, Luo C, Shi H, Kou Y. MoWhi2 Mediates Mitophagy to Regulate Conidiation and Pathogenesis in Magnaporthe oryzae. International Journal of Molecular Sciences. 2022; 23(10):5311. https://doi.org/10.3390/ijms23105311
Chicago/Turabian StyleMeng, Shuai, Jane Sadhna Jagernath, Chaoxi Luo, Huanbin Shi, and Yanjun Kou. 2022. "MoWhi2 Mediates Mitophagy to Regulate Conidiation and Pathogenesis in Magnaporthe oryzae" International Journal of Molecular Sciences 23, no. 10: 5311. https://doi.org/10.3390/ijms23105311
APA StyleMeng, S., Jagernath, J. S., Luo, C., Shi, H., & Kou, Y. (2022). MoWhi2 Mediates Mitophagy to Regulate Conidiation and Pathogenesis in Magnaporthe oryzae. International Journal of Molecular Sciences, 23(10), 5311. https://doi.org/10.3390/ijms23105311





