Remediation and Micro-Ecological Regulation of Cadmium and Arsenic Co-Contaminated Soils by Rotation of High-Biomass Crops and Sedum alfredii Hance: A Field Study
Abstract
:1. Introduction
2. Materials and Methods
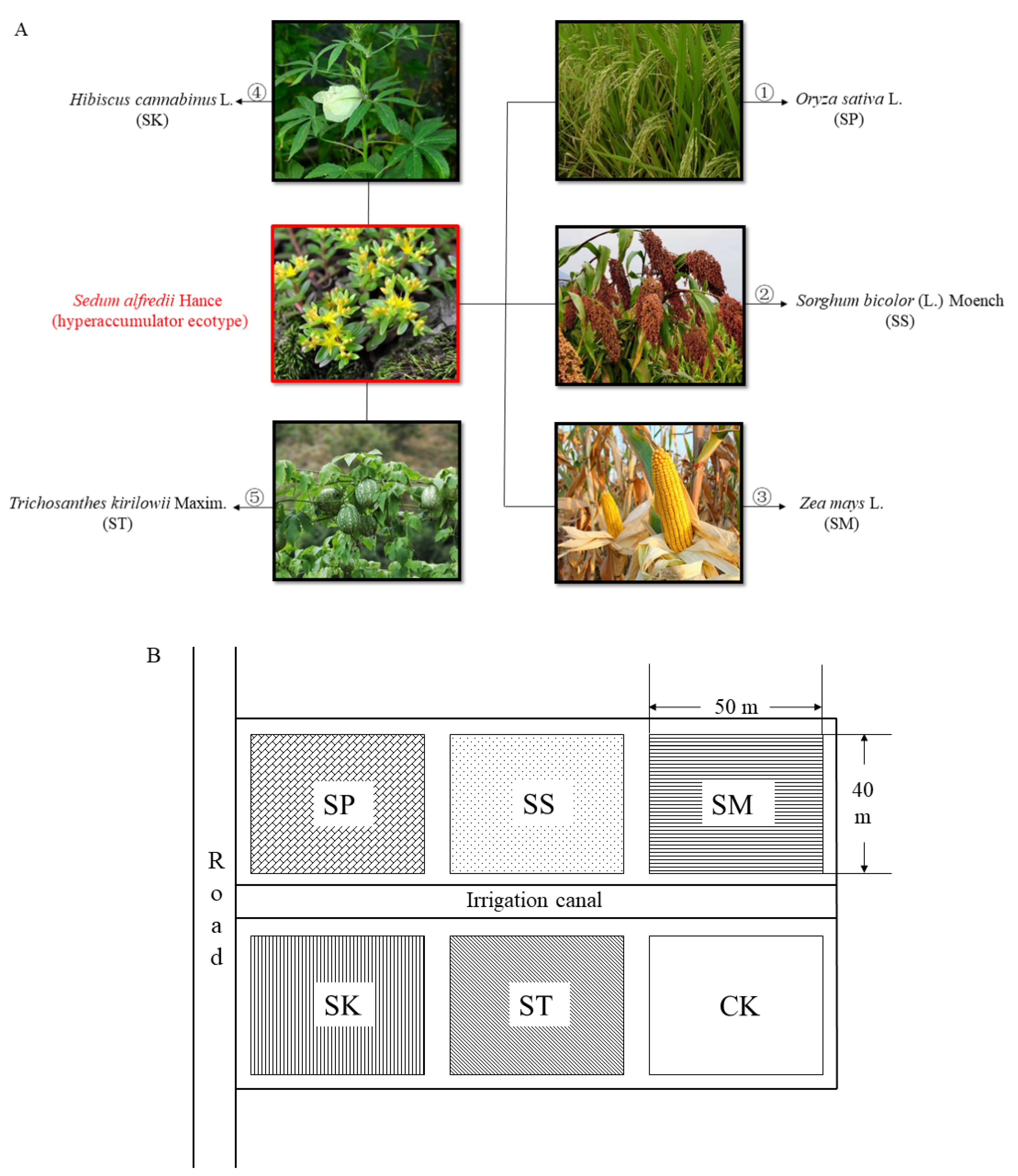
2.1. Experimental Site
2.2. Experimental Design
2.3. Soil Properties Analysis
2.4. Soil Microbe Analysis
2.5. Statistical Analysis
3. Results
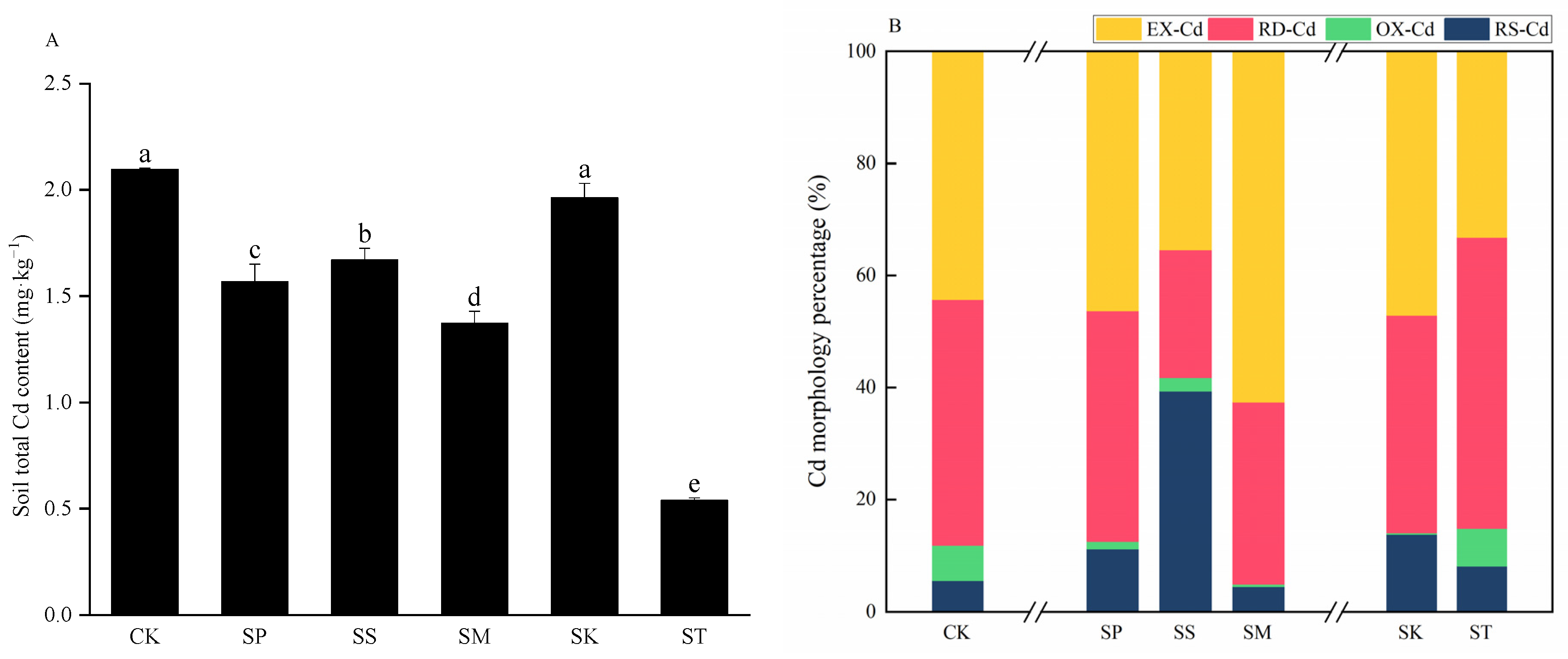
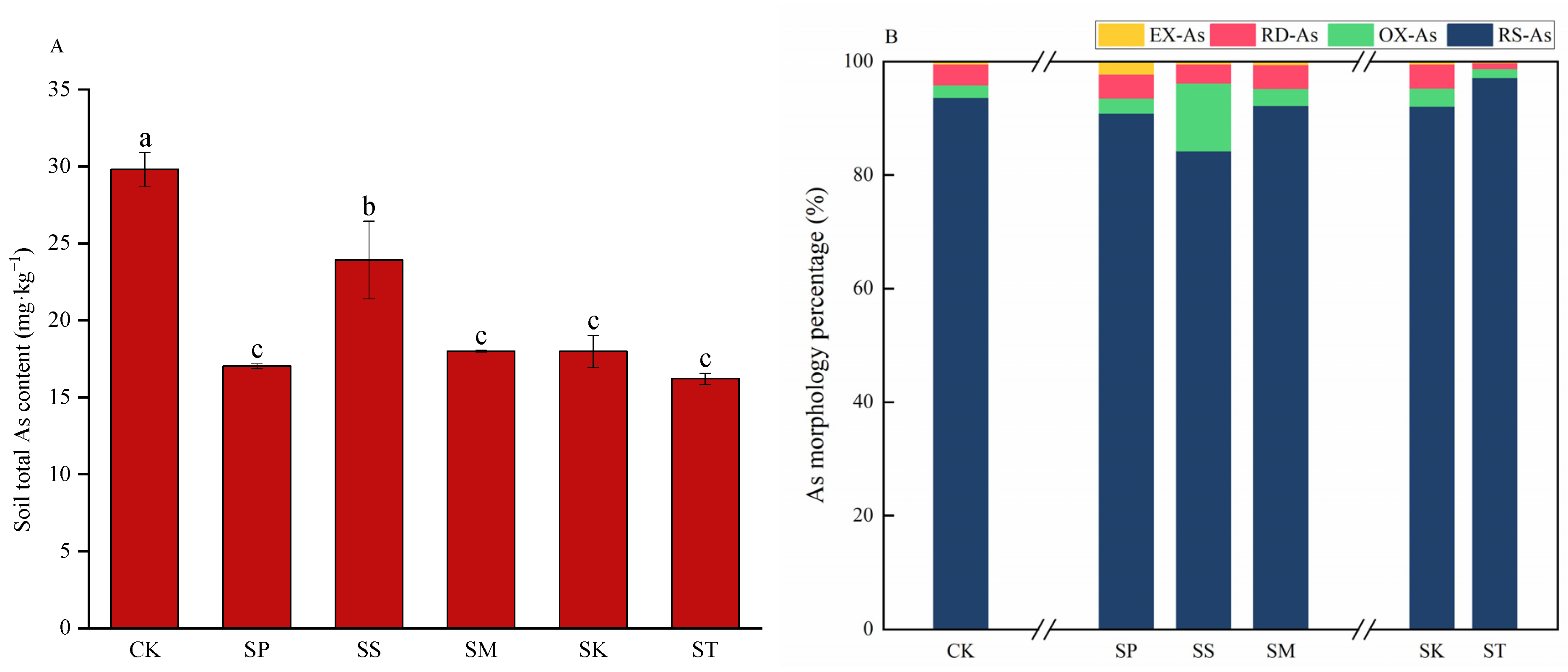
3.1. Total Cd/As and Fraction Contents
3.2. Soil Physicochemical Characteristics
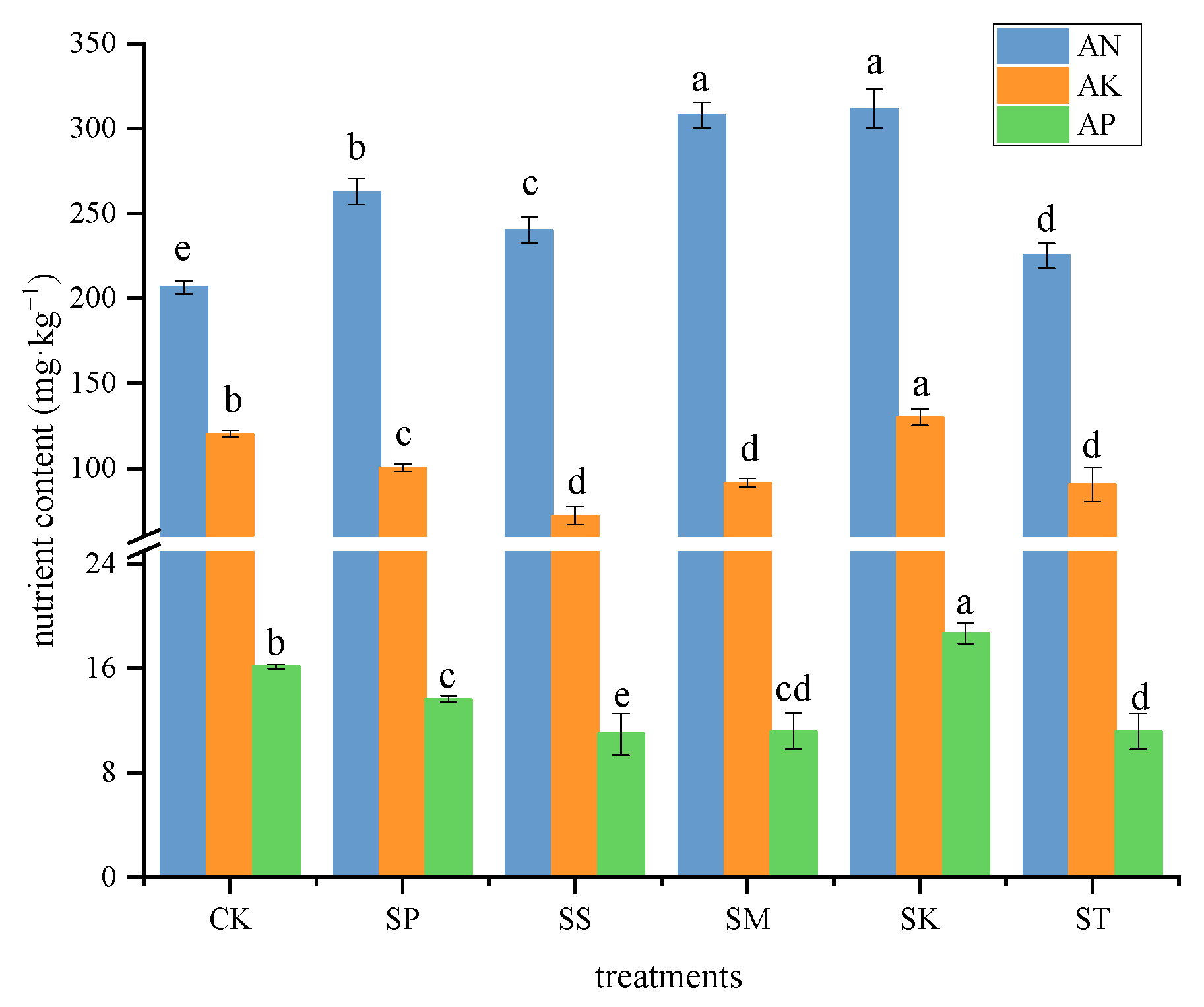
3.3. Soil Nutrient Content
3.4. Soil Enzyme Activities
3.5. Soil Bacterial Properties
3.5.1. Microbial Community Composition
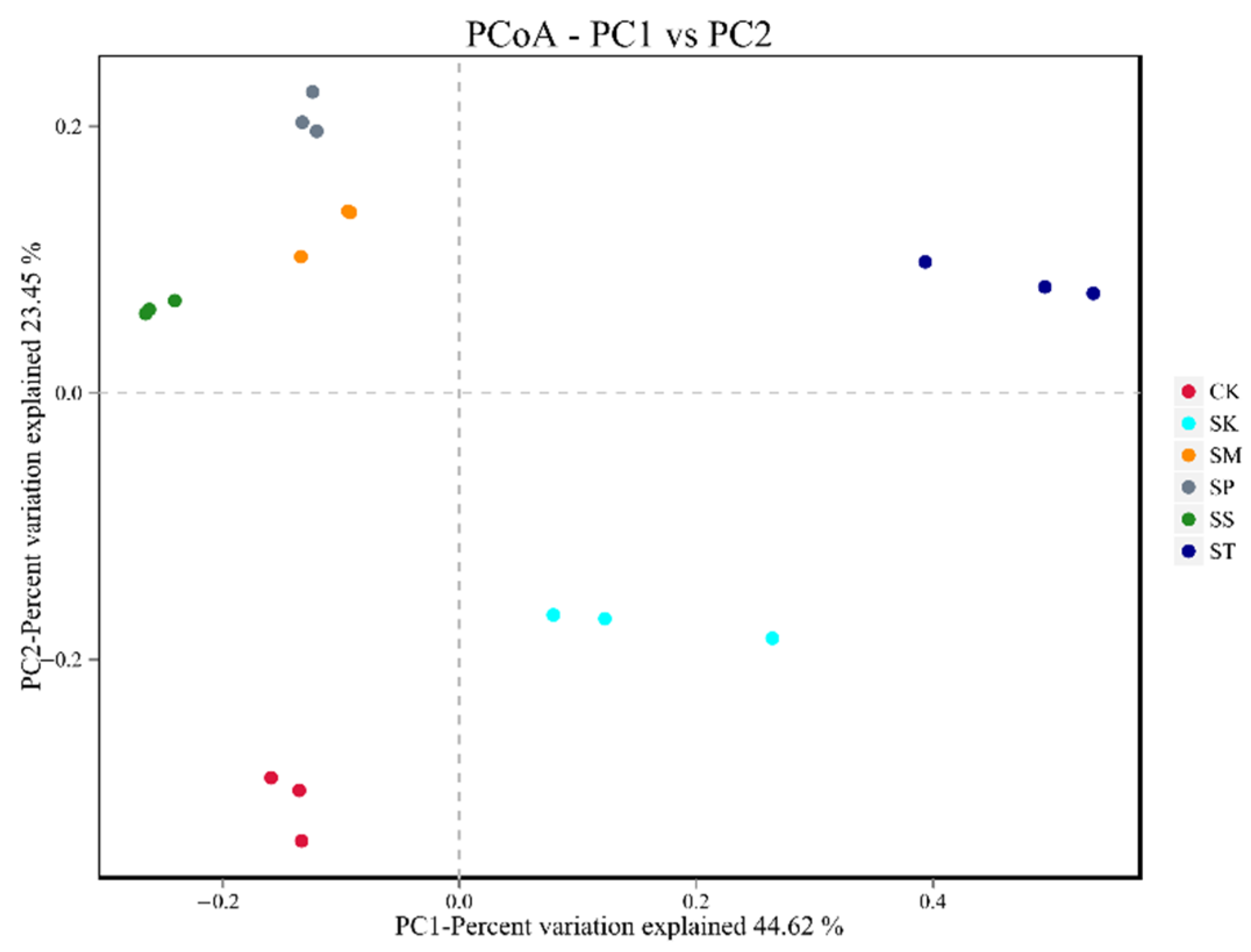
3.5.2. Diversity of Bacterial Community
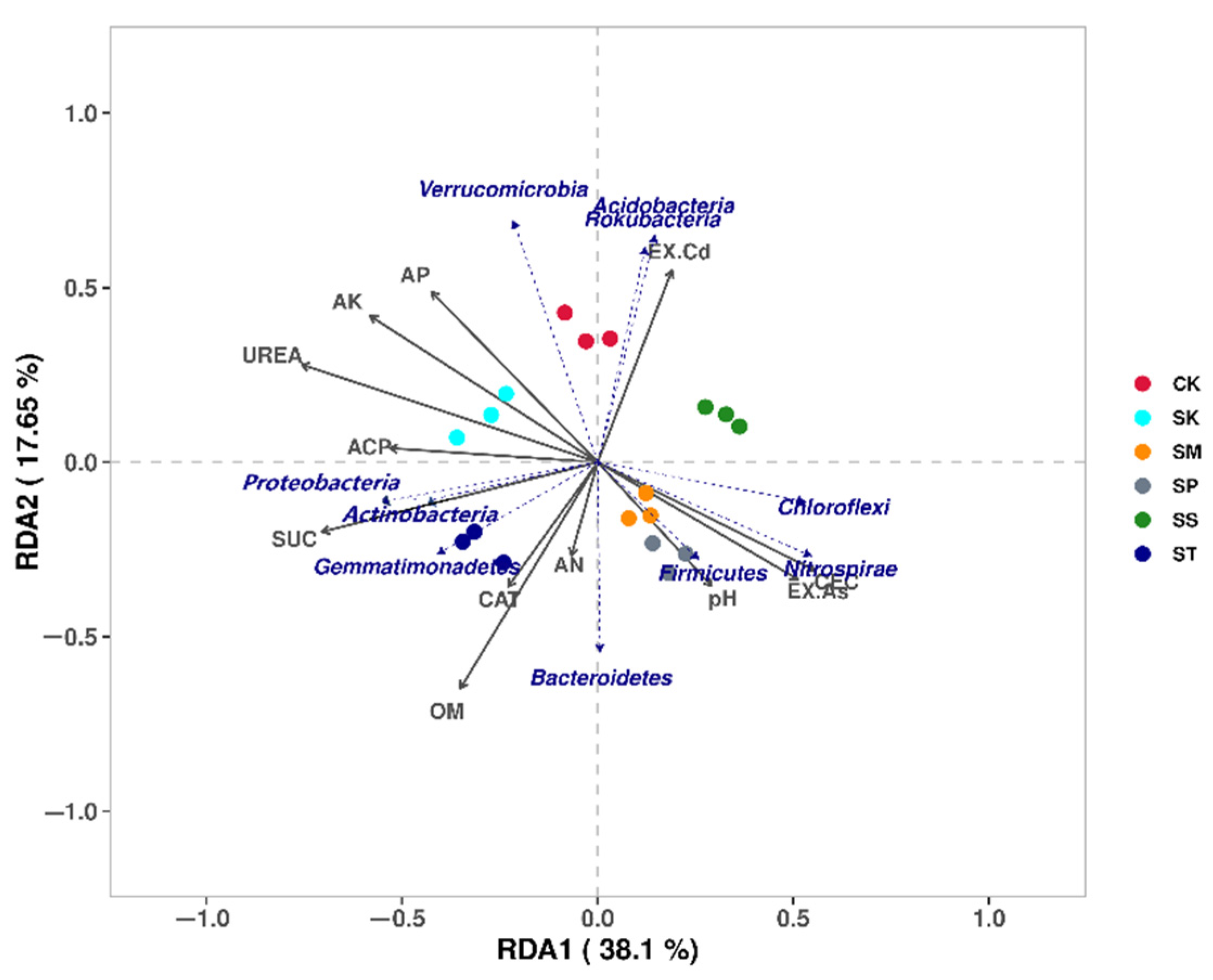
3.5.3. Relationship between Microbial Properties and Environmental Variables
4. Discussion
4.1. Effect of Rotation Modes on Soil Cd/As Content
4.2. Effect of Rotation Modes on Soil Microecology
4.3. Cost-Benefit Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Zhong, L.; Meng, J.; Wang, F.; Zhang, J.; Zhi, Y.; Zeng, L.; Tang, X.; Xu, J. A multi-medium chain modeling approach to estimate the cumulative effects of cadmium pollution on human health. Environ. Pollut. 2018, 239, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Zhou, Y.; Dou, L.; Cai, L.; Mo, L.; You, J. Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: A case study in the Pearl River Delta, South China. Environ. Pollut. 2018, 235, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Kumar, C.; Patel, N.K. Integrated micro-biochemical approach for phytoremediation of cadmium and lead contaminated soils using Gladiolus grandiflorus L cut flower. Ecotoxicol. Environ. Saf. 2016, 124, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kumar Yadav, K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A.; Kahlon, C.S.; Brar, A.S.; Grover, K.K.; Dia, M.; Steiner, R.L. The Role of Cover Crops towards Sustainable Soil Health and Agriculture—A Review Paper. Am. J. Plant Sci. 2018, 9, 1935–1951. [Google Scholar] [CrossRef] [Green Version]
- Marques, E.; Kur, A.; Bueno, E.; Von Wettberg, E. Defining and improving the rotational and intercropping value of a crop using a plant–soil feedbacks approach. Crop Sci. 2020, 60, 2195–2203. [Google Scholar] [CrossRef]
- Cheng, F.; Ali, M.; Liu, C.; Deng, R.; Cheng, Z. Garlic Allelochemical Diallyl Disulfide Alleviates Autotoxicity in the Root Exudates Caused by Long-Term Continuous Cropping of Tomato. J. Agric. Food Chem. 2020, 68, 11684–11693. [Google Scholar] [CrossRef]
- Yu, L.; Tang, Y.; Wang, Z.; Gou, Y.; Wang, J. Nitrogen-cycling genes and rhizosphere microbial community with reduced nitrogen application in maize/soybean strip intercropping. Nutr. Cycl. Agroecosystems 2018, 113, 35–49. [Google Scholar] [CrossRef]
- Medeiros, E.V.; Notaro, K.d.A.; de Barros, J.A.; Duda, G.P.; dos Santos Moraes, M.d.C.H.; de Queiroz Ambrósio, M.M.; Negreiros, A.M.P.; Júnior, R.S. Soils from intercropped fields have a higher capacity to suppress black root rot in cassava, caused by Scytalidium lignicola. J. Phytopathol. 2019, 167, 209–217. [Google Scholar] [CrossRef]
- Ali, A.; Ghani, M.I.; Elrys, A.S.; Ding, H.; Iqbal, M.; Cheng, Z.; Cai, Z. Different cropping systems regulate the metabolic capabilities and potential ecological functions altered by soil microbiome structure in the plastic shed mono-cropped cucumber rhizosphere. Agric. Ecosyst. Environ. 2021, 318, 107486. [Google Scholar] [CrossRef]
- Gao, Y.; Miao, C.; Xia, J.; Mao, L.; Wang, Y.; Zhou, P. Plant diversity reduces the effect of multiple heavy metal pollution on soil enzyme activities and microbial community structure. Front. Environ. Sci. Eng. 2011, 6, 213–223. [Google Scholar] [CrossRef]
- Lu, S.; Lepo, J.E.; Song, H.X.; Guan, C.-Y.; Zhang, Z.-H. Increased rice yield in long-term crop rotation regimes through improved soil structure, rhizosphere microbial communities, and nutrient bioavailability in paddy soil. Biol. Fertil. Soils 2018, 54, 909–923. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Chen, Z.; Lin, Q.; Shohag, J.I.; He, Z.; Yang, X. A phytoremediation coupled with agro-production mode suppresses Fusarium wilt disease and alleviates cadmium phytotoxicity of cucumber (Cucumis sativus L.) in continuous cropping greenhouse soil. Chemosphere 2020, 270, 128634. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, P.; Comolli, R.; Ferrè, C.; Ghiani, A.; Gentili, R.; Citterio, S. The rotation of white lupin (Lupinus albus L.) with metal-accumulating plant crops: A strategy to increase the benefits of soil phytoremediation. J. Environ. Manag. 2014, 145, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Saad, R.F.; Kobaissi, A.; Echevarria, G.; Kidd, P.; Calusinska, M.; Goux, X.; Benizri, E. Influence of new agromining cropping systems on soil bacterial diversity and the physico-chemical characteristics of an ultramafic soil. Sci. Total Environ. 2018, 645, 380–392. [Google Scholar] [CrossRef]
- Saad, R.F.; Kobaissi, A.; Amiaud, B.; Ruelle, J.; Benizri, E. Changes in physicochemical characteristics of a serpentine soil and in root architecture of a hyperaccumulating plant cropped with a legume. J. Soils Sediments 2018, 18, 1994–2007. [Google Scholar] [CrossRef]
- Long, X.; Ni, W.; Fu, C. Sedum alfredii H: A new Zn hyperaccumulating plant first found in China. Chin. Sci. Bull. 2002, 47, 1634–1637. [Google Scholar]
- Yang, X.; Li, T.; Yang, J.; He, Z.; Lu, L.; Meng, F. Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta 2005, 224, 185–195. [Google Scholar] [CrossRef]
- Deng, D.M.; Shu, W.S.; Zhang, J.; Zou, H.L.; Lin, Z.; Ye, Z.H.; Wong, M.H. Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environ. Pollut. 2007, 147, 381–386. [Google Scholar] [CrossRef]
- Chen, P.; Li, Z.; Luo, D.; Jia, R.; Lu, H.; Tang, M.; Hu, Y.; Yue, J.; Huang, Z. Comparative transcriptomic analysis reveals key genes and pathways in two different cadmium tolerance kenaf (Hibiscus cannabinus L.) cultivars. Chemosphere 2020, 263, 128211. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Z.; Yang, X.L.; Zhu, Y.G.; Li, Y. Physiological response of Trichosanthes kirilowii eeedlings to Cd and Zn combined pollution in soil. Acta Bot. Boreali-Occident. Sin. 2007, 27, 1191–1196. [Google Scholar]
- Alguacil, M.M.; Torrecillas, E.; García-Orenes, F.; Roldán, A. Changes in the composition and diversity of AMF communities mediated by management practices in a Mediterranean soil are related with increases in soil biological activity. Soil Biol. Biochem. 2014, 76, 34–44. [Google Scholar] [CrossRef]
- Kahr, G.; Madsen, F.T. Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl. Environ. Microbiol. 1995, 9, 327–336. [Google Scholar] [CrossRef]
- Ma, C.; Ci, K.; Zhu, J.; Sun, Z.; Liu, Z.; Li, X.; Zhu, Y.; Tang, C.; Wang, P.; Liu, Z. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci. Total Environ. 2020, 759, 143501. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Cheng, J.; Su, J.; Bai, Y.; Jin, J. Changes in plant community composition and soil properties under 3-decade grazing exclusion in semiarid grassland. Ecol. Eng. 2014, 64, 171–178. [Google Scholar] [CrossRef]
- Lestari; Budiyanto, F.; Hindarti, D. Speciation of heavy metals Cu, Ni and Zn by modified BCR sequential extraction procedure in sediments from Banten Bay, Banten Province, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012059. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, F.; Chang, Y.; Cui, J.; He, D.; Du, J.; Chan, A.; Yao, D.; Li, Y.; Chen, Z.; et al. Effects of Soil Amendments on Microbial Activities in a Typical Cd-Contaminated Purple Field Soil, Southwestern China. Bull. Environ. Contam. Toxicol. 2020, 104, 380–385. [Google Scholar] [CrossRef]
- Milani, C.; Hevia-Gonzalez, A.; Foroni, E.; Duranti, S.; Turroni, F.; Lugli, G.A.; Sanchez, B.; Martin, R.; Gueimonde, M.; Van Sinderen, D.; et al. Assessing the Fecal Microbiota: An Optimized Ion Torrent 16S rRNA Gene-Based Analysis Protocol. PLoS ONE 2013, 8, e68739. [Google Scholar] [CrossRef]
- Duong, T.T.T.; Penfold, C.; Marschner, P. Amending soils of different texture with six compost types: Impact on soil nutrient availability, plant growth and nutrient uptake. Plant Soil 2011, 354, 197–209. [Google Scholar] [CrossRef]
- D’Acunto, L.; Andrade, J.F.; Poggio, S.L.; Semmartin, M. Diversifying crop rotation increased metabolic soil diversity and activity of the microbial community. Agric. Ecosyst. Environ. 2018, 257, 159–164. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar]
- Chen, C.; Li, Z.; Li, S.; Deng, N.; Mei, P. Effects of root exudates on the activation and remediation of cadmium ion in contaminated soils. Environ. Sci. Pollut. Res. 2019, 27, 2926–2934. [Google Scholar]
- Ajwa, H.A.; Dell, C.J.; Rice, C.W. Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol. Biochem. 1999, 31, 769–777. [Google Scholar] [CrossRef]
- Balezentiene, L.; Klimas, E. Effect of organic and mineral fertilizers and land management on soil enzyme activities. Agron. Res. 2009, 21, 1153–1159. [Google Scholar]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Zhu, T.; Shu, W.; Geng, L. Ecological improvement of antimony and cadmium contaminated soil by earthworm Eisenia fetida: Soil enzyme and microorganism diversity. Chemosphere 2021, 273, 129496. [Google Scholar] [CrossRef]
- Hussain, J.; Wei, X.; Xue-Gang, L.; Shah, S.R.U.; Aslam, M.; Ahmed, I.; Abdullah, S.; Babar, A.; Jakhar, A.M.; Azam, T. Garlic (Allium sativum) based interplanting alters the heavy metals absorption and bacterial diversity in neighboring plants. Sci. Rep. 2021, 11, 5833. [Google Scholar]
- Ha, M.T.; Phan, T.N.; Kim, J.A.; Oh, W.K.; Lee, J.H.; Woo, H.; Min, B.S. Trichosanhemiketal A and B: Two 13,14-seco-13,14-epoxyporiferastanes from the root of Trichosanthes kirilowii Maxim. Bioorg. Chem. 2019, 83, 105–110. [Google Scholar] [CrossRef]
- Liu, L.; Huang, X.; Zhang, J.; Cai, Z.; Jiang, K.; Chang, Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 2020, 148, 107909. [Google Scholar]
- Li, H.; Li, C.; Song, X.; Liu, Y.; Gao, Q.; Zheng, R.; Li, J.; Zhang, P.; Liu, X. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 2022, 12, 2758. [Google Scholar]
- Guo, H.; Nasir, M.; Lv, J.; Dai, Y.; Gao, J. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Y.; Xie, L.L.; Jin, D.C.; Mi, B.B.; Wang, D.H.; Li, X.F.; Dai, X.Z.; Zou, X.X.; Zhang, Z.; Ma, Y.Q.; et al. Bacterial community response to cadmium contamination of agricultural paddy soil. Appl. Soil Ecol. 2019, 139, 100–106. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, A.; Wu, J.; Fang, W.; Huang, B.; Yan, D.; Wang, Q.; Li, Y. Effects of chloropicrin fumigation combined with biochar on soil bacterial and fungal communities and Fusarium oxysporum. Ecotoxicol. Environ. Saf. 2021, 220, 112414. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Huang, X.Q.; Wu, W.X.; Xiang, Y.J.; Du, L.; Zhang, L.; Liu, Y. Effects of different rotation patterns on the occurrence of clubroot disease and diversity of rhizosphere microbes. J. Integr. Agric. 2020, 19, 2265–2273. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, Q.Z. Changes in fungal community and diversity in strawberry rhizosphere soil after 12 years in the greenhouse. J. Integr. Agric. 2019, 18, 677–687. [Google Scholar] [CrossRef]
- Pan, F.; Meng, Q.; Wang, Q.; Luo, S.; Chen, B.; Khan, K.Y.; Yang, X.; Feng, Y. Endophytic bacterium Sphingomonas SaMR12 promotes cadmium accumulation by increasing glutathione biosynthesis in Sedum alfredii Hance. Chemosphere 2016, 154, 358–366. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Rafiq, M.T.; Khan, K.Y.; Pan, F.; Yang, X.; Feng, Y. Improvement of cadmium uptake and accumulation in Sedum alfredii by endophytic bacteria Sphingomonas SaMR12: Effects on plant growth and root exudates. Chemosphere 2014, 117, 367–373. [Google Scholar] [CrossRef]
- Gremion, F.; Chatzinotas, A.; Harms, H. Comparative 16S rDNA and 16S rRNA sequence analysis indicates that Actinobacteria might be a dominant part of the metabolically active bacteria in heavy metal-contaminated bulk and rhizosphere soil. Environ. Microbiol. 2003, 5, 896–907. [Google Scholar] [CrossRef]
- Luo, J.; Tao, Q.; Jupa, R.; Liu, Y.; Wu, K.; Song, Y.; Li, J.; Huang, Y.; Zou, L.; Liang, Y.; et al. Role of Vertical Transmission of Shoot Endophytes in Root-Associated Microbiome Assembly and Heavy Metal Hyperaccumulation in Sedum alfredii. Environ. Sci. Technol. 2019, 53, 6954–6963. [Google Scholar] [CrossRef]
- Baderna, D.; Lomazzi, E.; Pogliaghi, A.; Ciaccia, G.; Lodi, M.; Benfenati, E. Acute phytotoxicity of seven metals alone and in mixture: Are Italian soil threshold concentrations suitable for plant protection? Environ. Res. 2015, 140, 102–111. [Google Scholar] [CrossRef] [PubMed]
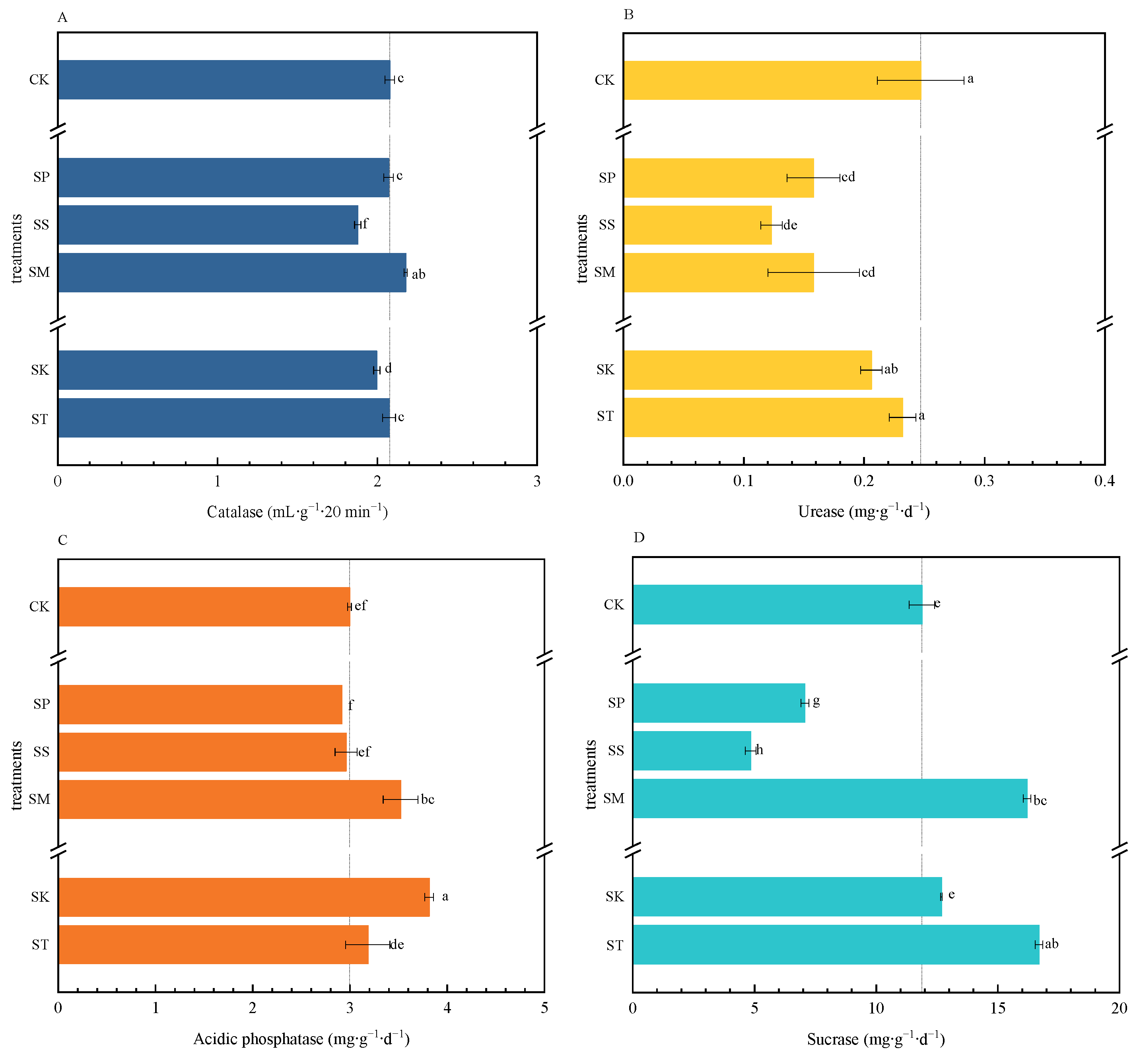

| Treatments | CK | SP | SS | SM | SK | ST |
|---|---|---|---|---|---|---|
| EX-Cd | 0.929 | 0.727 | 0.592 | 0.797 | 0.925 | 0.179 |
| RD-Cd | 0.919 | 0.647 | 0.381 | 0.413 | 0.763 | 0.280 |
| OX-Cd | 0.130 | 0.020 | 0.039 | 0.005 | 0.006 | 0.036 |
| RS-Cd | 0.117 | 0.175 | 0.657 | 0.057 | 0.269 | 0.044 |
| EX-As | 0.130 | 0.373 | 0.101 | 0.098 | 0.075 | 0.038 |
| RD-As | 1.098 | 0.727 | 0.808 | 0.764 | 0.773 | 0.164 |
| OX-As | 0.667 | 0.458 | 2.850 | 0.529 | 0.579 | 0.257 |
| RS-As | 27.916 | 15.461 | 20.163 | 16.605 | 16.555 | 15.740 |
| Treatment | Soil Properties | ||
|---|---|---|---|
| pH | OM (mg/kg) | CEC (cmol+/kg) | |
| CK | 5.97 ± 0.01 b | 18.17 ± 0.06 e | 12.05 ± 0.35 a |
| SP | 6.14 ± 0.05 a | 26.78 ± 0.61 d | 11.46 ± 0.27 b |
| SS | 5.44 ± 0.12 c | 27.79 ± 1.41 cd | 9.99 ± 0.31 c |
| SM | 5.84 ± 0.13 b | 29.46 ± 0.16 bc | 9.98 ± 0.23 c |
| SK | 4.98 ± 0.12 d | 31.42 ± 2.19 ab | 10.35 ± 0.23 c |
| ST | 5.85 ± 0.04 b | 33.12 ± 1.62 a | 11.55 ± 0.05 b |
| Treatment | Richness Estimators | Evenness Indices | Coverage | ||
|---|---|---|---|---|---|
| ACE | Chao1 | Simpson | Shannon | ||
| CK | 1416.14 ± 9.96 d | 1447.95 ± 7.58 d | 0.0081 ± 0.0014 b | 5.8040 ± 0.11 b | 1.0 |
| SP | 1691.82 ± 14.96 a | 1693.15 ± 14.33 a | 0.0036 ± 0.0004 e | 6.4780 ± 0.04 a | 1.0 |
| SS | 1531.99 ± 16.41 bc | 1565.51 ± 16.43 bc | 0.0043 ± 0.0003 d | 6.2668 ± 0.05 a | 1.0 |
| SM | 1663.51 ± 2.91 a | 1674.78 ± 7.56 a | 0.0037 ± 0.0002 e | 6.4557 ± 0.01 a | 1.0 |
| SK | 1550.34 ± 33.46 b | 1574.52 ± 42.2 b | 0.0079 ± 0.0020 c | 5.9639 ± 0.12 b | 1.0 |
| ST | 1466.50 ± 31.66 cd | 1499.36 ± 29.5 cd | 0.0141 ± 0.0047 a | 5.7576 ± 0.16 b | 1.0 |
| Treatment | Dry Weight (kg/arce) | Economic Benefit (CNY/arce) | ||
|---|---|---|---|---|
| Biomass Fuel | Biochar | Biothanol | ||
| SP | 925.3 | 1388.0 | 2705.9 | 3198.4 |
| SS | 1323.5 | 1984.5 | 3937.9 | 3246.9 |
| SM | 830.1 | 1245.2 | 2193.6 | 1819.4 |
| SK | 717.7 | 1076.6 | 2111.3 | 1746.4 |
| ST | 1561.8 | 2342.7 | 4326.3 | 4348.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, Y.; Zhu, J.; Wang, P.; Tang, C.; Liu, Z. Remediation and Micro-Ecological Regulation of Cadmium and Arsenic Co-Contaminated Soils by Rotation of High-Biomass Crops and Sedum alfredii Hance: A Field Study. Sustainability 2022, 14, 5717. https://doi.org/10.3390/su14095717
Li X, Zhu Y, Zhu J, Wang P, Tang C, Liu Z. Remediation and Micro-Ecological Regulation of Cadmium and Arsenic Co-Contaminated Soils by Rotation of High-Biomass Crops and Sedum alfredii Hance: A Field Study. Sustainability. 2022; 14(9):5717. https://doi.org/10.3390/su14095717
Chicago/Turabian StyleLi, Xinyi, Yelin Zhu, Jian Zhu, Ping Wang, Cheng Tang, and Zhiming Liu. 2022. "Remediation and Micro-Ecological Regulation of Cadmium and Arsenic Co-Contaminated Soils by Rotation of High-Biomass Crops and Sedum alfredii Hance: A Field Study" Sustainability 14, no. 9: 5717. https://doi.org/10.3390/su14095717





